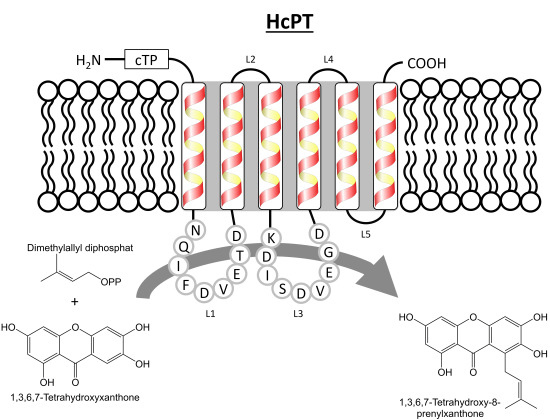
Molecular Cloning and Characterization of a Xanthone Prenyltransferase from Hypericum calycinum Cell Cultures
Abstract
:1. Introduction
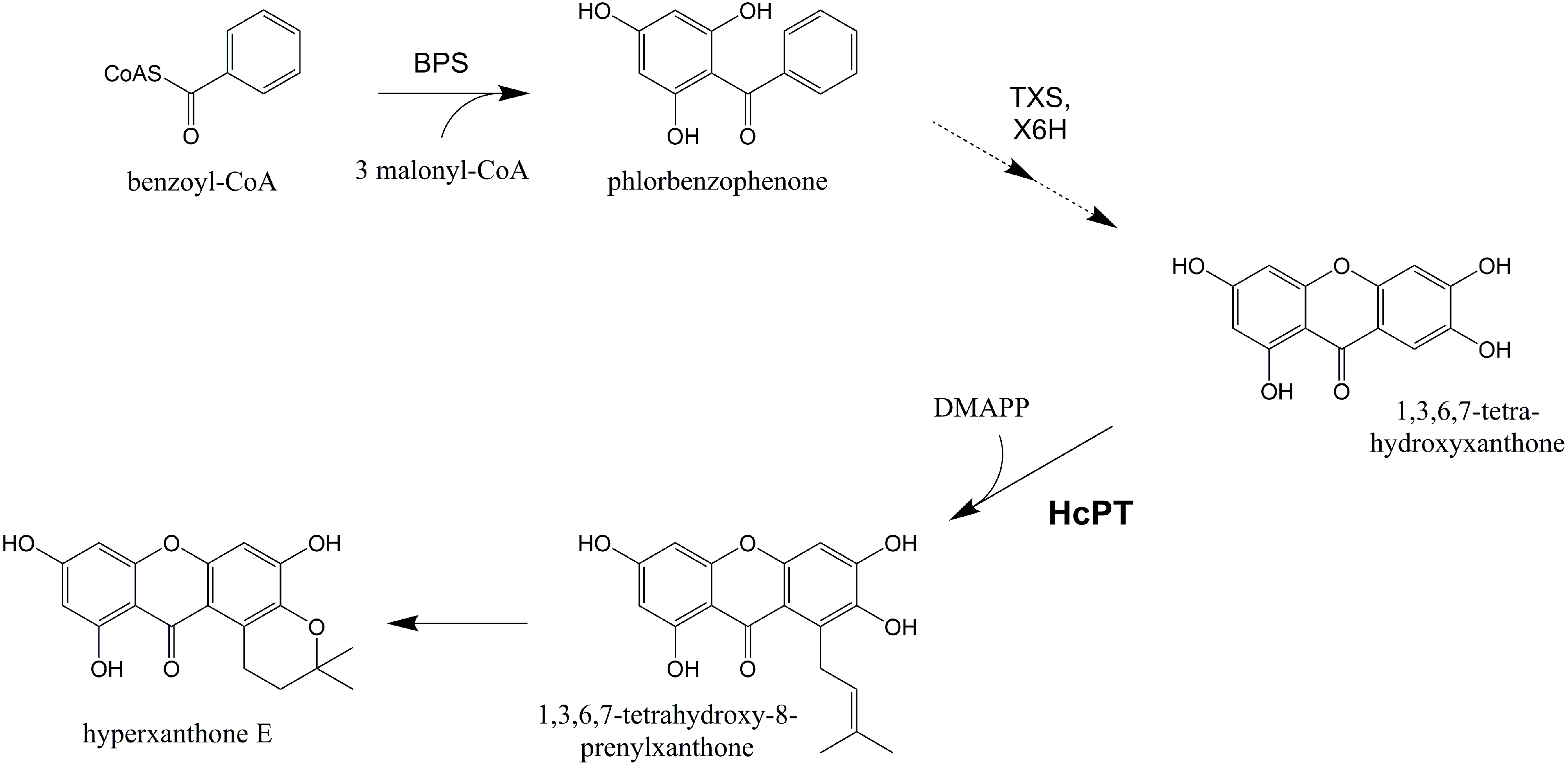
2. Results and Discussion
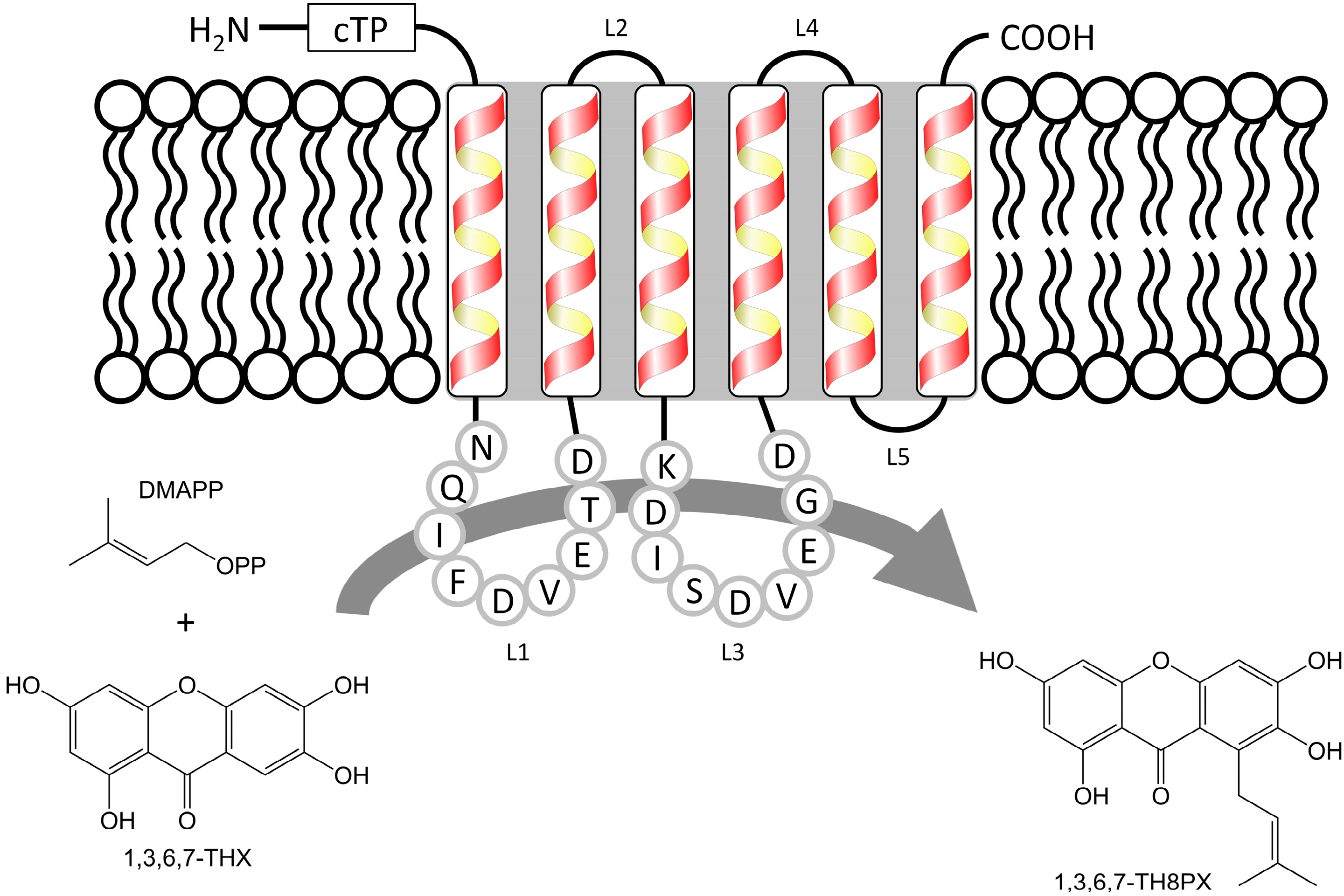
2.1. Isolation and Structural Analysis of a cDNA Encoding an Aromatic Prenyltransferase
| No. | Sequence * |
|---|---|
| HcPT-specific cloning primers | |
| 1 | 5′-TAGTACAAGTGATAAGAAATTCGG-3′ |
| 2 | 5′-TGTACTTAATGGTAAATCGGGTTTG-3′ |
| 3 | 5′-ACTGTATTGACAAGAAGGTTGATGG-3′ |
| 4 | 5′-AACGGCATGTATCTTGATGAACATAGG-3′ |
| HcCNL-specific primers for transcript level inspection | |
| 5 | 5′-TCAGCAGCGTGGAGGTCGAGTCG-3′ |
| 6 | 5′-TTAAAGACGAGACATGGCAAG-3′ |
| Hc18S-rRNA-specific primers for transcript level normalization | |
| 7 | 5′-TGATGGTATCTACTACTCGG-3′ |
| 8 | 5′-AATATACGCTATTGGAGCTGG-3′ |
| HcPT-specific overexpression primers | |
| 9 | 5′-AATGCTAGCATGGAGGTTTCTCGATTGCCATCG-3′ |
| 10 | 5′-AATAAGCTTCTAGATGAAGGGGAGCAAGATAAATTGC-3′ |
| 11 | 5′-ATTGAATTCATGGAGGTTTCTCGATTGC-3′ |
| 12 | 5′-ATTTTAATTAACTAGATGAAGGGGAGCAAGATAAATTGC-3′ |
| 13 | 5′-ATTGAATTCATGGAGGTTTCTCGATTGCCATCG-3′ |
2.2. Elicitor-Induced Changes at the Metabolite and Transcript Levels in H. calycinum Cell Cultures

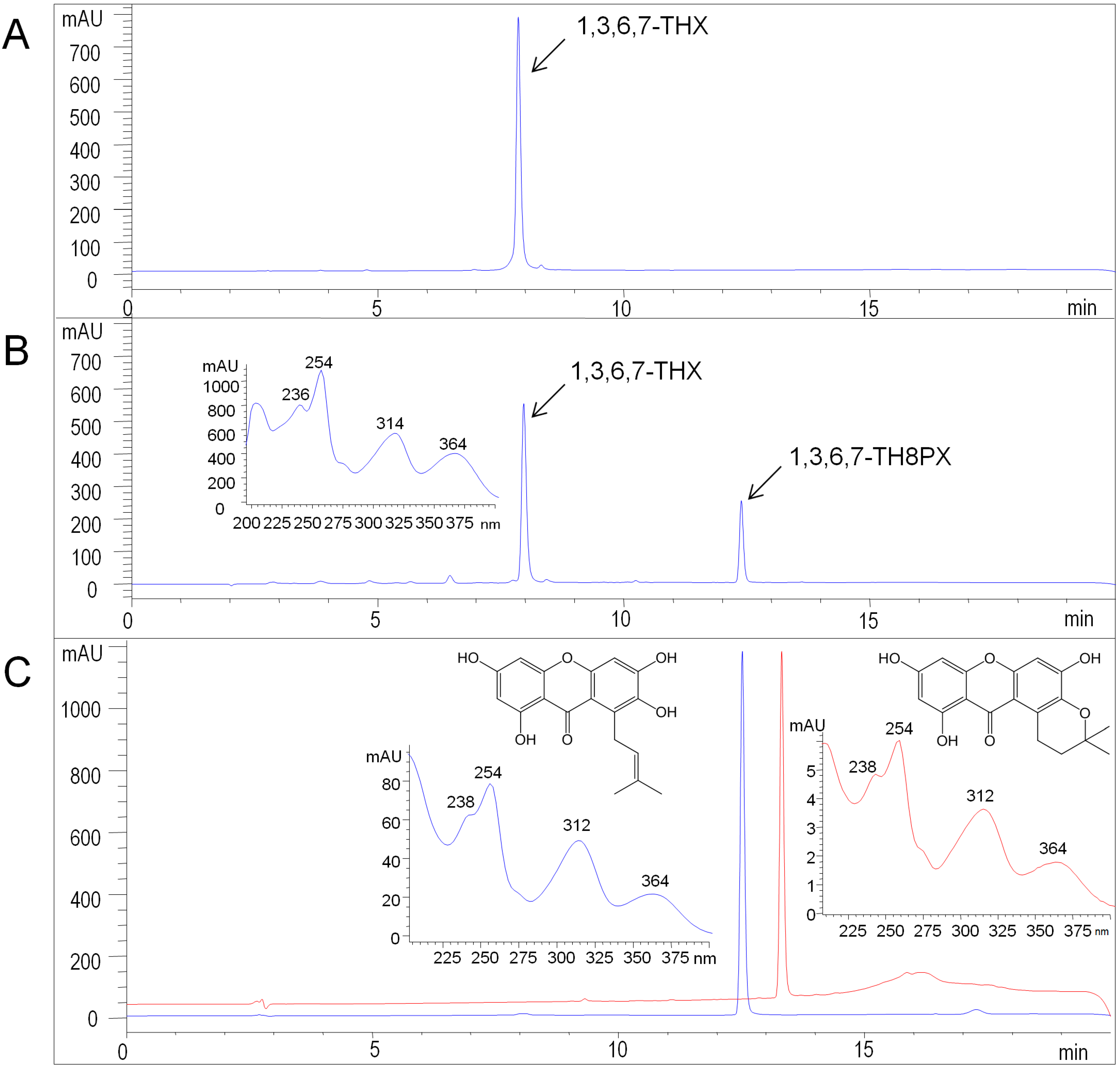
2.3. Functional Expression of HcPT
| Proton Position | δH * | J (Hz) | |
|---|---|---|---|
| 1–OH | 14.03 [1H] | s |  |
| 5–H | 6.54 [1H] | s | |
| 4–H | 6.30 [1H] | d, 2 | |
| 2–H | 6.15 [1H] | d, 2 | |
| =CH– | 5.47 [1H] | t, 6 | |
| 8–CH2 | 4.04 [2H] | d, 6 | |
| =C(CH3) | 1.80 [3H] | s | |
| =C(CH3) | 1.63 [3H] | s |
3. Experimental Section
3.1. Reagents
3.2. Synthesis of Trisammonium Dimethylallyl Diphosphate and 1,3,6,7-Tetrahydroxyxanthone
3.3. Plant Material and Elicitation
3.4. Isolation of an HcPT cDNA
3.5. Semi-Quantitative Reverse Transcription PCR
3.6. Heterologous Expression of HcPT
3.7. Microsomal Preparation from Insect Cells
3.8. In Vitro Enzyme Assay
3.9. HPLC and LC-ESI-MS Analyses
3.10. 1H-NMR Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beerhues, L. Biosynthesis of the Active Hypericum perforatum Constituents. In Medicinal and Aromatic Plant Science and Biotechnology; Odabas, M.S., Cirak, C., Eds.; Global Science Books: Isleworth, UK, 2011; Volume 5, (Special Issue 1: Hypericum); pp. 70–77. [Google Scholar]
- Tocci, N.; Simonetti, G.; D’Auria, F.D.; Panella, S.; Palamara, A.N.; Valletta, A.; Pasqua, G. Root cultures of Hypericum perforatum subsp. angustifolium elicited with chitosan and production of xanthone-rich extracts with antifungal activity. Appl. Microbiol. Biotechnol. 2011, 91, 977–987. [Google Scholar] [PubMed]
- El-Seedi, H.R.; El-Barbary, M.A.; El-Ghorab, D.M.H.; Bohlin, L.; Borg-Karlson, A.K.; Goransson, U.; Verpoorte, R. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar] [CrossRef] [PubMed]
- Na, Y. Recent cancer drug development with xanthone structures. J. Pharm. Pharmacol. 2009, 61, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Lee, H.H. Xanthones from Guttiferae. Phytochemistry 1989, 28, 967–998. [Google Scholar] [CrossRef]
- Chantarasriwong, O.; Cho, W.C.; Batova, A.; Chavasiri, W.; Moore, C.; Rheingold, A.L.; Theodorakis, E.A. Evaluation of the pharmacophoric motif of the caged Garcinia xanthones. Org. Biomol. Chem. 2009, 7, 4886–4894. [Google Scholar] [CrossRef] [PubMed]
- Anantachoke, N.; Tuchinda, P.; Kuhakarn, C.; Pohmakotr, M.; Reutrakul, V. Prenylated caged xanthones: Chemistry and biology. Pharm. Biol. 2012, 50, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Sasaki, K.; Tsurumaru, Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 2009, 70, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Karamat, F.; Olry, A.; Munakata, R.; Koeduka, T.; Sugiyama, A.; Paris, C.; Hehn, A.; Bourgaud, F.; Yazaki, K. A coumarin-specific prenyltransferase catalyzes the crucial biosynthetic reaction for furanocoumarin formation in parsley. Plant J. 2014, 77, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Gaid, M.M.; Sircar, D.; Müller, A.; Beuerle, T.; Liu, B.; Ernst, L.; Hänsch, R.; Beerhues, L. Cinnamate:CoA ligase initiates the biosynthesis of a benzoate-derived xanthone phytoalexin in Hypericum calycinum cell cultures. Plant Physiol. 2012, 160, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Scicchitano, F.; Whalley, B.J.; Mazzitello, C.; Ciriaco, M.; Esposito, S.; Patanè, M.; Upton, R.; Pugliese, M.; Chimirri, S.; et al. Hypericum perforatum: Pharmacokinetic, mechanism of action, tolerability, and clinical drug-drug interactions. Phytother. Res. 2014, 28, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Falkenstein-Paul, H.; Schmidt, W.; Beerhues, L. Benzophenone synthase and chalcone synthase from Hypericum androsaemum cell cultures: cDNA cloning, functional expression, and site-directed mutagenesis of two polyketide synthases. Plant J. 2003, 34, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Schmidt, W.; Beerhues, L. Regioselective oxidative phenol couplings of 2,3′,4,6-tetrahydroxybenzophenone in cell cultures of Centaurium erythraea RAFN and Hypericum androsaemum L. Planta 1997, 204, 64–69. [Google Scholar] [CrossRef]
- Schmidt, W.; Beerhues, L. Alternative pathways of xanthone biosynthesis in cell cultures of Hypericum androsaemum L. FEBS Lett. 1997, 420, 143–146. [Google Scholar] [CrossRef]
- Beerhues, L.; Berger, U. Xanthones in cell suspension cultures of two Centaurium species. Phytochemistry 1994, 35, 1227–1231. [Google Scholar] [CrossRef]
- Schmidt, W.; Abd El-Mawla, M.A.; Wolfender, J.L.; Hostettmann, K.; Beerhues, L. Xanthones in cell cultures of Hypericum androsaemum. Planta Med. 2000, 66, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Peters, S.; Beerhues, L. Xanthone 6-hydroxylase from cell cultures of Centaurium erythraea RAFN and Hypericum androsaemum L. Phytochemistry 2000, 53, 427–431. [Google Scholar] [CrossRef]
- Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 3 June 2015).
- Tsurumaru, Y.; Sasaki, K.; Miyawaki, T.; Uto, Y.; Momma, T.; Umemoto, N.; Momose, M.; Yazaki, K. HlPT-1, a membrane-bound prenyltransferase responsible for the biosynthesis of bitter acids in hops. Biochem. Biophys. Res. Commun. 2012, 417, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Mito, K.; Ohara, K.; Yamamoto, H.; Yazaki, K. Cloning and characterization of naringenin 8-prenyltransferase, a flavonoid-specific prenyltransferase of Sophora flavescens. Plant Physiol. 2008, 146, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Sasaki, K.; Aoki, T.; Ayabe, S.; Yazaki, K. Molecular cloning and characterization of a cDNA for pterocarpan 4-dimethylallyltransferase catalyzing the key prenylation step in the biosynthesis of glyceollin, a soybean phytoalexin. Plant Physiol. 2009, 149, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Mito, K.; Yazaki, K. Homogeneous purification and characterization of LePGT1— A membrane-bound aromatic substrate prenyltransferase involved in secondary metabolism of Lithospermum erythrorhizon. FEBS J. 2013, 280, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Muroya, A.; Fukushima, N.; Yazaki, K. Functional characterization of LePGT1, a membrane-bound prenyltransferase involved in the geranylation of p-hydroxybenzoic acid. Biochem. J. 2009, 421, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Compute pI/Mw Tool. Available online: http://web.expasy.org/compute_pi (accessed on 3 June 2015).
- Membrane Topology Prediction. Available online: http://harrier.nagahama-i-bio.ac.jp/sosui/sosui_submit.html (accessed on 3 June 2015).
- Subcellular Location Prediction. Available online: http://www.cbs.dtu.dk/services/TargetP (accessed on 3 June 2015).
- Chloroplast Transit Peptide Prediction. Available online: http://www.cbs.dtu.dk/services/ChloroP (accessed on 3 June 2015).
- Emanuelsson, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2007, 2, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Localization Prediction for N-Terminal Sorting Signals. Available online: http://ipsort.hgc.jp (accessed on 3 June 2015).
- Tsurumaru, Y.; Sasaki, K.; Miyawaki, T.; Momma, T.; Umemoto, N.; Yazaki, K. An aromatic prenyltransferase-like gene HlPT-1 preferentially expressed in lupulin glands of hop. Plant Biotechnol. 2010, 27, 199–204. [Google Scholar] [CrossRef]
- Munakata, R.; Inoue, T.; Koeduka, T.; Karamat, F.; Olry, A.; Sugiyama, A.; Takanashi, K.; Dugrand, A.; Froelicher, Y.; Tanaka, R.; et al. Molecular cloning and characterization of a geranyl diphosphate-specific aromatic prenyltransferase from lemon. Plant Physiol. 2014, 166, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, S.K. Beta-barrel proteins from bacterial outer membranes: Structure, function and refolding. Curr. Opin. Struct. Biol. 1999, 9, 455–461. [Google Scholar] [CrossRef]
- Rogl, H.; Kosemund, K.; Kuhlbrandt, W.; Collinson, I. Refolding of Escherichia coli produced membrane protein inclusion bodies immobilized by nickel chelating chromatography. FEBS Lett. 1998, 432, 21–26. [Google Scholar] [CrossRef]
- Kiefer, H. In vitro folding of alpha-helical membrane proteins. Biochim. Biophys. Acta 2003, 1610, 57–62. [Google Scholar] [CrossRef]
- Nielsen, H.; Arends, P. Xanthone constituents of Hypericum androsaemum. J. Nat. Prod. 1979, 42, 301–304. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Rattanaburi, S.; Phongpaichit, S.; Kanjana-Opas, A. Antibacterial and cytotoxic xanthones from Cratoxylum cochinchinense. Phytochem. Lett. 2008, 1, 211–214. [Google Scholar] [CrossRef]
- Chin, Y.W.; Kinghorn, A.D. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev. Org. Chem. 2008, 5, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, A.; Paiva, A.; Sousa, E.; Seca, H.; Almeida, G.M.; Lima, R.T.; Fernandes, M.X.; Pinto, M.; Vasconcelos, M.H. Insights into the in vitro antitumor mechanism of action of a new pyranoxanthone. Chem. Biol. Drug Des. 2010, 76, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.M.; Castanheiro, R. Natural prenylated xanthones: Chemistry and biological activities. In Natural Products: Chemistry, Biochemistry and Pharmacology, 1st ed.; Brahmachari, G., Ed.; Narosa Publishing House Pvt. Ltd: New Delhi, India, 2009; pp. 520–675. [Google Scholar]
- Castanheiro, R.A.P.; Pinto, M.M.M.; Silva, A.M.S.; Cravo, S.M.M.; Gales, L.; Damas, A.M.; Nazareth, N.; Nascimentoa, M.S.J.; Graham, E. Dihydroxyxanthone prenylated derivatives: Synthesis, structure elucidation, and growth inhibitory activity on human tumor cell lines with improvement of selectivity for MCF-7. Bioorg. Med. Chem. 2007, 15, 6080–6088. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.K.; Choi, S.B.; Tanc, S.C.; Wahab, H.A.; Chane, K.L.; Murugaiyaha, V. Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies. Phytomedicine 2014, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Gujral, V.; Gupta, S.R. Synthesis of some 3-methylbut-2-enylated 1,3,5-trihydroxyxanthones. Bull. Chem. Soc. Jpn. 1979, 52, 3679–3681. [Google Scholar] [CrossRef]
- Helesbeux, J.J.; Duval, O.; Dartiguelongue, C.; Séraphin, D.; Oger, J.M.; Richomme, P. Synthesis of 2-hydroxy-3-methylbut-3-enyl substituted coumarins and xanthones as natural products. Application of the Schenck ene reaction of singlet oxygen with ortho-prenylphenol precursors. Tetrahedron 2004, 60, 2293–2300. [Google Scholar] [CrossRef]
- Woodside, A.B.; Huang, Z.; Poulter, C.D. Trisammonium geranyl diphosphate. Org. Synth. 1988, 66, 211. [Google Scholar] [CrossRef]
- Genoux-Bastide, E.; Lorendeau, D.; Nicolle, E.; Yahiaoui, S.; Magnard, S.; di Pietro, A.; Baubichon-Cortay, H.; Boumendjel, A. Identification of xanthones as selective killers of cancer cells overexpressing the ABC transporter MRP1. Chem. Med. Chem. 2011, 6, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Klingauf, P.; Beuerle, T.; Mellenthin, A.; El-Moghazy, S.A.M.; Boubakir, Z.; Beerhues, L. Biosynthesis of the hyperforin skeleton in Hypericum calycinum cell cultures. Phytochemistry 2005, 66, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Sircar, D.; Gaid, M.M.; Chizzali, C.; Reckwell, D.; Kaufholdt, D.; Beuerle, T.; Broggini, G.A.L.; Flachowsky, H.; Liu, B.; Hänsch, R.; et al. Biphenyl-4-hydroxylases involved in aucuparin biosynthesis in rowan and apple are CYP736A proteins. Plant Physiol. 2015, 168, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J. Trioxygenated naturally occurring xanthones. Phytochemistry 1997, 44, 191–214. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J.; de Oliveira, F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar] [CrossRef]
- Sousa, M.E.; Pinto, M.M.M. Synthesis of xanthones: An overview. Curr. Med. Chem. 2005, 12, 2447–2479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Ye, S.; Zhang, Y.; Tao, L.; Gao, Y.; Gong, D.; Xi, M.; Meng, H.; Zhang, M.; et al. Synthesis, SAR and biological evaluation of natural and non-natural hydroxylated and prenylated xanthones as antitumor agents. Med. Chem. 2012, 8, 1012–1025. [Google Scholar] [PubMed]
- Pinto, M.M.; Sousa, M.E.; Nascimento, M.S. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Bohle, D.S. Pharmacological and biological activities of xanthones. Anti-Infect. Agents Med. Chem. 2006, 5, 15–31. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, L.; Chen, X.H.; Zhu, X.F.; Qian, X.J.; Feng, G.K.; Lan, W.J.; Li, H.J. Cytotoxic prenylated xanthones from the pericarp of Garcinia mangostana. Molecules 2014, 19, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1,3,6,7-tetrahydroxyxanthone and 1,3,6,7-tetrahydroxy-8-prenylxanthone are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiesel, T.; Gaid, M.; Müller, A.; Bartels, J.; El-Awaad, I.; Beuerle, T.; Ernst, L.; Behrends, S.; Beerhues, L. Molecular Cloning and Characterization of a Xanthone Prenyltransferase from Hypericum calycinum Cell Cultures. Molecules 2015, 20, 15616-15630. https://doi.org/10.3390/molecules200915616
Fiesel T, Gaid M, Müller A, Bartels J, El-Awaad I, Beuerle T, Ernst L, Behrends S, Beerhues L. Molecular Cloning and Characterization of a Xanthone Prenyltransferase from Hypericum calycinum Cell Cultures. Molecules. 2015; 20(9):15616-15630. https://doi.org/10.3390/molecules200915616
Chicago/Turabian StyleFiesel, Tobias, Mariam Gaid, Andreas Müller, Joana Bartels, Islam El-Awaad, Till Beuerle, Ludger Ernst, Sönke Behrends, and Ludger Beerhues. 2015. "Molecular Cloning and Characterization of a Xanthone Prenyltransferase from Hypericum calycinum Cell Cultures" Molecules 20, no. 9: 15616-15630. https://doi.org/10.3390/molecules200915616
APA StyleFiesel, T., Gaid, M., Müller, A., Bartels, J., El-Awaad, I., Beuerle, T., Ernst, L., Behrends, S., & Beerhues, L. (2015). Molecular Cloning and Characterization of a Xanthone Prenyltransferase from Hypericum calycinum Cell Cultures. Molecules, 20(9), 15616-15630. https://doi.org/10.3390/molecules200915616