Adsorption and Corrosion Inhibition Studies of Some Selected Dyes as Corrosion Inhibitors for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, Quantum Chemical Studies and Synergistic Effect with Iodide Ions
Abstract
:1. Introduction
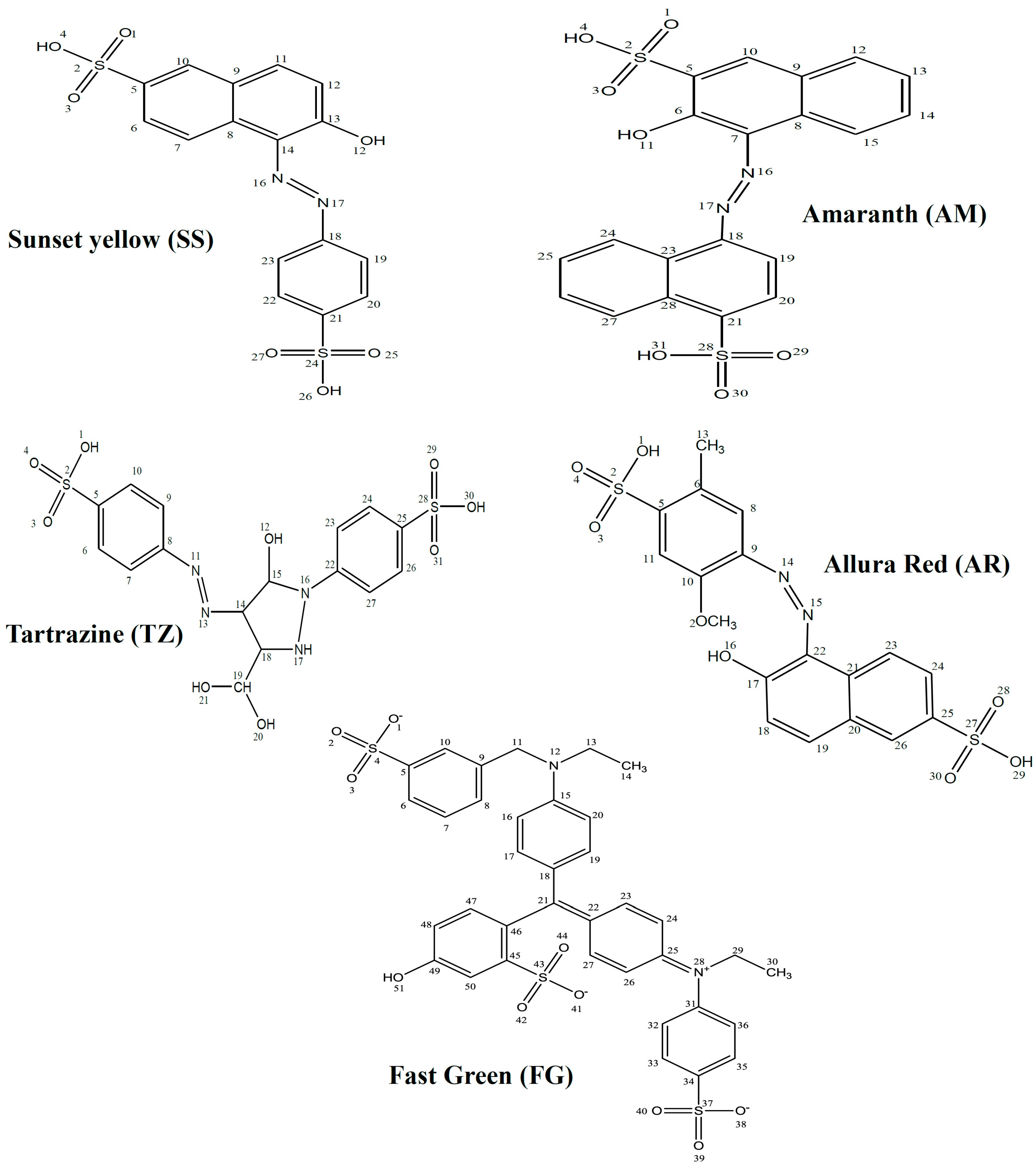
2. Results and Discussion
2.1. Gravimetric Method
2.1.1. Effect of the Inhibitor Concentration and Temperature

2.1.2. Thermodynamic and Activation Parameters
| Inhibitor | Inhibitor Concentration (ppm) | Ea (kJ·mol−1) | ΔH* (kJ·mol−1) | ΔS* (JK−1·mol−1) |
|---|---|---|---|---|
| - | 58.28 | 55.26 | −115.47 | |
| SS | 25 | 77.72 (90.96) a | 75.15 (88.15) a | −58.52 (−20.94) a |
| 50 | 78.09 (90.94) a | 75.46 (88.00) a | −57.61 (−22.02) a | |
| 75 | 79.28 (91.91) a | 77.08 (89.48) a | −52.70 (−17.78) a | |
| 100 | 81.27 (94.15) a | 78.43 (91.46) a | −48.79 (−12.30) a | |
| 125 | 82.38 (93.77) a | 80.03 (91.70) a | −44.06 (−6.39) a | |
| 150 | 82.76 (95.98) a | 80.53 (93.13) a | −42.64 (−8.31) a | |
| AM | 25 | 81.50 (91.88) a | 78.83 (89.40) a | −46.55 (−18.62) a |
| 50 | 81.33 (92.95) a | 78.75 (60.60) a | −47.05 (−15.12) a | |
| 75 | 84.63 (96.10) a | 81.84 (93.17) a | −37.74 (−7.64) a | |
| 100 | 85.05 (95.89) a | 79.78 (93.30) a | −44.22 (−7.56) a | |
| 125 | 85.99 (95.56) a | 83.53 (92.60) a | −32.92 (−9.80) a | |
| 150 | 87.81 (95.70) a | 85.15 (93.21) a | −28.09 (−6.73) a | |
| AR | 25 | 58.28 (96.14) a | 91.94 (87.69) a | −9.14 (−7.14) a |
| 50 | 81.69 (102.16) a | 79.64 (89.46) a | −39.82 (−6.81) a | |
| 75 | 83.64 (97.44) a | 81.99 (89.99) a | −37.49 (−2.57) a | |
| 100 | 85.20 (98.75) a | 82.96 (87.16) a | −34.66 (9.40) a | |
| 125 | 86.22 (99.58) a | 84.59 (91.59) a | −29.84 (9.24) a | |
| 150 | 87.61 (98.05) a | 88.91 (91.20) a | −16.87 (−2.15) a | |
| TZ | 25 | 77.33 (89.47) a | 74.73 (87.69) a | −59.19 (−23.69) a |
| 50 | 81.86 (85.65) a | 107.39 (89.46) a | 36.84 (−18.53) a | |
| 75 | 82.88 (92.85) a | 106.97 (89.99) a | 34.76 (−17.37) a | |
| 100 | 83.47 (92.85) a | 101.74 (87.16) a | 23.12 (−25.77) a | |
| 125 | 85.42 (94.44) a | 82.35 (91.59) a | −36.41 (−13.04) a | |
| 150 | 85.63 (93.90) a | 90.91 (91.20) a | −10.30 (−14.96) a | |
| FG | 25 | 108.63 (59.26) a | 106.08 (57.03) a | 33.01 (−129.52) a |
| 50 | 109.78 (66.77) a | 107.39 (64.44) a | 36.84 (−107.41) a | |
| 75 | 109.95 (63.54) a | 106.97 (60.95) a | 34.76 (−119.96) a | |
| 100 | 105.78 (69.77) a | 105.85 (66.95) a | 30.27 (−102.17) a | |
| 125 | 108.75 (67.78) a | 106.07 (64.58) a | 30.52 (−109.99) a | |
| 150 | 106.23 (68.47) a | 104.38 (64.14) a | 23.87 (−106.33) a |
2.2. Adsorption Isotherms
| Inhibitor | T/K | Kads (103 × mol−1) | −Δ (kJ·mol−1) |
|---|---|---|---|
| SS | 303 | 0.15 (0.34) a | −5.40 (−7.44) a |
| 313 | 0.09 (0.10) a | −4.40 (−4.39) a | |
| 323 | 0.03 (0.08) a | −1.37 (−3.94) a | |
| 333 | 0.05 (0.05) a | −2.81 (−2.81) a | |
| AM | 303 | 0.14 (0.55) a | −5.13 (−8.60) a |
| 313 | 0.09 (0.17) a | −4.43 (−5.89) a | |
| 323 | 0.03 (0.12) a | −1.64 (−5.02) a | |
| 333 | 0.07 (0.07) a | −3.79 (−3.79) a | |
| AR | 303 | 0.12 (0.63) a | −4.87 (−8.95) a |
| 313 | 0.06 (0.28) a | −3.07 (−7.18) a | |
| 323 | 0.03 (0.12) a | −0.93 (−5.11) a | |
| 333 | 0.11 (0.11) a | −4.86 (−4.86) a | |
| TZ | 303 | 0.14 (0.49) a | −5.21 (−8.32) a |
| 313 | 0.097 (0.23) a | −4.39 (−6.59) a | |
| 323 | 0.03 (0.09) a | −0.93 (−4.49) a | |
| 333 | 0.10 (0.10) a | −4.71 (−4.61) a | |
| FG | 303 | 0.40 (0.39) a | −8.04 (−7.76) a |
| 313 | 0.47 (3.57) a | −8.49 (−13.77) a | |
| 323 | 0.01 (0.41) a | −1.17 (−8.37) a | |
| 333 | 0.01 (0.50) a | −1.69 (−8.89) a |

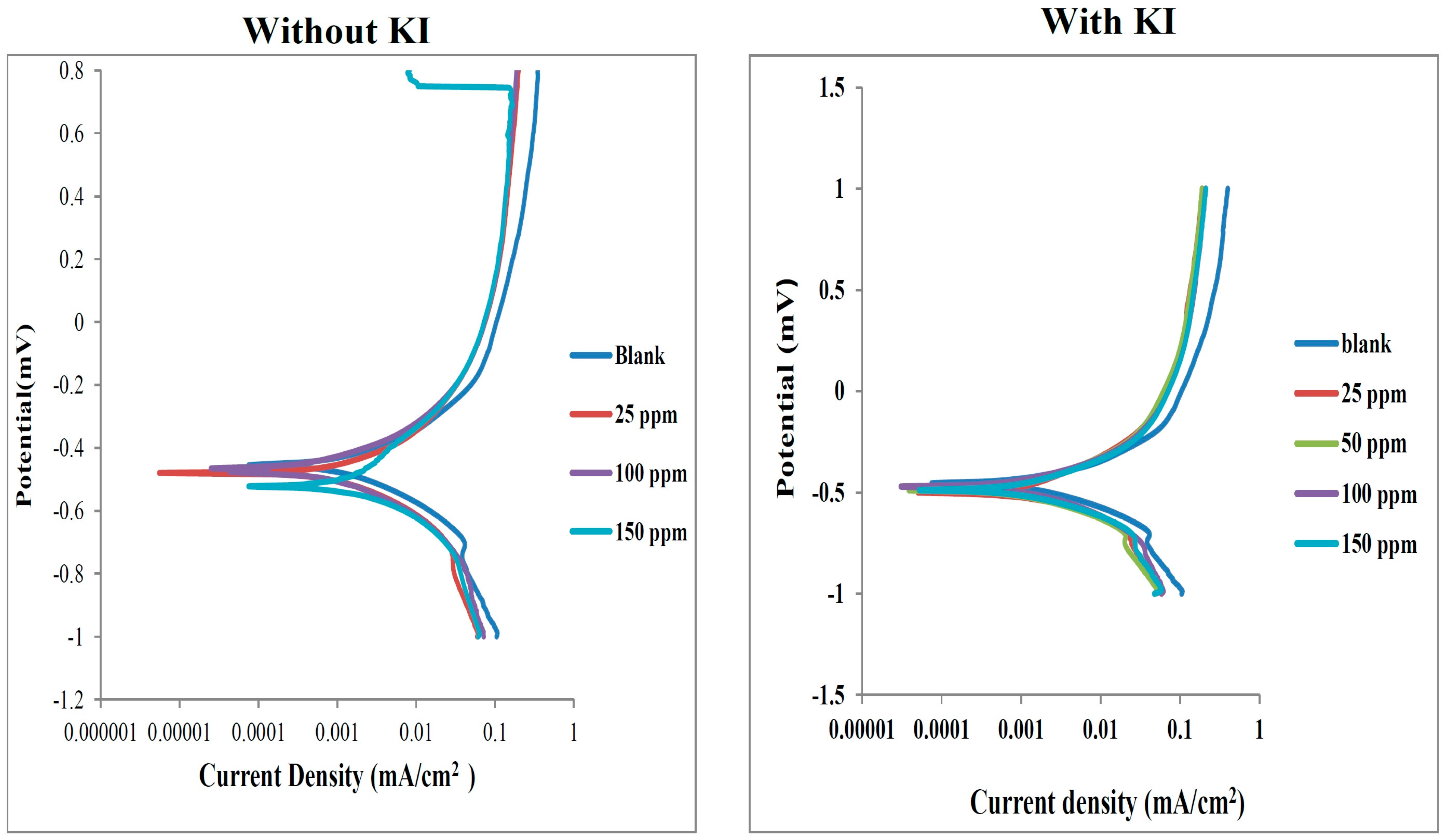
2.3. Electrochemical Measurements
Potentiodynamic Polarization Measurements
| Inhibitor/Blank | Conc. of Inhibitor (ppm) | icorr (A cm−2) × 10−4 | ba (mV·dec−1) | bc (mV·dec−1) | −Ecorr (mV) | % IEP |
|---|---|---|---|---|---|---|
| Blank | - | 16.86 | 148 | 147 | 459 | |
| SS | 25 | 8.190 (7.275) a | 111 (80) a | 101 (150) a | 481 (505) a | 51.42 (56.85) a |
| 100 | 5.588 (6.459) a | 93 (78) a | 100 (76) a | 478 (490) a | 66.86 (61.69) a | |
| 150 | 4.613 (4.720) a | 89 (76) a | 75 (89) a | 477 (488) a | 72.94 (72.04) a | |
| AM | 25 | 5.982 (3.846) a | 100 (67) a | 93 (122) a | 437 (490) a | 64.52 (77.19) a |
| 50 | 5.122 (3.463) a | 82 (81) a | 93 (133) a | 458 (491) a | 72.16 (79.46) a | |
| 150 | 4.489 (5.078) a | 79 (87) a | 81 (103) a | 460 (481) a | 73.37 (69.88) a | |
| AR | 25 | 7.797 (1.659) a | 122 (168) a | 109 (189) a | 463 (475) a | 53.75 (90.16) a |
| 100 | 7.425 (5.086) a | 57 (97) a | 96 (109) a | 524 (460) a | 55.96 (69.83) a | |
| 150 | 7.272 (4.598) a | 76 (80) a | 95 (89) a | 483 (483) a | 56.86 (72.73) a | |
| TZ | 25 | 4.504 (1.388) a | 112 (154) a | 125 (151) a | 461 (465) a | 73.29 (91.77) a |
| 100 | 3.554 (4.422) a | 72 (79) a | 100 (91) a | 481 (476) a | 78.92 (73.77) a | |
| 150 | 2.902 (7.256) a | 49 (91) a | 61 (122) a | 510 (489) a | 82.79 (56.96) a | |
| FG | 25 | 9.327 (7.333) a | 102 (101) a | 144 (137) a | 486 (485) a | 44. 76 (56.51) a |
| 100 | 5.800 (3.953) a | 90 (64) a | 112 (116) a | 473 (482) a | 65.59 (76.55) a | |
| 150 | 5.152 (1.763) a | 69 (36) a | 121 (80) a | 490 (480) a | 69.44 (89.53) a |
2.4. Synergism Consideration
| Inhibitor | SI Values at Various Concentrations of Inhibitor | |||||
|---|---|---|---|---|---|---|
| 25 ppm | 50 ppm | 75 ppm | 100 ppm | 125 ppm | 150 ppm | |
| SS | 1.50 (1.83) b | 1.50 | 1.49 | 1.50 (1.94) b | 1.52 | 1.50 (1.74) b |
| AM | 1.45 (1.52) b | 1.45 | 1.48 | 1.48 (1.00) b | 1.49 | 1.50 (1.80) b |
| AR | 1.47 (1.18) b | 1.49 | 1.50 | 1.50 (1.55) b | 1.52 | 1.55 (1.50) b |
| TZ | 1.46 (1.37) b | 1.51 | 1.52 | 1.52 (1.78) b | 1.53 | 1.53 (2.39) b |
| FG | 1.57 (1.72) b | 1.54 | 1.53 | 1.52 (1.54) b | 1.52 | 1.52 (1.36) b |
2.5. Quantum Chemical Calculation
2.5.1. Results of the Study on Neutral Species

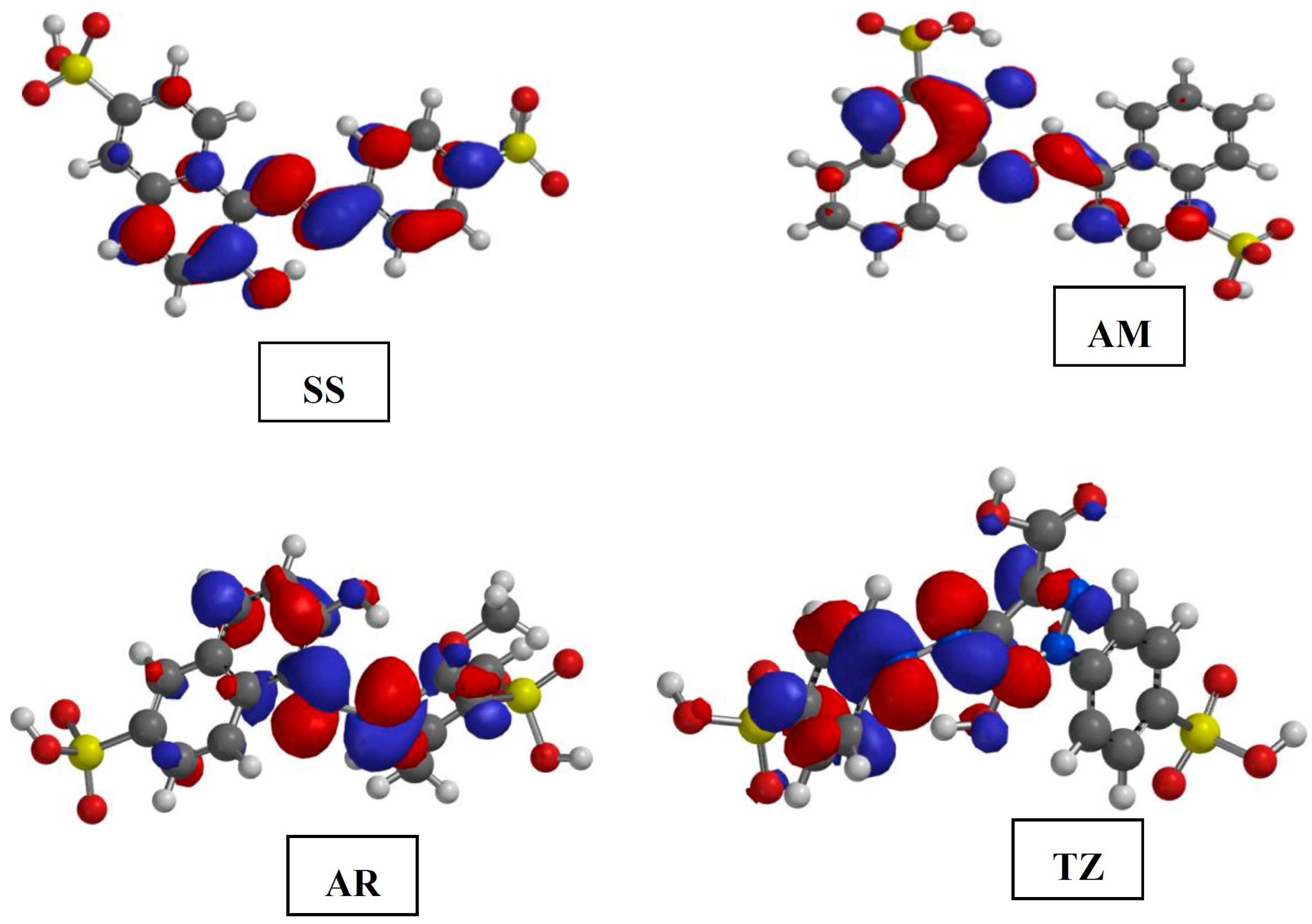
| Parameters | AR | AM | SS | TZ | ||||
|---|---|---|---|---|---|---|---|---|
| Neutral | Protonated | Neutral | Protonated | Neutral | Protonated | Neutral | Protonated | |
| EHOMO (eV) | −6.15 | −9.59 | −6.35 | −9.29 | −6.46 | −9.96 | −6.62 | −9.05 |
| ELUMO (eV) | −2.62 | −7.09 | −3.63 | −7.12 | −3.27 | −7.46 | −2.87 | −7.55 |
| ΔE (eV) | 3.53 | 2.5 | 2.72 | 2.17 | 3.19 | 2.5 | 3.75 | 1.5 |
| η (eV) | 1.77 | 1.25 | 1.36 | 1.085 | 1.60 | 1.25 | 1.88 | 0.75 |
| σ (eV) | 0.57 | 0.8 | 0.74 | 0.92 | 0.63 | 0.8 | 0.53 | 1.33 |
| ΔN | −0.74 | −0.54 | −0.74 | −0.56 | −0.67 | −0.68 | −0.60 | −0.87 |
| ω | 5.45 | 27.82 | 9.15 | 31.02 | 7.42 | 30.35 | 6.00 | 45.93 |
| μ (Debye) | 6.14 | 5.65 | 6.73 | 8.53 | 3.74 | 8.11 | 5.28 | 12.40 |
| IE (%) | 87.94 | 87.65 | 86.03 | 85.06 | ||||
2.5.2. Results of the Study on Protonated Species

3. Experimental Section
3.1. Materials and Aggressive Solutions
3.2. Weight Loss Measurements
3.3. Electrochemical Measurements
3.4. Quantum Chemical Calculations
4. Conclusions
- (a)
- All the studied dyes showed appreciable inhibition efficiency for mild steel corrosion in 0.5 M HCl and their inhibition efficiencies increase with increasing concentration, and decrease with increasing temperature.
- (b)
- The results of weight loss measurements suggest possible aggregates formation of the dyes molecules at some concentrations and temperatures leading to a non-uniform trend of inhibition efficiency.
- (c)
- Addition of KI synergistically improved the inhibition efficiency of all the studied dyes.
- (d)
- Potentiodynamic polarization results revealed that the studied dyes are mixed-type inhibitors both in the absence and presence of KI.
- (e)
- The adsorption of the studied dyes on mild steel surface with and without KI obeys the Langmuir adsorption isotherm and involves physical adsorption mechanism.
- (f)
- The quantum chemically derived EHOMO results give a good correlation with the trend of the inhibition efficiencies of the dyes at 150 ppm and 303 K as obtained from the weight loss measurements. The HOMO, LUMO and Fukui indices revealed the most probable sites of adsorption of the dyes to the steel surface.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ulaeto, S.B.; Ekpe, U.J.; Chidiebere, M.A.; Oguzie, E.E. Corrosion inhibition of mild steel in hydrochloric acid by acid extracts of eichhornia crassipes. Int. J. Mater. Chem. 2012, 2, 158–164. [Google Scholar]
- De la Fuente, D.; Diaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Xometl, O.; Lopez-Aguilar, C.; Herrasti-Gonzalez, P.; Likhanova, N.V.; Lijanova, I.; Martinez-Palou, R.; Rivera-Marquez, J.A. Adsorption and corrosion inhibition performance by three new ILs on API 5L X52 steel surface in acid media. Ind. Eng. Chem. Res. 2014, 53, 9534–9543. [Google Scholar] [CrossRef]
- Mashuga, M.E.; Olasunkanmi, L.O.; Adekunle, A.S.; Yesudass, S.; Kabanda, M.M.; Ebenso, E.E. Adsorption, thermodynamic and quantum chemical studies of 1-hexyl-3-methylimidazolium based ionic liquids as corrosion inhibitors for mild steel in HCl. Materials 2015, 8, 3607–3632. [Google Scholar] [CrossRef]
- Sastri, V.S. Corrosion Inhibitors Principles and Applications; Jonh Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Asegbeloyin, J.N.; Ejikeme, P.M.; Olasunkanmi, L.O.; Adekunle, A.S.; Ebenso, E.E. A novel Schiff base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2′-(ethylenedioxy)diethylamine as Potential Corrosion Inhibitor for Mild Steel in acidic medium. Materials 2015, 8, 2918–2934. [Google Scholar] [CrossRef]
- Bouayed, M.; Rabaa, H.; Srhiri, A.; Saillard, J.Y.; Bachiran, A.B.; Beuze, A.L. Experimental and theoretical study of organic corrosion inhibitors on iron in acidic medium. Corros. Sci. 1998, 41, 501–517. [Google Scholar] [CrossRef]
- Bommersbach, P.; Dumont, C.A.; Millet, J.P.; Normand, B. Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical methods. Electrochim. Acta 2005, 51, 1076–1084. [Google Scholar] [CrossRef]
- Ortega, D.T.; Pandiyan, T.; Cruz, J.; Ochoa, E.G. Interaction of imidazoline compounds with Fen (n = 1–4 atoms) as a model for corrosion inhibition: DFT and electrochemical studies. J. Phys. Chem. C 2007, 27, 9853–9866. [Google Scholar] [CrossRef]
- Paul, S.; Kar, B. Mitigation of mild steel corrosion in acid by green inhibitors: Yeast, pepper, garlic, and coffee. ISRN Corros. 2012. [Google Scholar] [CrossRef]
- Matad, P.B.; Mokshanatha, P.B.; Hebbar, N.; Venkatesha, V.T.; Tandon, H.C. Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. Ind. Eng. Chem. Res. 2014, 53, 8436–8444. [Google Scholar] [CrossRef]
- Shivakumar, S.S.; Mohana, K.N. Centella asiatica extracts as green corrosion inhibitor for mild steel in 0.5 M sulphuric acid medium. Adv. Appl. Sci. Res. 2012, 3, 3097–3106. [Google Scholar]
- Rajendran, S.; Sri, V.G.; Arockiaselvi, J.; Amalraj, A.J. Corrosion inhibition by plant extracts-an overview. Bull. Electrochem. 2005, 21, 367–377. [Google Scholar]
- Lame, A.; Kokalari, E.; Jano, A. Use of green inhibitors for concrete armor protection against H2SO4 corrosion. Asian J. Chem. 2013, 25, 4017–4021. [Google Scholar]
- Dotto, G.L.; Pinto, L.A.A. Adsorption of food dyes onto chitosan: Optimization process and kinetic. Carbohydr. Polym. 2011, 84, 231–238. [Google Scholar] [CrossRef]
- Elwakeel, K.Z. Removal of reactive Black 5 from aqueous solutions using magnetic chitosan resins. J. Hazard. Mater. 2009, 167, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, S.; Sohrabi, B.; Tehrani-Bagha, A.R. The study of sunset yellow anionic dye interaction with gemini and conventional cationic surfactants in aqueous solution. Dyes Pigment. 2012, 95, 768–775. [Google Scholar] [CrossRef]
- Snehalatha, M.; Ravikumar, C.; Joe, I.H.; Sekar, N.; Jayakumar, V.S. Spectroscopic analysis and DFT calculations of a food additive carmoisine. Spectrochim. Acta A 2009, 72, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Zeini-Isfahani, A.; Habibi, M.H. Molecular exciton theory calculations based on experimental results for solophenyl red 3BL azo dye-surfactants interactions. Spectrochim. Acta A 2006, 64, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Oguzie, E.E. Influence of halide ions on the inhibitive effect of Congo red dye on the corrosion of mild steel in sulphuric acid solution. Mater. Chem. Phys. 2004, 87, 212–217. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Okolue, B.N.; Ebenso, E.E.; Onuoha, G.N.; Onuchukwu, A.I. Evaluation of the inhibitory effect of methylene blue dye on the corrosion of aluminium in hydrochloric acid. Mater. Chem. Phys. 2004, 87, 394–401. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Okolue, B.N.; Ogukwe, C.E.; Onuchukwu, A.I.; Unaegbu, C. Inhibition of corrosion of aluminium in alkaline solution by methylene blue. Bull. Electrochem. 2004, 20, 421–425. [Google Scholar]
- Oguzie, E.E.; Unaegbu, C.; Ogukwe, C.E.; Okolue, B.N.; Onuchukwu, A.I. Inhibition of mild steel corrosion in sulphuric acid using indigo dye and synergistic halide additives. Mater. Chem. Phys. 2004, 84, 363–368. [Google Scholar] [CrossRef]
- Onuchukwu, A.I.; Njemanze, G.N. The corrosion susceptibility of dyes and dye-bath auxiliaries on galvanized steel pipes. J. Chem. Soc. Niger. 1997, 22, 1500–1504. [Google Scholar]
- Ebenso, E.E. Inhibition of corrosion of mild steel hydrochloric acid by some azo dyes. Niger. J. Chem. Res. 2001, 6, 8–12. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Okafor, P.C.; Ibok, U.J.; Ekpe, U.J.; Onuchukwu, A.I. The joint effects of halide ions and methylene blue on the corrosion inhibition of aluminium and mild steel in acid corrodent. J. Chem. Soc. Niger. 2004, 29, 15–25. [Google Scholar]
- Ebenso, E.E.; Kabanda, M.M.; Murulana, L.C.; Singh, A.K.; Shukla, S.K. Electrochemical and quantum chemical investigation of some azine and thiazine dyes as potential corrosion inhibitors for mild steel in hydrochloric acid solutions. Ind. Eng. Chem. Res. 2012, 51, 12940–12958. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Udosoro, I.I.; Udoh, A.P. Synergistic and antagonistic effects between halide ions and carboxylmethyl cellulose for the corrosion inhibition of mild steel in sulphuric acid solution. Cellulose 2010, 17, 635–648. [Google Scholar] [CrossRef]
- Eddy, N.O.; Odoemelam, S.A.; Odiongenyi, A.O. Joint effect of halides and ethanol extract of Lasianthera africana on inhibition corrosion of mild steel in H2SO4. J. Appl. Electrochem. 2009, 39, 349–357. [Google Scholar] [CrossRef]
- Prabhu, R.A.; Venkateshab, T.V.; Shanbhag, A.V. Carmine and fast green as corrosion inhibitors for mild steel in hydrochloric acid solution. J. Iran. Chem. Soc. 2009, 6, 353–363. [Google Scholar] [CrossRef]
- Pandya, J.M.; Joshi, S.D.; Hathi, M.V.; Acharya, G.D. Food colorant as corrosion inhibitors for aluminium-copper alloy in trichloroacetic acid. Asian J. Chem. 2001, 13, 158–162. [Google Scholar]
- Talati, J.D.; Patel, A.S. Corrosion of copper by food acids containing colourants and sweetening agents—Part IV: Corrosion by lactic acid. Mater. Corros. 1988, 39, 27–33. [Google Scholar] [CrossRef]
- Renshaw, M.P.; Day, I.J. NMR characterization of the aggregation state of the azo dye Sunset Yellow in the isotropic phase. J. Phys. Chem. B 2010, 114, 10032–10038. [Google Scholar] [CrossRef] [PubMed]
- Snehalatha, M.; Sekar, N.; Jayakumar, V.S.; Hubert, J.I. Quantum chemical computations and Fourier transform infrared spectral studies of a nonlinear food dye E110. Spectrochim. Acta A 2008, 69, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Chami, F.; Wilson, M.R. Molecular order in a chromonic liquid crystal: A molecular simulation study of the anionic azo dye sunset yellow. J. Am. Chem. Soc. 2010, 132, 7794–7802. [Google Scholar] [CrossRef] [PubMed]
- Marmion, D.M. Handbook of U.S. Colorants: Foods, Drugs, Cosmetics, and Medical Devices, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1991. [Google Scholar]
- Omar, A.; el Seoud, O.A.; Pires, P.A.R.; Abdel-Moghny, T.; Bastos, E.L. Synthesis and micellar properties of surface-active ionic liquids: 1-Alkyl-3-methylimidazolium chlorides. J. Colloid Interface Sci. 2007, 313, 296–304. [Google Scholar]
- Singh, T.; Kumar, A. Aggregation behavior of ionic liquids in aqueous solutions: Effect of alkyl chain length, cations, and anions. J. Phys. Chem. B 2007, 111, 7843–7851. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Zhang, S.; Xuan, X. Structural effects of anions and cations on the aggregation behavior of ionic liquids in aqueous solutions. J. Phys. Chem. B 2008, 112, 16682–16689. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Hashim, M.A.; Nabi, F.; AL-Thabaiti, S.A.; Khan, Z. Anti-corrosion ability of surfactants: A review. Int. J. Electrochem. Sci. 2011, 6, 1927–1948. [Google Scholar]
- Fuchs-Godec, R. The adsorption, CMC determination and corrosion inhibition of some N-alkyl quaternary ammonium salts on carbon steel surface in 2 M H2SO4. Colloids Surf. A 2006, 280, 130–139. [Google Scholar] [CrossRef]
- Negm, N.A.; Elkholy, Y.M.; Zahran, M.K.; Tawfik, S.M. Corrosion inhibition efficiency and surface activity of benzothiazol-3-ium cationic Schiff base derivatives in hydrochloric acid. Corros. Sci. 2010, 52, 3523–3536. [Google Scholar] [CrossRef]
- Negm, N.A.; Mohamed, A.S. Surface and thermodynamic properties of diquaternary Bola-form amphiphiles containing an aromatic spacer. J. Surfactant Deterg. 2004, 7, 23–30. [Google Scholar] [CrossRef]
- Negm, N.A.; Ismail, A.; Aiad, I.A. Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J. Surfactant Deterg. 2007, 10, 87–92. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. The Porphyrin Handbook: Phthalocyanines: Properties and Materials; Elsevier Science: San Diego, CA, USA, 2003. [Google Scholar]
- Dibetsoe, M.; Olasunkanmi, L.O.; Fayemi, O.E.; Yesudass, S.; Ramaganthan, B.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Some phthalocyanine and naphthalocyanine derivatives as corrosion inhibitors for aluminium in acidic medium: Experimental, quantum chemical calculations, qsar studies and synergistic effect of iodide ions. Molecules 2015, 20, 15701–15734. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ebenso, E.E. The synergistic effect of polyacrylamide and iodide on the corrosion inhibition of mild steel in H2SO4. Mater. Chem. Phys. 2007, 106, 387–393. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Alemu, H.; Umoren, S.A.; Obot, I.B. Inhibition of mild steel corrosion in sulphuric acid using Alizarin yellow GG dye and synergistic iodide addictive. Int. J. Electrochem. Sci. 2008, 3, 1325–1339. [Google Scholar]
- Fuchs-Godec, R.; Pavlovic, M.G.; Tomic, M.V. Effect of temperature on the corrosion inhibition of nonionic surfactant TRITON-X-405 on ferric stainless steel in 1.0 M H2SO4. Ind. Eng. Chem. Res. 2012, 51, 274–284. [Google Scholar] [CrossRef]
- Singh, A.K.; Quraishi, M.A. Effect of 2,2-benzothiazolyl disulfide on the corrosion of mild steel in acidic medium. Corros. Sci. 2009, 51, 2752–2760. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Gengembre, L.M. A new triazole derivative as inhibitor of the acidcorrosion of mild steel: Electrochemical studies, weight loss determination, SEM and XPS. Appl. Surf. Sci. 1999, 152, 237–249. [Google Scholar] [CrossRef]
- Kertit, S.; Hammouti, B. Corrosion inhibition of iron in 1 M HCl by 1-phenyl-5-mercapto-1,2,3,4-tetrazole. Appl. Surf. Sci. 1996, 93, 59–66. [Google Scholar] [CrossRef]
- Obot, I.B.; Ebenso, E.E.; Obi-Egbedi, N.O.; Afolabi, A.S.; Gasem, Z.M. Experimental and theoretical investigations of adsorption characteristics of itraconazole as green corrosion inhibitor at a mild steel/ hydrochloric acid interface. Res. Chem. Intermed. 2012, 38, 1761–1779. [Google Scholar] [CrossRef]
- Herrag, L.; Hammouti, B.; Elkadiri, S.; Aouniti, A.; Jama, C.; Vezin, H.; Bentiss, F. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010, 52, 3042–3051. [Google Scholar] [CrossRef]
- Hoar, T.P.; Holliday, R.D. The inhibition by quinolines and thioureas of the acid dissolution of mild steel. J. Appl. Chem. 1953, 3, 502–513. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr.; Hurd, R.M. Temperature coefficient of corrosion inhibition. Corrosion 1967, 23, 252–258. [Google Scholar] [CrossRef]
- Benali, O.; Larabi, L.; Merah, S.; Harek, Y. Influence of the methylene blue dye (MBD) on the corrosion inhibition of mild steel in 0.5 M sulphuric acid, Part I: Weight loss and electrochemical studies. J. Mater. Environ. Sci. 2011, 2, 39–48. [Google Scholar]
- Szauer, T.; Brandt, A. Adsorption of oleates of various amines on iron in acidic solution. Electrochim. Acta 1981, 26, 1209–1217. [Google Scholar] [CrossRef]
- Dahmani, M.; Et-Touhami, A.; Al-Deyab, S.S.; Hammouti, B.; Bouyanzer, A. Corrosion inhibition of C38 steel in 1 M HCl: A comparative study of black pepper extract and its isolated piperine. Int. J. Electrochem. Sci. 2010, 5, 1060–1069. [Google Scholar]
- Saliyan, V.R.; Adhikari, A.V. Inhibition of corrosion of mild steel in acid media by N′-benzylidene-3-(quinolin-4-ylthio)propanohydrazide. Bull. Mater. Sci. 2007, 31, 699–711. [Google Scholar] [CrossRef]
- Ateya, B.; El-Anadauli, B.E.; El-Nizamy, F.M. The adsorption of thiourea on mild steel. Corros. Sci. 1984, 24, 509–515. [Google Scholar] [CrossRef]
- Emregül, K.C.; Hayvalì, M. Studies on the effect of a newly synthesized Schiff base compound from phenazone and vanillin on the corrosion of steel in 2M HCl. Corros. Sci. 2006, 48, 797–812. [Google Scholar] [CrossRef]
- Mohammed, K.Z.; Hamdy, A.; Abdel-wahab, A.; Farid, N.A. Temperature effect on corrosion inhibition of carbon steel in formation water by Non-ionic synergistic influence of halide ions. Life Sci. J. 2012, 9, 424–434. [Google Scholar]
- Behpour, M.; Ghoreishi, S.M.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M.; Gandomi, A. Electrochemical and theoretical investigation on the corrosion of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid. Corros. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Murulana, L.C.; Kabanda, M.M.; Ebenso, E.E. Experimental and theoretical studies on the corrosion inhibition of mild steel by some sulphonamides in aqueous HCl. RSC Adv. 2015, 5, 28743–28761. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, S.; Guo, L.; Zhang, S.; Lu, H.; Gong, Y.; Gao, G. Evaluating two new Schiff bases synthesized on the inhibition of corrosion of copper in NaCl solutions. RSC Adv. 2015, 5, 14804–14813. [Google Scholar] [CrossRef]
- De Souza, F.S.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Murulana, L.C.; Singh, A.K.; Shukla, S.K.; Kabanda, M.M.; Ebenso, E.E. Experimental andquantum chemical studies of some bis(trifluoromethyl-sulfonyl) imide imidazolium-based ionic liquids as corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 13282–13299. [Google Scholar] [CrossRef]
- Solmaz, R.; Altunbas, E.; Kardas, G. Adsorption and corrosion inhibition effect of 2-((5-mercapto-1,3,4-thidiazol-2-ylimino)methyl)phenol Schiff base mild steel. Mater. Chem. Phys. 2011, 125, 796–801. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Gomez, B.; Likhanova, N.V.; Dominguez-Aguilar, M.A.; Martinez-Palou, R.; Vela, A.; Gazquez, J.L. Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles. J. Phys. Chem. B 2006, 110, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Zhang, Y.; Wang, P.; Zhang, J. Theoretical evaluation of inhibition performance of purine corrosion inhibitors. Mol. Simul. 2013, 39, 1034–1041. [Google Scholar] [CrossRef]
- Echegaray, E.; Cárdenas, C.; Rabi, S.; Rabi, N.; Lee, S.; Zadeh, F.H.; Toro-Labbe, A.; Ayers, P.W.; Anderson, J.S.M. In pursuit of negative Fukui functions: Examples where the highest occupied molecular orbital fails to dominate the chemical reactivity. J. Mol. Model. 2013, 19, 2779–2783. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Mortier, W.J. The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peme, T.; Olasunkanmi, L.O.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Adsorption and Corrosion Inhibition Studies of Some Selected Dyes as Corrosion Inhibitors for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, Quantum Chemical Studies and Synergistic Effect with Iodide Ions. Molecules 2015, 20, 16004-16029. https://doi.org/10.3390/molecules200916004
Peme T, Olasunkanmi LO, Bahadur I, Adekunle AS, Kabanda MM, Ebenso EE. Adsorption and Corrosion Inhibition Studies of Some Selected Dyes as Corrosion Inhibitors for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, Quantum Chemical Studies and Synergistic Effect with Iodide Ions. Molecules. 2015; 20(9):16004-16029. https://doi.org/10.3390/molecules200916004
Chicago/Turabian StylePeme, Thabo, Lukman O. Olasunkanmi, Indra Bahadur, Abolanle S. Adekunle, Mwadham M. Kabanda, and Eno E. Ebenso. 2015. "Adsorption and Corrosion Inhibition Studies of Some Selected Dyes as Corrosion Inhibitors for Mild Steel in Acidic Medium: Gravimetric, Electrochemical, Quantum Chemical Studies and Synergistic Effect with Iodide Ions" Molecules 20, no. 9: 16004-16029. https://doi.org/10.3390/molecules200916004





