Digestive Gland from Aplysia depilans Gmelin: Leads for Inflammation Treatment
Abstract
:1. Introduction

2. Results and Discussion
2.1. Metabolic Analysis
2.1.1. Fatty Acids

| Peak | Fatty Acids | mg/kg (Dry Basis) |
|---|---|---|
| 1 | Dodecanoic | 7.67 (0.26) |
| 2 | Tetradecanoic | 55.60 (0.64) |
| 3 | Pentadecanoic | 26.08 (1.40) |
| 4 | cis-9-Hexadecenoic | 46.71 (1.27) |
| 5 | Hexadecanoic | 525.48 (4.54) |
| 6 | Heptadecanoic | 16.24 (0.55) |
| 7 | cis-6,9,12-Octadecatrienoic | 109.88 (0.76) |
| 8 | cis-9,12-Octadecadienoic | 132.65 (0.75) |
| 9 | cis-9-Octadecenoic | 214.90 (4.50) |
| 10 | trans-9-Octadecenoic | 171.13 (2.54) |
| 11 | Octadecanoic | 355.70 (2.57) |
| 12 | cis-5,8,11,14-Eicosatetraenoic | 367.30 (5.26) |
| 13 | cis-5,8,11,14,17-Eicosapentaenoic | 270.35 (1.35) |
| 14 | cis-8,11,14-Eicosatrienoic | 200.66 (3.36) |
| 15 | cis-11,14-Eicosadienoic | 91.58 (0.70) |
| 16 | cis-9,12,15-Octadecatrienoic | 115.49 (1.56) |
| 17 | Eicosanoic | 21.88 (0.95) |
| 18 | cis-4,7,10,13,16,19-Docosahexaenoic | 7.43 (0.07) |
| 19 | cis-7,10,13,16-Docosatetraenoic | 355.99 (3.08) |
| 20 | cis-13-Docosenoic | 14.18 (0.40) |
| 21 | Docosanoic | 21.42 (0.45) |
| 22 | Tetracosanoic | 3.33 (0.10) |
| Total | 3131.65 | |
| SFA | 1033.40 | |
| MUFA | 446.92 | |
| PUFA | 1651.33 | |
| ω3 | 393.27 | |
| ω6 | 1258.06 | |
2.1.2. Carotenoids
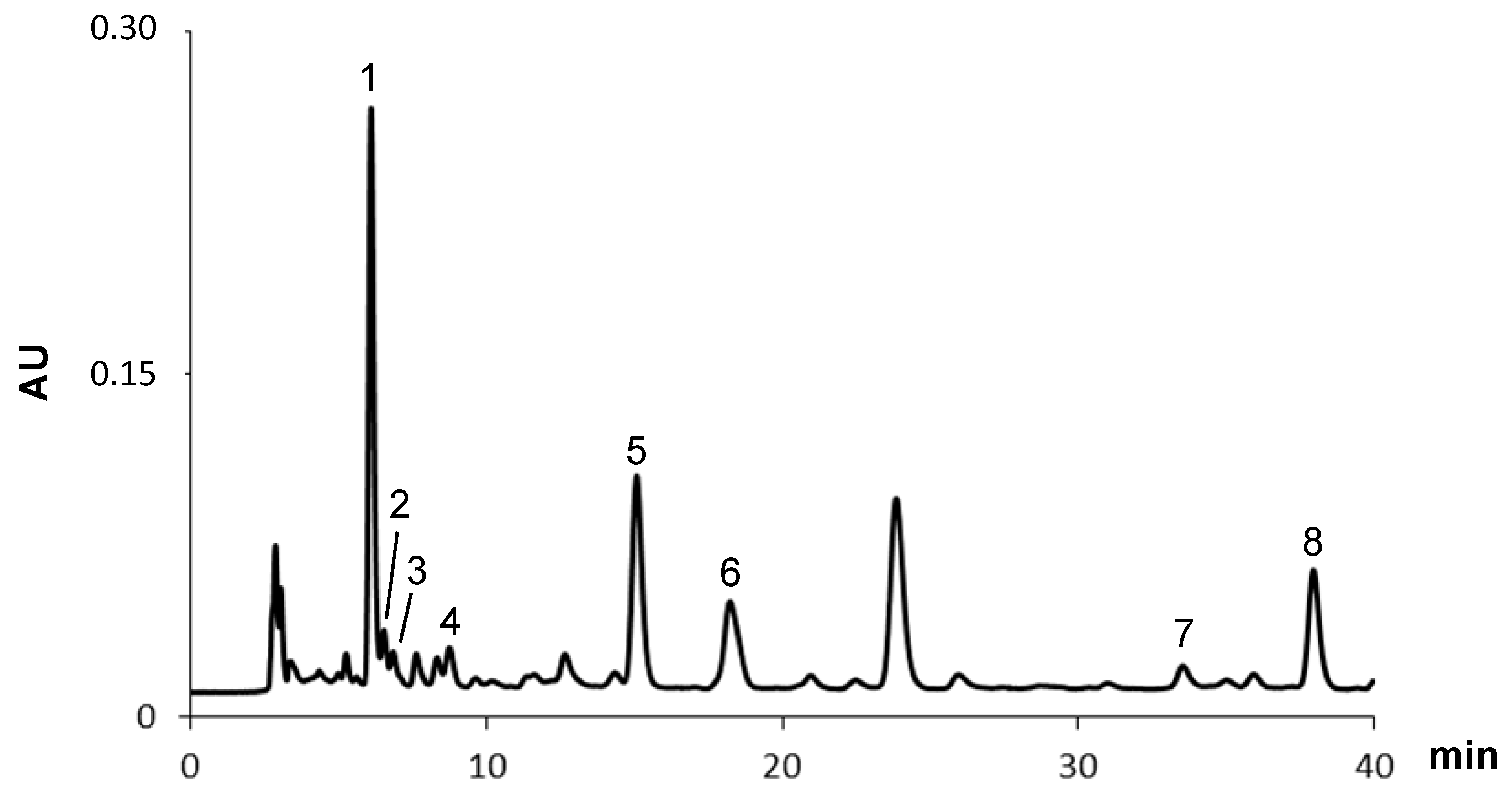
| Peak | Carotenoids | μg/g (Dry Basis) |
|---|---|---|
| 1 | Fucoxanthin | 215.80 (0.58) |
| 2 | Fucoxanthin cis isomer 1 | 24.50 (0.11) |
| 3 | Fucoxanthin cis isomer 2 | 15.60 (0.20) |
| 4 | Neoxanthin | 12.80 (0.03) |
| 5 | Lutein | 33.80 (0.04) |
| 6 | Zeaxanthin | 403.40 (0.50) |
| 7 | α-Carotene | 5.70 (0.01) |
| 8 | β-Carotene | 18.90 (0.26) |
| Total | 730.50 |
2.2. Anti-Inflammatory Potential
2.2.1. Cell Viability and Membrane Integrity

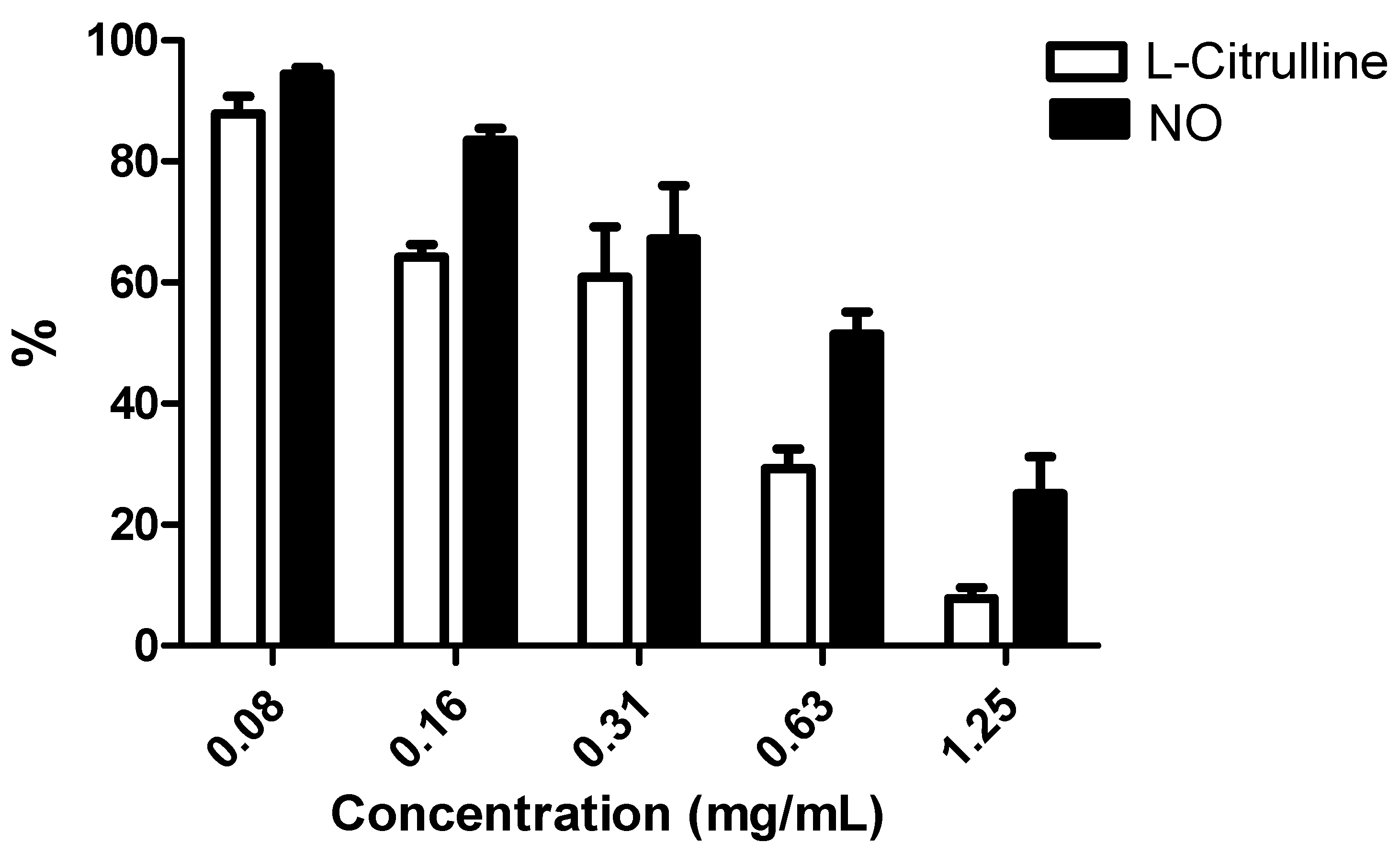
2.2.2. NO and L-Citrulline Levels
3. Experimental Section
3.1. Standards and Reagents
3.2. Sampling
3.3. Extraction
3.4. Fatty Acids Determination
3.4.1. Derivatization Process
3.4.2. GC-MS Analysis
3.5. Carotenoids Determination
HPLC-DAD Analysis
3.6. Anti-Inflammatory Potential
3.6.1. Cell Culture
3.6.2. Cell Viability
3.6.3. Membrane Integrity
3.6.4. NO Determination
3.6.5. L-Citrulline Determination
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jha, R.K.; Zi-Rong, X. Biomedical compounds from marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef]
- Eales, N.B. Revision of the world species of Aplysia (Gastropoda, Opisthobranchia). Bull. Br. Mus. Zool. 1960, 5, 266–404. [Google Scholar]
- Carefoot, T.H. Aplysia: Its biology and ecology. Oceanogr. Mar. Biol. Ann. Rev. 1987, 25, 167–284. [Google Scholar]
- Lobo-da-Cunha, A.; Batista-Pinto, C. Ultrastructural, histochemical and cytochemical characterization of intestinal epithelial cells in Aplysia depilans (Gastropoda, Opisthobranchia). Acta Zool. (Stockh.) 2007, 88, 211–221. [Google Scholar] [CrossRef]
- Fretter, V.; Ko, B.H. The specialization of the Aplysiid gut. Malacologia 1979, 18, 7–11. [Google Scholar]
- Jiménez, C.; Quiñoá, E.; Castedo, L.; Riguera, R. Epidioxy sterols from the tunicates Dendrodoa grossularia and Ascidiella aspersa and the gastropoda Aplysia depilans and Aplysia punctata. J. Nat. Prod. 1986, 49, 905–909. [Google Scholar] [CrossRef]
- Spinella, A.; Zubía, E.; Martînez, E.; Ortea, J.; Cimino, G. Structure and stereochemistry of aplyolides A–E, lactonized dihydroxy fatty acids from the skin of the marine mollusk Aplysia depilans. J. Org. Chem. 1997, 62, 5471–5475. [Google Scholar] [CrossRef]
- Petraki, A.; Ioannou, E.; Papazafiri, P.; Roussis, V. Dactylomelane diterpenes from the sea hare Aplysia depilans. J. Nat. Prod. 2015, 78, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Lobo-da-Cunha, A. Ultrastructural and cytochemical aspects of the basophilic cells in the hepatopancreas of Aplysia depilans (Mollusca, Opisthobranchia). Tissue Cell 1999, 31, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lobo-da-Cunha, A. The digestive cells of the hepatopancreas in Aplysia depilans (Mollusca, Opistobranchia): Ultrastructural and cytochemical study. Tissue Cell 2000, 32, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Fong, C. The resolution of inflammation: Anti-inflammatory roles for NF-κB. Int. J. Biochem. Cell Biol. 2010, 42, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayaski, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.K.; Laurent, L.G. Pulmonary fibrosis: Cytokines in the balance. Eur. Resp. J. 1998, 11, 1218–1221. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.B.; Sousa, C.; Taveira, M.; Valentão, P.; Andrade, P.B. Fatty acids from edible sea hares: Anti-inflammatory capacity in LPS-stimulated RAW 264.7 cells involves iNOS modulation. RSC Adv. 2015, 5, 8981–8987. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Teixeira, N.; Andrade, P.B. Amino acids, fatty acids and sterols profile of some marine organisms from Portuguese waters. Food Chem. 2013, 141, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Natural marine anti-inflammatory products. Mini Rev. Med. Chem. 2008, 8, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Monroig, O.; Tocher, D.R.; Navarro, J.C. Biosynthesis of polyunsaturated fatty acids in marine invertebrates: Recent advances in molecular mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Marine natural pigments: Chemistry, distribution and analysis. Dyes Pigment. 2014, 111, 124–134. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Czeczuga, B. Investigations of carotenoids in some animals of Adriatic sea—VI. Representatives of sponges, annelids, molluscs and echinodermates. Comp. Biochem. Physiol. 1984, 78, 259–264. [Google Scholar] [CrossRef]
- Naczk, M.; Williams, J.; Brennan, K.; Liyanapathirana, C.; Shahidi, F. Compositional characteristics of green crab (Carcinus maenas). Food Chem. 2004, 88, 429–434. [Google Scholar] [CrossRef]
- Bellik, Y.; Hammoudi, S.M.; Abdellah, F.; Iguer-Ouada, M.; Boukraâ, L. Phytochemicals to prevent inflammation and allergy. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, S1505–S1519. [Google Scholar]
- Pereira, D.M.; Correia-da-Silva, G.; Valentão, P.; Teixeira, N.; Andrade, P.B. Palmitic acid and ergosta-7,22-dien-3-ol contribute to the apoptotic effect and cell cycle arrest of an extract from Marthasterias glacialis L. in neuroblastoma cells. Molecules 2014, 12, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.-H.; Affan, A.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Shafaie, Y. Dietary lutein modulates inducible nitric oxide synthase (iNOS) gene and protein expression in mouse macrophage cells (RAW 264.7). Mol. Nutr. Food Res. 2007, 51, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ambrozova, G.; Pekarova, M.; Lojek, A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by Raw 264.7 macrophages. Eur. J. Nutr. 2010, 49, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostagland. Leuk. Essent. Fatty Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Saw, C.L.L.; Lin, W.; Wu, T.; Huang, Y.; Kong, A.-N.T. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol. Pharm. 2010, 7, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Ibrahim, A.; Mbodji, K.; Coëffier, M.; Ziegler, F.; Bounoure, F.; Chardigny, J.M.; Skiba, M.; Savoye, G.; Déchelotte, P.; et al. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J. Nutr. 2010, 140, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef] [PubMed]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: An update. Molecules 2013, 18, 322–353. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.A.; Lee, H.; Kwon, Y.G.; Chung, C.K.; Kim, Y.M. Beta-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-κB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.; Guedes de Pinho, P.; Andrade, P.B.; Baptista, P.; Valentão, P. Fatty acid composition of wild edible mushrooms species: A comparative study. Microchem. J. 2009, 93, 29–35. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Pereira, D.M.; Mercadante, A.Z.; Valentão, P.; Teixeira, N.; Andrade, P.B. Further insights on the carotenoid profile of the echinoderm Marthasterias glacialis L. Mar. Drugs 2012, 10, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar]
- Sample Availability: Samples of the reference compounds of fatty acids and carotenoids are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.P.; Lobo-da-Cunha, A.; Taveira, M.; Ferreira, M.; Valentão, P.; Andrade, P.B. Digestive Gland from Aplysia depilans Gmelin: Leads for Inflammation Treatment. Molecules 2015, 20, 15766-15780. https://doi.org/10.3390/molecules200915766
Oliveira AP, Lobo-da-Cunha A, Taveira M, Ferreira M, Valentão P, Andrade PB. Digestive Gland from Aplysia depilans Gmelin: Leads for Inflammation Treatment. Molecules. 2015; 20(9):15766-15780. https://doi.org/10.3390/molecules200915766
Chicago/Turabian StyleOliveira, Andreia P., Alexandre Lobo-da-Cunha, Marcos Taveira, Marta Ferreira, Patrícia Valentão, and Paula B. Andrade. 2015. "Digestive Gland from Aplysia depilans Gmelin: Leads for Inflammation Treatment" Molecules 20, no. 9: 15766-15780. https://doi.org/10.3390/molecules200915766






