Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods
Abstract
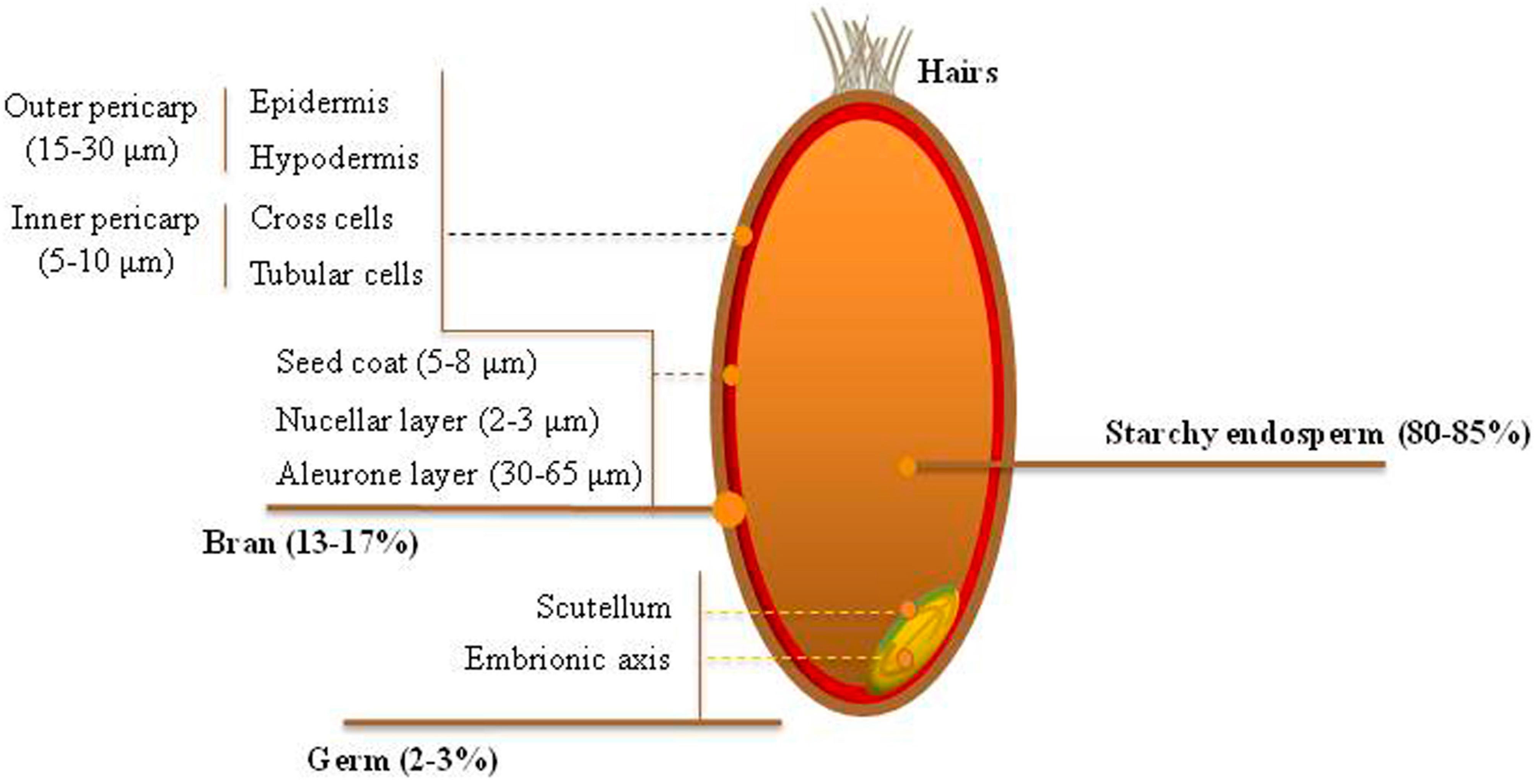
:1. Introduction
2. Biosynthesis, Chemistry, and Distribution of Phenolic Acids across the Wheat Grain Tissues
| Wheat Bran Tissues | FA | DHD | DHT | SA | p-CA | Total |
|---|---|---|---|---|---|---|
| Bran | 5.26 | 1.01 | 0.24 | 0.25 | 0.09 | 6.85 |
| Endosperm | 0.10 | 0.03 | 0.00 | 0.01 | 0.00 | 0.14 |
| Aleurone | 8.17 | 1.07 | 0.11 | 0.44 | 0.21 | 10 .00 |
| Intermediate layer | 5.92 | 0.91 | 0.07 | 0.08 | 0.07 | 7.05 |
| Pericarp | 8.18 | 5.12 | 1.21 | 0.01 | 0.04 | 14.56 |
| Scutellum | 3.48 | 0.37 | 0.03 | 0.01 | 0.01 | 3.90 |
| Embryonic axis | 0.31 | 0.09 | 0.01 | 0.00 | 0.00 | 0.41 |
3. Biological Activity and Bioavailability of Phenolic Acids
4. Strategies to Increase Phenolic Acid Content and Bioavailability in Wheat-Based Products
5. Wheat Bran as a Source of Phenolic Acids for the Food Industry
6. Stability of Phenolic Acids in Wheat-Based Foods
| Product | Ingredients | Baking/Cooking | Extraction Method | Increase/Decrease of Free Phenolic Acids | Increase/Decrease of Bound Phenolic Acids | Increase/Decrease of TPC | Reference | |
|---|---|---|---|---|---|---|---|---|
| Temp. | Time | |||||||
| Unleavened flat bread | Whole grain Einkorn wheat flour, quick yeast, sugar, gluten, water | 175 °C | 25′ | 80% methanol; NaOH-hydrolysis | +32% Product vs. flour * | −38% Product vs. flour | n.a. | [111] |
| Cookie | Whole grain 1:1 einkorn:corn flour, sugar, salt, sodium bicarbonate, water | 175 °C | 13′ | 80% methanol; NaOH-hydrolysis | +35% Product vs. flour * | +11% Product vs. flour | n.a. | [111] |
| Muffin | Whole grain 1:1 einkorn:corn flour, sugar, salt, baking powder, margarine, water | 175 °C | 30′ | 80% methanol; NaOH-hydrolysis | +99% Product vs. flour * | −1% Product vs. flour | n.a. | [111] |
| Fortified unleavened flat bread | Whole grain Einkorn wheat flour, quick yeast, sugar, gluten, water + lutein | 175 °C | 25′ | 80% methanol; NaOH-hydrolysis | +17% Product vs. flour * | −36% Product vs. flour | n.a. | [111] |
| Fortified cookie | Whole grain 1:1 einkorn:corn flour, sugar, salt, sodium bicarbonate, water + lutein | 175 °C | 13′ | 80% methanol; NaOH-hydrolysis | +31% Product vs. flour * | +12% Product vs. flour | n.a. | [111] |
| Fortified muffin | Whole grain 1:1 einkorn:corn flour, sugar, salt, baking powder, margarine, water + lutein | 175 °C | 30′ | 80% methanol; NaOH-hydrolysis | +95% Product vs. flour * | −6% Product vs. flour | n.a. | [111] |
| Bread | Refined wheat flour, salt, brewer yeast, water | 215 °C | 45′ | NaOH-hydrolysis | n.a. | n.a. | −36% Product vs. flour | [90] |
| Enriched bread | Refined wheat flour +10% of second debranning fraction of soft wheat, salt, brewer yeast, water | 215 °C | 45′ | NaOH-hydrolysis | n.a. | n.a. | −35% Product vs. flour | [90] |
| White bread | Commercial wheat flour, salt, sucrose, shortening, dry yeast, water | 215 °C | 24′ | 95% ethanol/HCl | n.a. | n.a. | −33% Product vs. flour | [113] |
| Whole wheat bread | Commercial whole wheat flour, salt, sucrose, shortening, dry yeast, water | 215 °C | 24′ | 95% ethanol/HCl | n.a. | n.a | −28% Product vs. flour | [113] |
| Purple wheat bran muffins | Purple wheat bran, flour, sugar, baking soda, salt, oil, buttermilk, eggs, molasses | 177 °C | 20′ | 100% methanol | n.a. | n.a. | +92% Product vs. flour | [114] |
| Wheat bran Muffins | Flour, sugar, baking soda, salt, oil, buttermilk, eggs, molasses | 177 °C | 20′ | 100% methanol | n.a. | n.a. | +90% Product vs. flour | [114] |
| White bread | Commercial wheat flour, salt, sugar, fat, ascorbic acid, fresh yeast, water | 220 °C | 30′ | 80% methanol; NaOH-hydrolysis | −20% Product vs. flour | 17% Product vs. flour | n.a. | [31] |
| Enriched bread | Commercial wheat flour + 6% of second and third debranning fraction of soft wheat, salt, sugar, fat, ascorbic acid, fresh yeast, water | 220 °C | 30′ | 80% methanol; NaOH-hydrolysis | −21% Product vs. flour | −6% Product vs. flour | n.a. | [31] |
| Enriched bread | Commercial wheat flour + 10% of second and third debranning fraction of soft wheat, salt, sugar, fat, ascorbic acid, fresh, yeast, water | 220 °C | 30′ | 80% methanol; NaOH-hydrolysis | −42% Product vs. flour | −14% Product vs. flour | n.a. | [31] |
| Enriched pasta | Durum wheat semolina + 6% of first debranning fraction, water | Boiling water | n.a. | 80% methanol/1% HCl; NaOH-hydrolysis | −39% cooked pasta vs. flour (+0.1% cooked vs. uncooked pasta) | -32% cooked pasta vs. flour (+26% cooked vs. uncooked pasta) | n.a. | [91] |
| Enriched pasta | Durum wheat semolina + 10% of first debranning fraction, water | Boiling water | n.a. | 80% methanol/1% HCl; NaOH-hydrolysis | −36% cooked pasta vs. flour; (−30% cooked vs. uncooked pasta) | −33% cooked pasta vs. flour (+30% cooked vs. uncooked pasta) | n.a. | [91] |
| Enriched pasta | Durum wheat semolina + 6% of second and third debranning fraction, water | Boiling water | n.a. | 80% methanol/1% HCl; NaOH-hydrolysis | −37% cooked pasta vs. flour (−18% cooked vs. uncooked pasta) | −44% cooked pasta vs. flour (+27% cooked vs. uncooked pasta) | n.a. | [91] |
| Enriched pasta | Durum wheat semolina + 10% of second and third debranning fraction, water | Boiling water | n.a. | 80%methanol/acetic acid; NaOH-hydrolysis | −35% cooked pasta vs. flour (−6.8% cooked vs. uncooked pasta) | −21% cooked pasta vs. flour (+18% cooked vs. uncooked pasta) | n.a. | [91] |
| Regular spaghetti | Durum wheat semolina, water | Boiling water | 12′20′′ | NaOH-hydrolysis | n.a. | n.a. | −57% cooked vs. uncooked pasta | [116] |
| Spaghetti | Whole durum wheat semolina, water | Boiling water | 12′20′′ | NaOH-hydrolysis | n.a. | n.a. | −67% cooked vs. uncooked pasta | [116] |
| Fortified spaghetti | Durum wheat semolina, water, inulin | Boiling water | 12′20′′ | NaOH-hydrolysis | n.a. | n.a. | −68% cooked vs. uncooked pasta | [116] |
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women. J. Am. Diet. Assoc. 2006, 106, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- He, M.; van Dam, R.M.; Rimm, E.; Hu, F.B.; Qi, L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010, 121, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Rimm, E.; Manson, J.E.; Hennekens, C.H.; Willett, W.C. Whole grain consumption and risk of coronary heart disease: Results from the nurses’ health study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar] [PubMed]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R., Jr.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, incident type 2 diabetes mellitus in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [PubMed]
- Parker, E.D.; Liu, S.; van Horn, L.; Tinker, L.F.; Shikany, J.M.; Eaton, C.B.; Margolis, K.L. The association of whole grain consumption with incident type 2 diabetes: The women’s health initiative observational study. Ann. Epidemiol. 2013, 23, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E.; Rémésy, C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? J. Cereal Sci. 2008, 48, 258–276. [Google Scholar] [CrossRef]
- Björck, I.; Östman, E.; Kristensen, M.; Anson, N.M.; Price, R.; Haenen, G.R.M.M.; Havenaar, R.; Knudsen, K.E.B.; Frid, A.; Mykkanen, H.; et al. Cereal grains for nutrition and health benefits: Overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Technol. 2012, 25, 87–100. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.; Liu, R. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.S.; Tudorica, C.M. Fresh pasta quality as affected by enrichment of non-starch polysaccharides. J. Food Sci. 2007, 72, S659–S665. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. Food Sci. Technol. 2015, 61, 41–46. [Google Scholar] [CrossRef]
- Penella, J.M.S.; Collar, C.; Haros, M. Effect of wheat bran and enzyme addition on dough functional performance and phytic acid levels in bread. J. Cereal Sci. 2008, 48, 715–721. [Google Scholar] [CrossRef]
- De Kock, S.; Taylor, J.; Taylor, J.R.N. Effect of heat treatment and particle size of different brans on loaf volume of brown bread. LWT-Food Sci. Technol. 1999, 32, 349–356. [Google Scholar] [CrossRef]
- Salmenkallio-Marttila, M.; Katina, K.; Autio, K. Effects of bran fermentation on quality and microstructure of high-fiber wheat bread. Cereal Chem. 2001, 78, 429–435. [Google Scholar] [CrossRef]
- Dey, T.B.; Kuhad, R.C. Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 2014, 4, 120–127. [Google Scholar]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Pasqualone, A.; Delvecchio, L.N.; Gambacorta, G.; Laddomada, B.; Urso, V.; Mazzaglia, A.; Ruisi, P.; di Miceli, G. Effect of supplementation with wheat bran aqueous extracts obtained by ultrasound-assisted technologies on the sensory properties and the antioxidant activity of dry pasta. Nat. Prod. Commun. 2015, in press. [Google Scholar]
- Shewry, P.R.; Piironen, V.; Lampi, A.M.; Edelmann, M.; Kariluoto., S.; Nurmi, T.; Fernandez-Orozco, R.; Ravel, C.; Charmet, G.; Andersson, A.A.M.; et al. The HEALTHGRAIN wheat diversity screen: Effects of genotype and environment on phytochemicals and dietary fiber components. J. Agric. Food Chem. 2010, 58, 9291–9298. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of environment and genotype on phenolic acids in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Durante, M.; Mangini, G.; D’Amico, L.; Lenucci, M.S.; Simeone, R.; Piarulli, L.; Mita, G.; Blanco, A. Genetic variability for individual and total phenolic acids in a tetraploid wheat collection. J. Sci. Food Agric. 2015. submitted. [Google Scholar]
- Dicko, M.H.; Gruppen, H.; Barro, C.; Traore, A.S.; van Berkel, W.J.H.; Voragen, A.G.J. Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 2005, 31, 2671–2688. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, R.; Singh, D.V.; Srivastava, K.D. Phenols as a biochemical basis of resistance in wheat against Karnal bunt. Plant Pathol. 2001, 50, 470–476. [Google Scholar] [CrossRef]
- Liu, L.; Gitz, D.C.; McClure, J.W. Effects of UV-B on flavonoids, ferulic acid, growth and photosynthesis in barley primary leaves. Physiol. Plant 1995, 93, 725–733. [Google Scholar] [CrossRef]
- Carver, T.L.W.; Zeyen, R.J.; Robbins, M.P.; Dearne, G.A. Effects of the PAL inhibitor, AOPP, on oat, barley and wheat cell responses to appropriate and inappropriate formae specials of Erysiphe graminis DC. Physiol. Mol. Plant Pathol. 1992, 41, 392–409. [Google Scholar] [CrossRef]
- Kruger, W.M.; Carver, T.L.W.; Zeyen, R.J. Effects of inhibiting phenolic biosynthesis on penetration resistance of barley isolines containing seven powdery mildew resistance genes or alleles. Physiol. Mol. Plant Pathol. 2002, 61, 41–51. [Google Scholar] [CrossRef]
- Heldt, H.W. Phenylalanine ammonia lyase catalyzes the initial reaction of phenylpropanoid metabolism. In Plant Biochemistry; Heldt, D.H., Heldt, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 437–454. [Google Scholar]
- Yu, L.; Zhou, K. Antioxidant properties of bran extracts for ‘‘Platte’’ wheat grown at different locations. Food Chem. 2004, 90, 311–316. [Google Scholar] [CrossRef]
- Gelinas, P.; McKinnon, C. Effect of wheat variety, farming site, and bread-baking on total phenolics. Int. J. Food Sci. Technol. 2006, 41, 329–332. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli, A.; Baiano, A. Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. Int. J. Food Sci. Technol. 2010, 45, 7–16. [Google Scholar] [CrossRef]
- Kim, K.H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Sosulski, F.; Krygier, K.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. Composition of phenolic acids in cereal and potato flours. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Barron, C.; Surget, A.; Rouau, X. Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J. Cereal Sci. 2007, 45, 88–96. [Google Scholar] [CrossRef]
- Antoine, C.; Peyron, S.; Lullien-Pellerin, V.; Abecassis, J.; Rouau, X. Wheat bran tissue fractionation using biochemical markers. J. Cereal Sci. 2004, 39, 387–393. [Google Scholar] [CrossRef]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic content and antioxidant activity of pearled wheat and roller-mill fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Surget, A.; Barron, C. Histologie du grain de blé. Ind. Cér. 2005, 145, 3–7. [Google Scholar]
- Esposito, F.; Arlotti, G.; Bonifati, A.M.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Dexter, J.; Shahidi, F. Antioxidant properties of wheat as affected by pearling. J. Agric. Food Chem. 2006, 54, 6177–6184. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Young, G. Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet. Assoc. 1999, 99, 213–218. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem. 2008, 85, 544–549. [Google Scholar] [CrossRef]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Juurlink, B.H.J.; Azouz, H.J.; Aldalati, A.M.Z.; AlTinawi, B.M.H.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Hadži-Tašković Šukalović, V.; Dodig, D.; Maksimović, V.; Maksimović, M.; Basić, Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble Antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Janković, M.; Gökmen, V. Distributions of phenolic compounds, yellow pigments and oxidative enzymes in wheat grains and their relation to antioxidant capacity of bran and debranned flour. J. Cereal Sci. 2012, 56, 652–658. [Google Scholar] [CrossRef]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Kanaski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Rukkumani, R.; Aruna, K.; Suresh Varma, P.; Menon, V.P. Influence of ferulic acid on circulatory prooxidant-antioxidant status during alcohol and PUFA induced toxicity. J. Physiol. Pharmacol. 2004, 55, 551–561. [Google Scholar] [PubMed]
- Meyer, A.S.; Donovan, J.L.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Fruit hydroxycinnamic acids inhibit human low density lipoprotein oxidation in vitro. J. Agric. Food Chem. 1998, 46, 1783–1787. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, K.; Parry, J.W. Inhibitory effects of wheat bran extracts on human LDL oxidation and free radicals. LWT 2005, 38, 463–470. [Google Scholar]
- Benzie, I.F. Evolution of antioxidant defence mechanisms. Eur. J. Nutr. 2000, 39, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R. Dietary and pharmacological antioxidants in atherosclerosis. Curr. Opin. Lipidol. 1999, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Novel Chelators for Cancer Treatment: Where Are We Now? Antioxid. Redox Signal. 2013, 18, 973–1006. [Google Scholar] [CrossRef] [PubMed]
- Mateo Anson, N.; Hemery, Y.M.; Basta, A.; Haenen, G.R.M.M. Optimizing the bioactive potential of wheat bran by processing. Food Funct. 2012, 3, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Durante, M.; Minervini, F.; Garbetta, A.; Cardinali, A.; D’Antuono, I.; Caretto, S.; Blanco, A.; Mita, G. Phytochemical characterization and anti-inflammatory activity of extracts from the whole-meal flour of Italian durum wheat cultivars. Int. J. Mol. Sci. 2015, 16, 3512–3527. [Google Scholar] [CrossRef] [PubMed]
- Whent, M.; Huang, H.; Xie, Z.; Lutterodt, H.; Yu, L.; Fuerst, E.P.; Craig, F.; Morris, C.F.; Yu, L.; Luthria, D. Phytochemical composition, anti-inflammatory, and antiproliferative activity of whole wheat flour. J. Agric. Food Chem. 2012, 60, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; la Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, A.; Danesi, F.; Verardo, V.; Serrazanetti, D.I.; Valli, V.; Russo, A.; Riciputi, Y.; Tossani, N.; Caboni, M.F.; Guerzoni, M.E.; et al. Role of cereal type and processing in whole grain in vivo protection from oxidative stress. Front. Biosci. 2011, 16, 1609–1618. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015. [CrossRef] [PubMed]
- Bourne, L.C.; Rice-Evans, C. Bioavailability of ferulic acid. Biochem. Biophys. Res. Commun. 1998, 253, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Bourne, L.; Paganga, G.; Baxter, D.; Hughes, P.; Rice-Evans, C. Absorption of ferulic acid from low-alcohol beer. Free Radic. Res. 2000, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.M.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.T. Absorption of hydroxycinnamates in humans after high-bran cereal consumption. J. Agric. Food Chem. 2003, 51, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef]
- Drankham, K.; Carter, J.; Madl, R.; Klopfenstein, C.; Padula, F.; Lu, Y.; Warren, T.; Schmitz, N.; Takemoto, D.J. Antitumor activity of wheats with high orthophenolic content. Nutr. Cancer 2003, 47, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Cerda, B.; Tomas-Barberan, F.A.; Espin, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Bosscher, D.; Breynaert, A.; Pieters, L.; Hermans, N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J. Physiol. Pharmacol. 2009, 60, 5–11. [Google Scholar] [PubMed]
- Kuznesof, S.; Brownlee, I.A.; Moore, C.; Richardson, D.P.; Jebb, S.A.; Seal, C.J. Whole heart study participant acceptance of wholegrain foods. Appetite 2012, 59, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Jonnalagadda, S.S.; Liu, S.; Marquart, L.; McKeown, N.; Reicks, M.; Riccardi, G.; Seal, C.; Slavin, J.; Thielecke, F.; et al. Developing a standard definition of whole-grain foods for dietary recommendations: Summary report of a multidisciplinary expert roundtable discussion. Adv. Nutr. 2014, 5, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Moore, W.R. Wheat bran particle size effects on bread baking performance and quality. J. Sci. Food Agric. 1999, 79, 805–809. [Google Scholar] [CrossRef]
- Alparce, N.K.M.; Anal, A.K. Food processing by-products as sources of functional foods and nutraceuticals. In Functional Foods and Dietary Supplements: Processing Effects and Health Benefits; Noomhorm, A., Ahmad, I., Anal, A.K., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2014; pp. 164–166. [Google Scholar]
- Edwards, N.M.; Biliaderis, C.G.; Dexter, J.E. Textural characteristics of wholewheat pasta and pasta containing non-starch polysaccharides. J. Food Sci. 1995, 60, 1321–1324. [Google Scholar] [CrossRef]
- Wang, J.; Rosell, C.M.; de Barber, C.B. Effect of the addition of different fibres on wheat dough performance and bread quality. Food Chem. 2002, 79, 221–226. [Google Scholar] [CrossRef]
- Wang, M.; Hamer, R.J.; van Vliet, T.; Gruppen, H.; Marseille, H.; Weegels, P.L. Effect of water unextractable solids on gluten formation and properties: Mechanistic considerations. J. Cereal Sci. 2003, 37, 55–64. [Google Scholar] [CrossRef]
- Gazzola, A.; Marinaccio, F.; Sovrani, V.; Blandino, M. Effect of adding wheat and barley pearled fractions on the rheological properties of doughs for bakery products. Tec. Mol. 2014, 65, 266–274. [Google Scholar]
- Peressini, D.; Sensidoni, A. Effect of soluble dietary fibre addition on rheological and breadmaking properties of wheat doughs. J. Cereal Sci. 2009, 49, 190–201. [Google Scholar] [CrossRef]
- Lebesi, D.M.; Tzia, C. Effect of the addition of different dietary fiber and edible cereal bran sources on the baking and sensory characteristics of cupcakes. Food Bioprocess Technol. 2011, 4, 710–722. [Google Scholar] [CrossRef]
- Mareo Anson, N.M.; van der Berg, R.; Havenaar, R.; Bast, A.; Haenen, G. Ferulic acid from aleurone determines the antioxidant potency of wheat grain (Triticum aestivum L.). J. Agric. Food Chem. 2008, 56, 5589–5594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Su, L.; Yu, L. Phytochemicals and antioxidant properties in wheat bran. J. Agric. Food Chem. 2004, 52, 6108–6114. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.A.; Rouau, X.; Courtin, C.M.; Poutanen, K.; Ranieri, R. Technologies for enhanced exploitation of the health-promoting potential of cereals. Trend Food Sci. Technol. 2012, 25, 78–86. [Google Scholar] [CrossRef]
- Hemery, Y.; Xavier Rouau, X.; Lullien-Pellerin, V.; Barron, J.C.; Abecassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Blandino, M.; Sovrani, V.; Marinaccio, F.; Reyneri, A.; Rolle, L.; Giacosa, S.; Locatelli, M.; Bordiga, M.; Travaglia, F.; Coïsson, J.D.; et al. Nutritional and technological quality of bread enriched with an intermediated pearled wheat fraction. Food Chem. 2013, 141, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Mazzacane, F.; Minervini, D.; Gobbetti, M. Micronized by-products from debranned durum wheat and sourdough fermentation enhanced the nutritional, textural and sensory features of bread. Food Res. Int. 2012, 46, 304–313. [Google Scholar] [CrossRef]
- Protonotariou, S.; Mandala, I.; Rosell, C.M. Jet milling effect on functionality, quality and in vitro digestibility of whole wheat flour and bread. Food Bioprocess Technol. 2015, 8, 1319–1329. [Google Scholar] [CrossRef]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Aman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbial. 2007, 24, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Mateo Anson, N.; Aura, A.M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R. Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts antiinflammatory effects ex vivo. J. Nutr. 2011, 141, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Larrea, M.A.; Chang, Y.K.; Martinez-Bustos, F. Some functional properties of extruded orange pulp and its effect on the quality of cookies. Food Sci. Technol. 2005, 38, 213–220. [Google Scholar] [CrossRef]
- Haros, M.; Rosell, C.M.; Benedito, C. Use of fungal phytase to improve breadmaking performance of whole wheat bread. J. Agric. Food Chem. 2001, 49, 5450–5454. [Google Scholar] [CrossRef] [PubMed]
- Sovrani, V.; Blandino, M.; Scarpino, V.; Reyneri, A.; Coïsson, J.D.; Travaglia, F.; Locatelli, M.; Bordiga, M.; Montella, R.; Arlorio, M. Bioactive compound content, antioxidant activity, deoxynivalenol and heavy metal contamination of pearled wheat fractions. Food Chem. 2012, 135, 39–46. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006. Commission Directive 2006/1881/EC of 19 December 2006, setting maximum levels for certain contaminants in food stuffs. Off. J. Eur. Commun. 2006, L364, 5–24. [Google Scholar]
- Shewry, P.R.; Charmet, G.; Branlard, G.; Lafiandra, D.; Gergely, S.; Salgó, A.; Saulnier, L.; Bedȍ, Z.; Mills, E.N.C.; Ward, J.L. Developing new types of wheat with enhanced health benefits. Trends Food Sci. Technol. 2012, 25, 70–77. [Google Scholar] [CrossRef]
- Rawat, N.; Laddomada, B.; Gill, B.S. Genomics of cereal-based functional foods. In Cereal Genomics II; Varshney, R.K., Gupta, P.K., Eds.; Springer Sciences + Business Media: Dordrecht, The Netherlands, 2013; pp. 247–274. [Google Scholar]
- Moure, A.; Cruz, J.M.; Franco, D.; Domìnguez, J.M.; Sineiro, J.; Dominguez, H.; Sineirob, J.; Domı́ngueza, H.; Núñezb, M.J.; Parajóa, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Fernàndez-Bolanos, J.; Rodrìguez, G.; Heredia, A. Production in large quantities of highly purified hydroxityrosol from liquid-solid waste of two-phase olive oil processing or Alperujo. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Dar, B.N.; Sharma, S. Total phenolic content of cereal bran using conventional and microwave assisted extraction. Am. J. Food Technol. 2011, 6, 1045–1053. [Google Scholar] [CrossRef]
- Delvecchio, L.N.; Pasqualone, A. Production trials of fresh pasta enriched with phenolic compounds extracted from wheat by KOH-induced hydrolysis. Progr. Nutr. 2013, 14, 247–251. [Google Scholar]
- Eisenmenger, M.; Dunford, N.T. Bioactive components of commercial and supercritical carbon dioxide processed wheat germ oil. J. Am. Oil Chem. Soc. 2008, 85, 55–61. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Rescio, L.; Mita, G.; Caretto, S. Durum wheat by-products as natural sources of valuable Nutrients. Phytochem. Rev. 2012, 11, 255–262. [Google Scholar] [CrossRef]
- Kwon, K.T.; Uddin, M.S.; Jung, G.W.; Sim, J.E.; Chun, B.S. Supercritical carbon dioxide extraction of phenolics and tocopherols enriched oil from wheat bran. World Acad. Sci. Eng. Technol. 2010, 64, 255–260. [Google Scholar]
- Bhandari, B.R.; D’Arcy, B.R.; Padukka, I. Encapsulation of lemon oil by paste method using beta-cyclodextrin: Encapsulation efficiency and profile of oil volatiles. J. Agric. Food. Chem. 1999, 47, 5194–5197. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Lenucci, M.S.; Laddomada, B.; Mita, G.; Caretto, S. Effects of sodium alginate bead encapsulation on the storage stability of durum wheat (Triticum durum Desf.) bran oil extracted by supercritical CO2. J. Agric. Food Chem. 2012, 60, 10689–10695. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.M.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Özer, E.A.; Herken, E.N.; Guzel, S.; Ainsworth, P.; Ibanoglu, S. Effect of extrusion process on the antioxidant activity and total phenolics in a nutritious snack food. Int. J. Food Sci. Technol. 2006, 41, 289–293. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Gómez, M.; Jiménez, S.; Ruiz, E.; Oliete, B. Effect of extruded wheat bran on dough rheology and bread quality. Food Sci. Technol. 2011, 44, 2231–2237. [Google Scholar] [CrossRef]
- Hirawan, R.; Ser, W.Y.; Arntfield, S.D.; Beta, T. Antioxidant properties of commercial, regular- and whole-wheat spaghetti. Food Chem. 2010, 119, 258–264. [Google Scholar] [CrossRef]
- Duodu, K.G. Effects of processing on antioxidant phenolics of cereal and legume grains. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.M., Piironen, V., Bean, S., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 31–54. [Google Scholar]
- Carini, E.; Curti, E.; Minucciani, M.; Antoniazzi, F.; Vittadini, E. Pasta. In Engineering Aspects of Cereal and Cereal-Based Products; De Pinho Ferreira Guinè, R., dos Reis Correira, P.M., Eds.; Taylor & Francis Group: LLC, Boca Raton, FL, USA, 2014; pp. 211–238. [Google Scholar]
- Mateo Anson, N.M.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.S.M. Impact of milling and thermal processing on phenolic compounds in cereal grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Xu, A.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; Seeram, N.P.; Hebe, D.; et al. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative Activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [PubMed]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666-15685. https://doi.org/10.3390/molecules200915666
Laddomada B, Caretto S, Mita G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules. 2015; 20(9):15666-15685. https://doi.org/10.3390/molecules200915666
Chicago/Turabian StyleLaddomada, Barbara, Sofia Caretto, and Giovanni Mita. 2015. "Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods" Molecules 20, no. 9: 15666-15685. https://doi.org/10.3390/molecules200915666








