Inorganic Phosphate Prevents Erk1/2 and Stat3 Activation and Improves Sensitivity to Doxorubicin of MDA-MB-231 Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
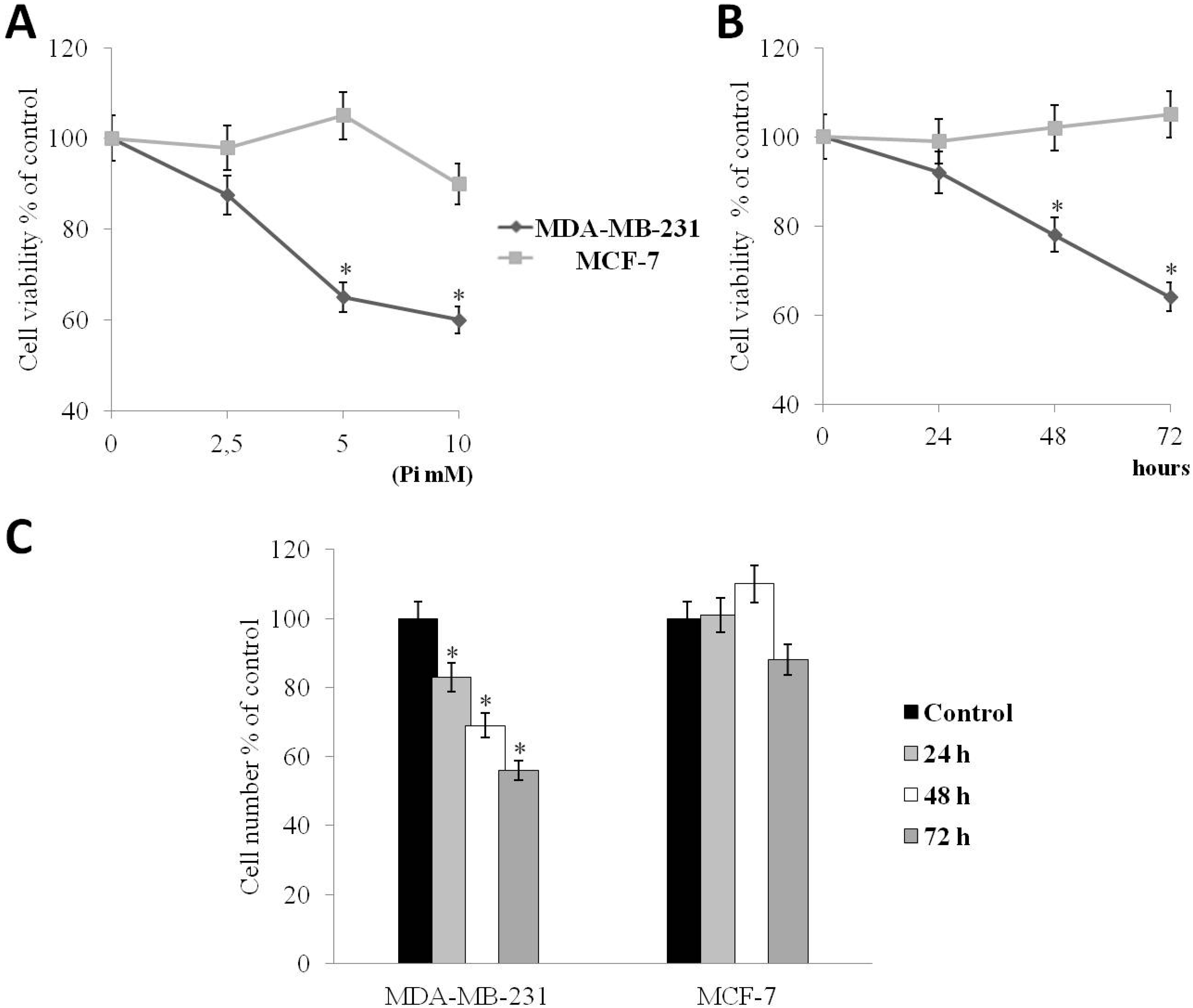
2.1. Pi Inhibits the Proliferation of Human MDA-MB-231 Triple-Negative Breast Cancer Cells
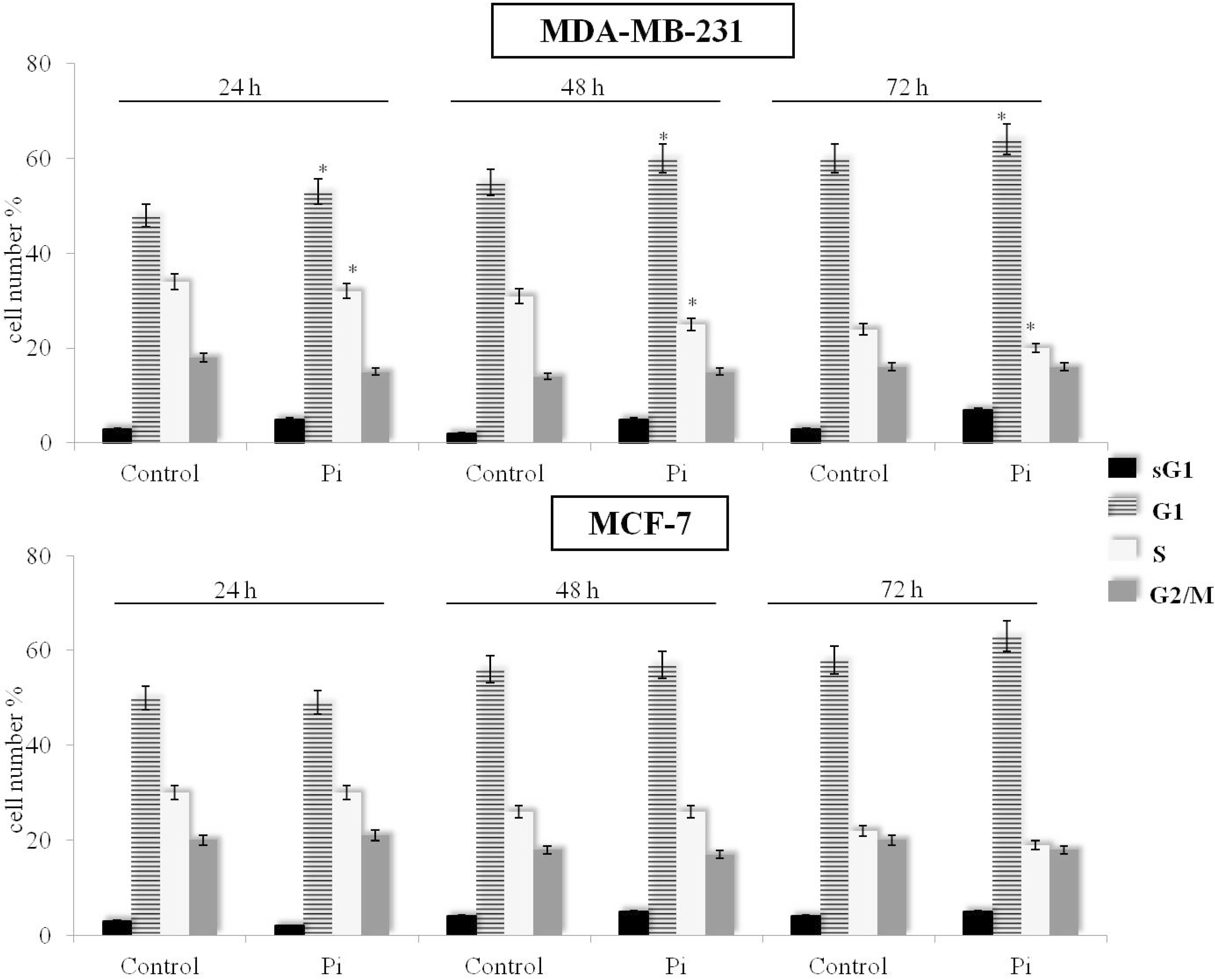
2.2. Pi Causes a Slowing Down of the Cell Division Cycle in MDA-MB-231 Cells
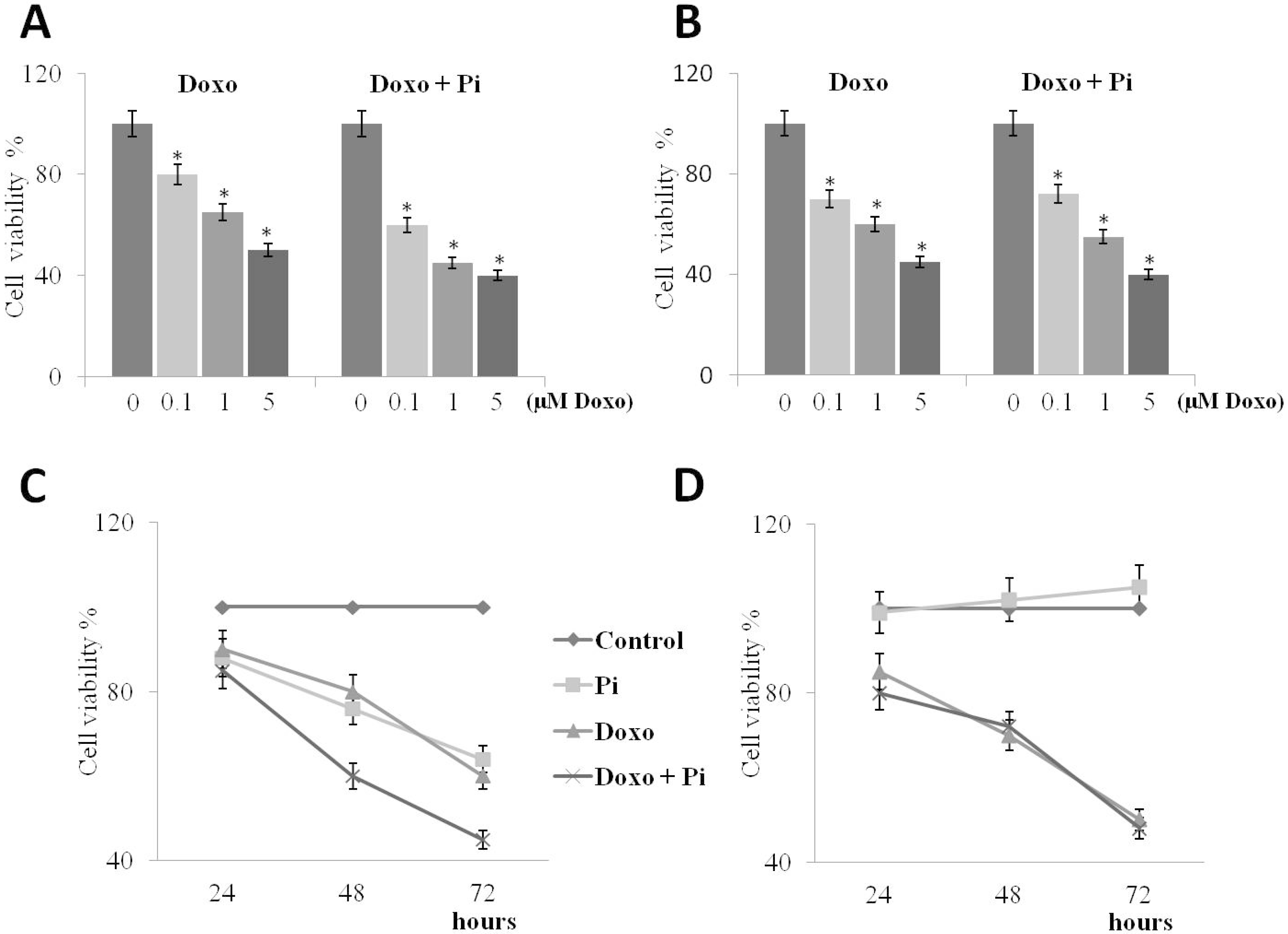
2.3. Pi Enhances Doxorubicin-Induced Cytotoxicity in MDA-MB-231 Cells
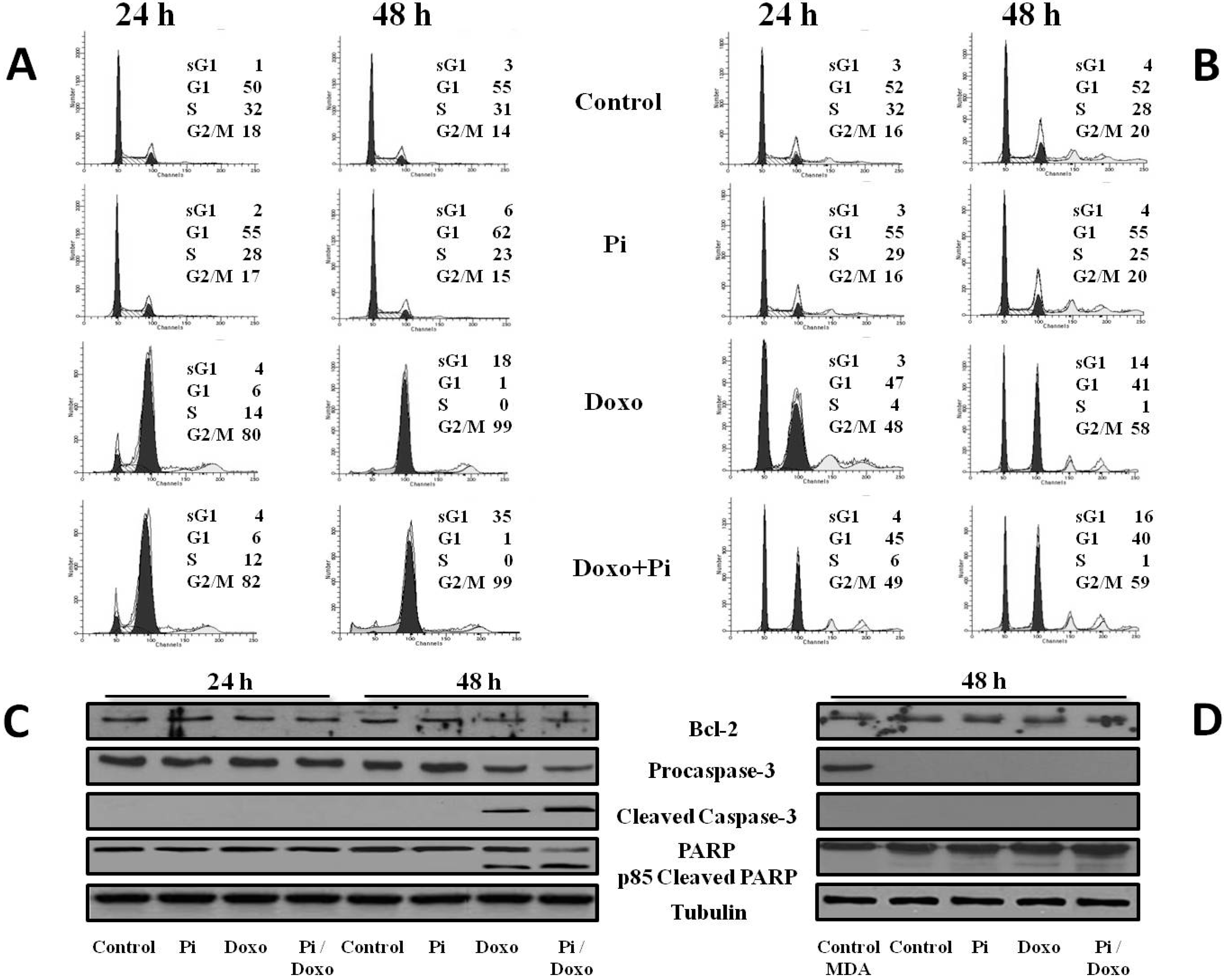
2.4. Pi Potentiates the Doxorubicin-Induced Cytotoxicity in MDA-MB-231 Cells by Inducing Apoptosis
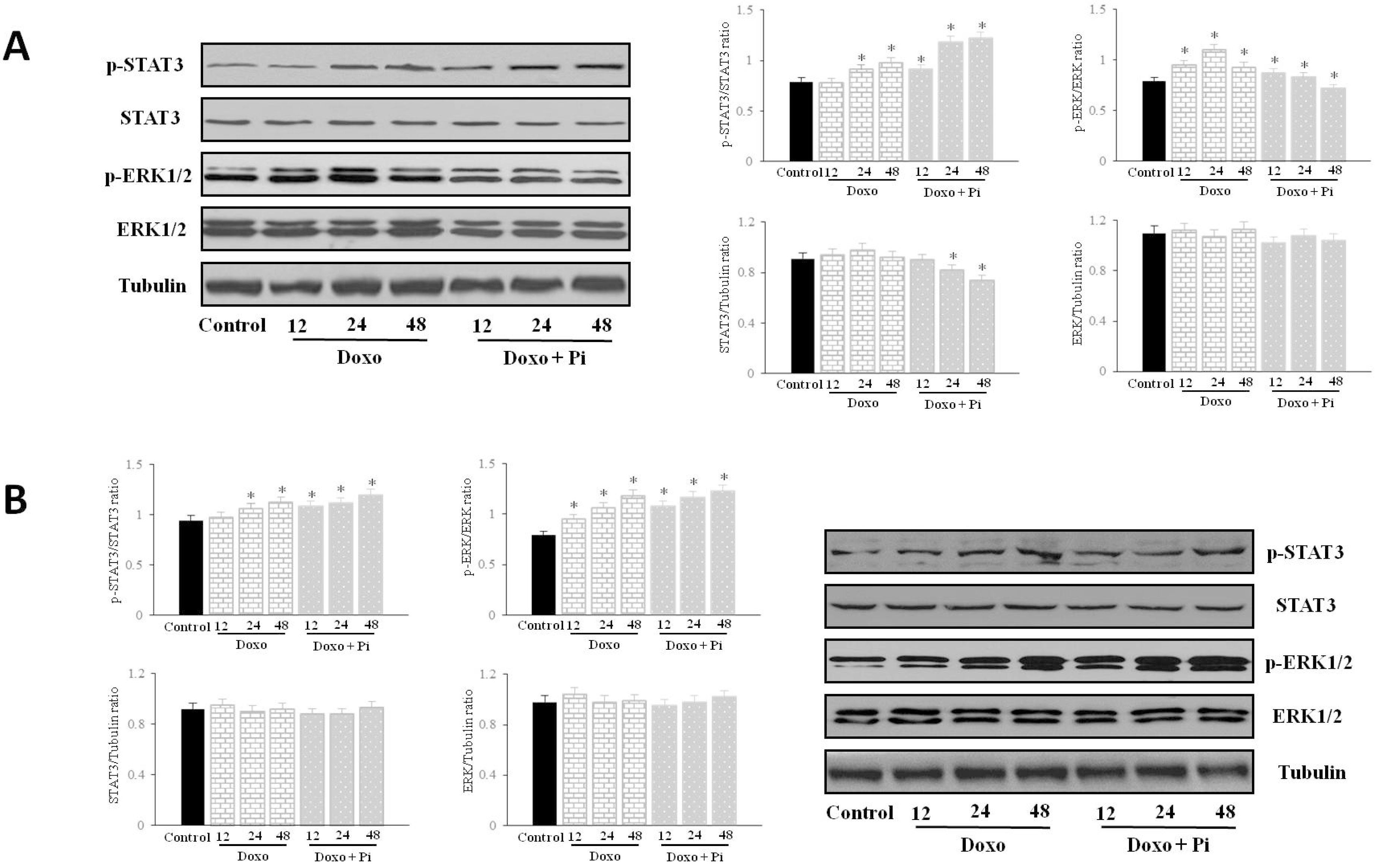
2.5. Pi Relevantly Affects Erk1/2 and Stat3 Protein and Phosphorylation Levels in Response to Doxorubicin in MDA-MB-231 Cells
2.6. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Culture and Treatments
3.3. Cell Viability Assay
3.4. Evaluation of Cell Cycle Phases by Flow Cytometry
3.5. Preparation of Cell Lysates
3.6. Immunodetection of Proteins
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rakha, E.A.; Chan, S. Metastatic Triple-negative Breast Cancer. Clin. Oncol. 2011, 23, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Reaves, D.K.; Fagan-Solis, K.D.; Dunphy, K.; Oliver, S.D.; Scott, D.W.; Fleming, J.M. The role of lipolysis stimulated lipoprotein receptor in breast cancer and directing breast cancer cell behavior. PLoS ONE 2014, 9, e91747. [Google Scholar] [CrossRef] [PubMed]
- Santana-Davila, R.; Perez, E.A. Treatment options for patients with triple-negative breast cancer. J. Hematol. Oncol. 2010, 3. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Childs, B.H.; Pegram, M. Triple negative breast cancer: Unmet medical needs. Breast Cancer Res. Treat. 2011, 125, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Abramson, V.G.; Lehmann, B.D.; Pietenpol, J.A. New strategies for triple-negative breast cancer-deciphering the heterogeneity. Clin. Cancer Res. 2014, 20, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.E.; Kummar, S.; Giaccone, G. Next generation oncology drug development: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2009, 6, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Nan, A. Combination drug delivery approaches in metastatic breast cancer. J. Drug Deliv. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, S.J. Triple-negative breast cancer role of specific chemotherapy agents. Cancer J. 2010, 16, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologie developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Chiosi, E.; Spina, A.; Sorrentino, A.; Romano, M.; Sorvillo, L.; Senatore, G.; D’Auria, R.; Abbruzzese, A.; Caraglia, M.; Naviglio, S.; et al. Change in TNF-alpha receptor expression is a relevant event in doxorubicin-induced H9c2 cardiomyocyte cell death. J. Interferon Cytokine Res. 2007, 27, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Agarwal, R. Combinatorial strategies for cancer eradication by silibinin and cytotoxic agents: Efficacy and mechanisms. Acta Pharmacol. Sin. 2007, 28, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Vibet, S.; Goupille, C.; Bougnoux, P.; Steghens, J.P.; Goré, J.; Mahéo, K. Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic. Biol. Med. 2008, 44, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, P.; Bai, J.; Zhang, J.; Kong, L.; Zhang, F.; Du, G.; Pei, S.; Zhang, L.; Jiang, Y.; et al. Ocotillol Enhanced the Antitumor Activity of Doxorubicin via p53-Dependent Apoptosis. Evid. -Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, S.; Della Ragione, F. Naturally occurring molecules and anticancer combination therapies in the era of personalized medicine and economic crisis. Curr. Pharm. Des. 2013, 19, 5325–5326. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Taketani, Y.; Sawada, N.; Sato, T.; Yamamoto, H. The regulation and function of phosphate in the human body. Biofactors 2004, 21, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Prié, D.; Beck, L.; Urena, P.; Friedlander, G. Recent findings in phosphate homeostasis. Curr. Opin. Nephrol. Hypertens. 2005, 14, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Khoshniat, S.; Bourgine, A.; Julien, M.; Weiss, P.; Guicheux, J.; Beck, L. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell. Mol. Life Sci. 2011, 68, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Sapio, L.; Esposito, A.; di Maiolo, F.; Sorvillo, L.; Naviglio, S. Inorganic phosphate as a novel signalling molecule with antiproliferative action in MDA-MB-231 breast cancer cells. BioResearch Open Access 2013, 2, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rahabi-Layachi, H.; Ourouda, R.; Boullier, A.; Massy, Z.A.; Amant, C. Distinct effects of inorganic phosphate on cell cycle and apoptosis in human vascular smooth muscle cells. J. Cell. Physiol. 2015, 230, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rendenbach, C.; Yorgan, T.A.; Heckt, T.; Otto, B.; Baldauf, C.; Jeschke, A.; Streichert, T.; David, J.P.; Amling, M.; Schinke, T.; et al. Effects of extracellular phosphate on gene expression in murine osteoblasts. Calcif. Tissue Int. 2014, 94, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Sorvillo, L.; di Maiolo, F.; Esposito, A.; D’Auria, R.; di Gesto, D.; Chiosi, E.; Naviglio, S. Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin via a p53-dependent pathway. J. Cell. Physiol. 2013, 228, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R., Jr. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, A.; Beck, L.; Khoshniat, S.; Wauquier, F.; Oliver, L.; Hue, E.; Alliot-Licht, B.; Weiss, P.; Guicheux, J.; Wittrant, Y. Inorganic phosphate stimulates apoptosis in murine MO6-G3 odontoblast-like cells. Arch. Oral Biol. 2011, 56, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Sorvillo, L.; Esposito, A.; Borgia, A.; Sapio, L.; Naviglio, S. Inorganic Phosphate as a Signaling Molecule: A Potential Strategy in Osteosarcoma Treatment. Curr. Pharm. Des. 2013, 19, 5394–5403. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics Adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Ravizza, R.; Molteni, R.; Osella, D.; Gabano, E.; Monti, E. Inhibition of Stat3 increases doxorubicin sensitivity in a human metastatic breast cancer cell line. Cancer Lett. 2007, 258, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Taherian, A.; Mazoochi, T. Different Expression of Extracellular Signal-Regulated Kinases (ERK) 1/2 and Phospho-Erk Proteins in MBA-MB-231 and MCF-7 Cells after Chemotherapy with Doxorubicin or Docetaxel. Iran. J. Basic Med. Sci. 2012, 15, 669–677. [Google Scholar] [PubMed]
- Tekedereli, I.; Alpay, S.N.; Tavares, C.D.; Cobanoglu, Z.E.; Kaoud, T.S.; Sahin, I.; Sood, A.K.; Lopez-Berestein, G.; Dalby, K.N.; Ozpolat, B. Targeted Silencing of Elongation Factor 2 Kinase Suppresses Growth and Sensitizes Tumors to Doxorubicin in an Orthotopic Model of Breast Cancer. PLoS ONE 2012, 7, e41171. [Google Scholar] [CrossRef] [PubMed]
- Varmeh, S.; Manfredi, J.J. Overexpression of the dual specificity phosphatase, Cdc25C, confers sensitivity on tumor cells to doxorubicin-induced cell death. Mol. Cancer Ther. 2008, 7, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, S.; di Gesto, D.; Romano, M.; Sorrentino, A.; Illiano, F.; Sorvillo, L.; Abbruzzese, A.; Marra, M.; Caraglia, M.; Chiosi, E.; et al. Leptin enhances growth inhibition by cAMP elevating agents through apoptosis of MDA-MB-231 breast cancer cells. Cancer Biol. Ther. 2009, 8, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Chen, P.S.; Prakash, E.; Hsu, H.C.; Huang, H.Y.; Lin, M.T.; Chang, K.J.; Kuo, M.L. Connective Tissue Growth Factor Confers Drug Resistance in Breast Cancer through Concomitant Up-regulation of Bcl-xL and cIAP1. Cancer Res. 2009, 69, 3482–3491. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Song, R.X.; McPherson, R.; Kumar, R.; Adam, L.; Jeng, M.H.; Yue, W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 239–256. [Google Scholar] [CrossRef]
- Haricharan, S.; Li, Y. STAT signaling in mammary gland differentiation, cell survival and tumorigenesis. Mol. Cell. Endocrinol. 2014, 382, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.L.; Steelman, L.S.; Shelton, J.G.; Wong, E.W.; Chappell, W.H.; Bäsecke, J.; Stivala, F.; Donia, M.; Nicoletti, F.; Libra, M.; et al. The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to targeted therapy. Cell Cycle 2010, 9, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Tentner, A.R.; Lee, M.J.; Ostheimer, G.J.; Samson, L.D.; Lauffenburger, D.A.; Yaffe, M.B. Combined experimental and computational analysis of DNA damage signaling reveals context-dependent roles for Erk in apoptosis and G1/S arrest after genotoxic stress. Mol. Syst. Biol. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Toniatti, C.; Jones, P.; Graham, H.; Pagliara, B.; Draetta, G. Oncology drug discovery: Planning a turnaround. Cancer Discov. 2014, 4, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bergsland, E.K. Is More Not Better? Combination Therapies in Colorectal Cancer Treatment. Hematol. Oncol. Clin. N. Am. 2015, 29, 85–116. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J. Potential health concerns of dietary phosphorus: Cancer, obesity, and hypertension. Ann. N. Y. Acad. Sci. 2013, 1301, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y. Phosphate as a sensor and signaling molecule. Clin. Nephrol. 2013, 79, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Sorvillo, L.; Chiosi, E.; Esposito, A.; di Maiolo, F.; Sapio, L.; Caraglia, M.; Naviglio, S. Synergistic cytotoxic effects of inorganic phosphate and chemotherapeutic drugs on human osteosarcoma cells. Oncol. Rep. 2013, 29, 1689–1696. [Google Scholar] [PubMed]
- Cox, R.F.; Hernandez-Santana, A.; Ramdass, S.; McMahon, G.; Harmey, J.H.; Morgan, M.P. Microcalcifications in breast cancer: Novel insights into the molecular mechanism and functional consequence of mammary mineralisation. Br. J. Cancer 2012, 106, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Tweardy, D.J. Targeting Stat3 in cancer therapy. Anticancer Drugs 2005, 16, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.; Frank, D.A. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin. Cancer Res. 2007, 13, 5665–5669. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.A.; Grandis, J.R. STAT3 signaling: Anticancer strategies and challenges. Mol. Interv. 2011, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. The Role of STAT3 Signaling in Mediating Tumor Resistance to Cancer Therapy. Curr. Drug Targets 2014, 15, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Liu, Y.; Jin, Z.; Hu, Q.; Lin, L.; Jou, D.; Yang, J.; Xu, Z.; Wang, H.; Li, C.; Lin, J. XZH-5 inhibits STAT3 phosphorylation and enhances the cytotoxicity of chemotherapeutic drugs in human breast and pancreatic cancer cells. PLoS ONE 2012, 7, e46624. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Scholz, C.; Zang, C.; Schefe, J.H.; Habbel, P.; Regierer, A.C.; Schulz, C.O.; Possinger, K.; Eucker, J. Metformin and the mTOR inhibitor everolimus (RAD001) sensitize breast cancer cells to the cytotoxic effect of chemotherapeutic drugs in vitro. Anticancer Res. 2012, 32, 1627–1637. [Google Scholar] [PubMed]
- Kulesza, D.W.; Carré, T.; Chouaib, S.; Kaminska, B. Silencing of the transcription factor STAT3 sensitizes lung cancer cells to DNA damaging drugs, but not to TNFα- and NK cytotoxicity. Exp. Cell Res. 2013, 319, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; di Maiolo, F.; Esposito, A.; D’Auria, R.; di Gesto, D.; Chiosi, E.; Sorvillo, L.; Naviglio, S. Integrating leptin and cAMP pathways in triple-negative breast cancer cells. Front. Biosci. (Landmark Ed.) 2013, 18, 133–144. [Google Scholar] [PubMed]
- Santarpia, L.; Lippman, S.M.; el-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Valero, V.; Hortobagyi, G.N. Emerging targeted therapies for breast cancer. J. Clin. Oncol. 2010, 28, 3366–3379. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; McGowan, P.M.; Crown, J. Targeted therapy for triple-negative breast cancer: Where are we? Int. J. Cancer 2012, 131, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Chambard, J.C.; Lefloch, R.; Pouyssegur, J.; Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta 2007, 1773, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, T.; Chen, C.; Chen, S.; Liu, Y.; Wu, J.; Shao, Z. Prognostic and predictive value of Phospho-p44/42 and pAKT in HER2-positive locally advanced breast cancer patients treated with anthracycline-based neoadjuvant chemotherapy. World J. Surg. Oncol. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Camirand, A.; Fadhil, I.; Luco, A.L.; Ochietti, B.; Kremer, R.B. Enhancement of taxol, doxorubicin and zoledronate anti-proliferation action on triple-negative breast cancer cells by a PTHrP blocking monoclonal antibody. Am. J. Cancer Res. 2013, 3, 500–508. [Google Scholar] [PubMed]
- Wang, F.; Yang, Y. Suppression of the xCT-CD44v antiporter system sensitizes triple negative breast cancer cells to doxorubicin. Breast Cancer Res. Treat. 2014, 147, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Loo, S.Y.; Huang, B.; Wong, L.; Tan, S.S.; Tan, T.Z.; Lee, S.C.; Thiery, J.P.; Lim, Y.C.; Yong, W.P.; et al. SPHK1 regulates proliferation and survival responses in triple negative breast cancer. Oncotarget 2014, 5, 5920–5933. [Google Scholar] [PubMed]
- Duan, Z.; Zhang, J.; Ye, S.; Shen, J.; Choy, E.; Cote, G.; Harmon, D.; Mankin, H.; Hua, Y.; Zhang, Y.; et al. A-770041 reverses paclitaxel and doxorubicin resistance in osteosarcoma cells. BMC Cancer 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Wei, W; Chen, Z.J.; Zhang, K.S.; Yang, X.L.; Wu, Y.M.; Chen, X.H.; Huang, H.B.; Liu, H.L.; Cai, S.H.; Du, J.; et al. The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Dis. 2014, 5, e1428. [Google Scholar] [CrossRef]
- Niu, D.; Wang, G.; Wang, X. Up-regulation of cyclin E in breast cancer via estrogen receptor pathway. Int. J. Clin. Exp. Med. 2015, 8, 910–915, eCollection 2015. [Google Scholar] [PubMed]
- Jun, W.; Lin, L.; Yurong, C.; Juming, Y. Recent advances of calcium phosphate nanoparticles for controlled drug delivery. Mini Rev. Med. Chem. 2013, 13, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Otsuka, M. Application of calcium phosphate as a controlled-release device. Biol. Pharm. Bull. 2013, 36, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapio, L.; Sorvillo, L.; Illiano, M.; Chiosi, E.; Spina, A.; Naviglio, S. Inorganic Phosphate Prevents Erk1/2 and Stat3 Activation and Improves Sensitivity to Doxorubicin of MDA-MB-231 Breast Cancer Cells. Molecules 2015, 20, 15910-15928. https://doi.org/10.3390/molecules200915910
Sapio L, Sorvillo L, Illiano M, Chiosi E, Spina A, Naviglio S. Inorganic Phosphate Prevents Erk1/2 and Stat3 Activation and Improves Sensitivity to Doxorubicin of MDA-MB-231 Breast Cancer Cells. Molecules. 2015; 20(9):15910-15928. https://doi.org/10.3390/molecules200915910
Chicago/Turabian StyleSapio, Luigi, Luca Sorvillo, Michela Illiano, Emilio Chiosi, Annamaria Spina, and Silvio Naviglio. 2015. "Inorganic Phosphate Prevents Erk1/2 and Stat3 Activation and Improves Sensitivity to Doxorubicin of MDA-MB-231 Breast Cancer Cells" Molecules 20, no. 9: 15910-15928. https://doi.org/10.3390/molecules200915910




