Ultrasound-Assisted Aqueous Two-Phase System for Extraction and Enrichment of Zanthoxylum armatum Lignans
Abstract
:1. Introduction
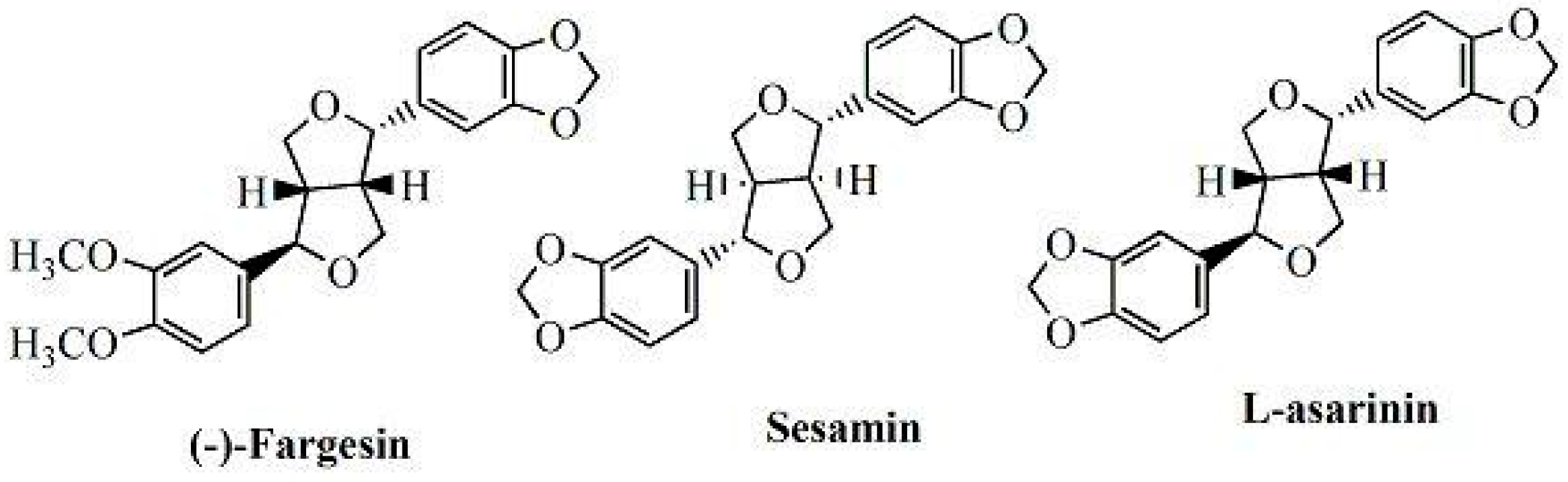
2. Results and Discussion
2.1. Phase Diagram of the Alcohol/Salts System

2.2. The Selection of the ATPS System
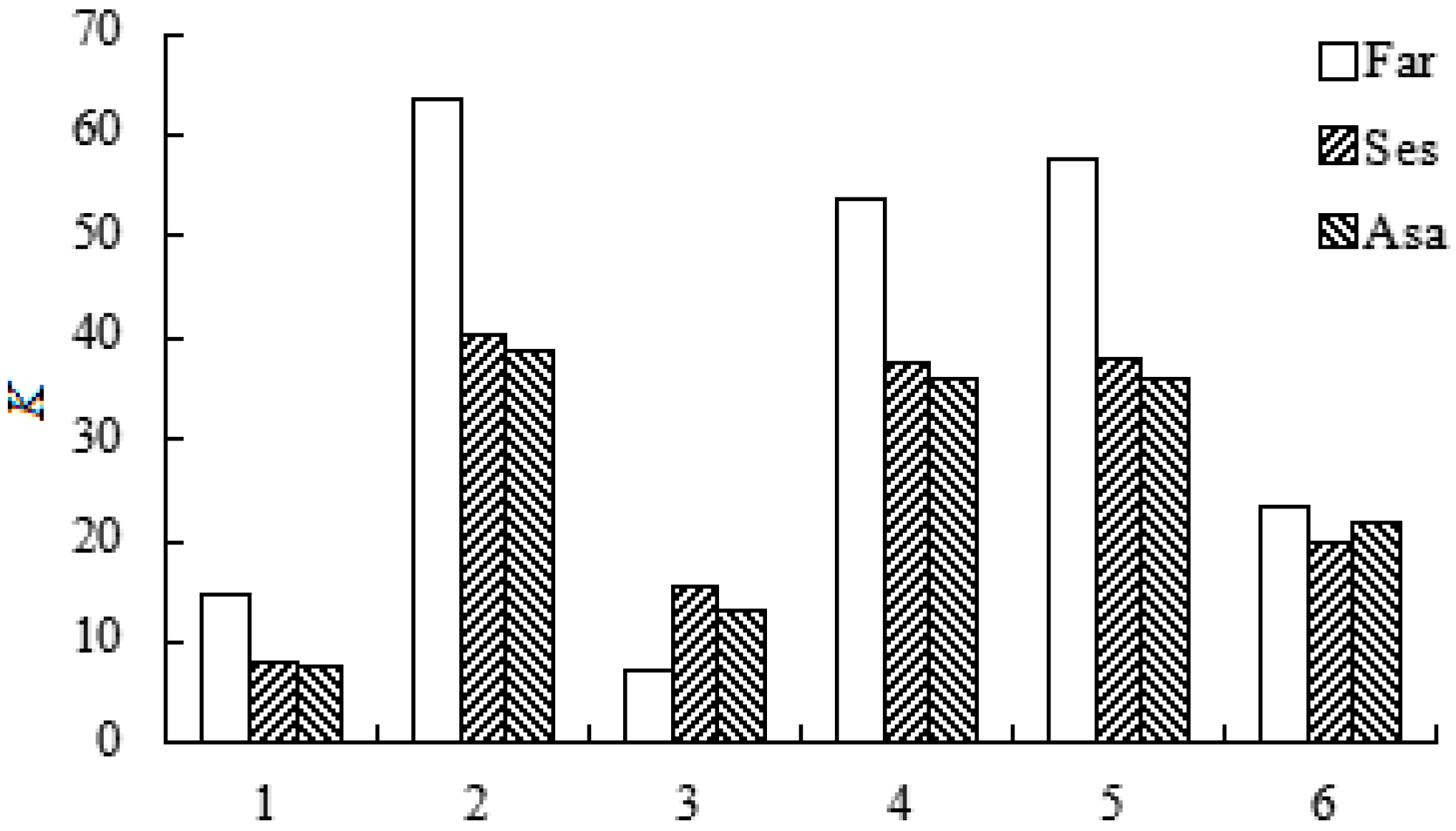
2.3. The Effects of (NH4)2SO4 on the Lignan Recoveries
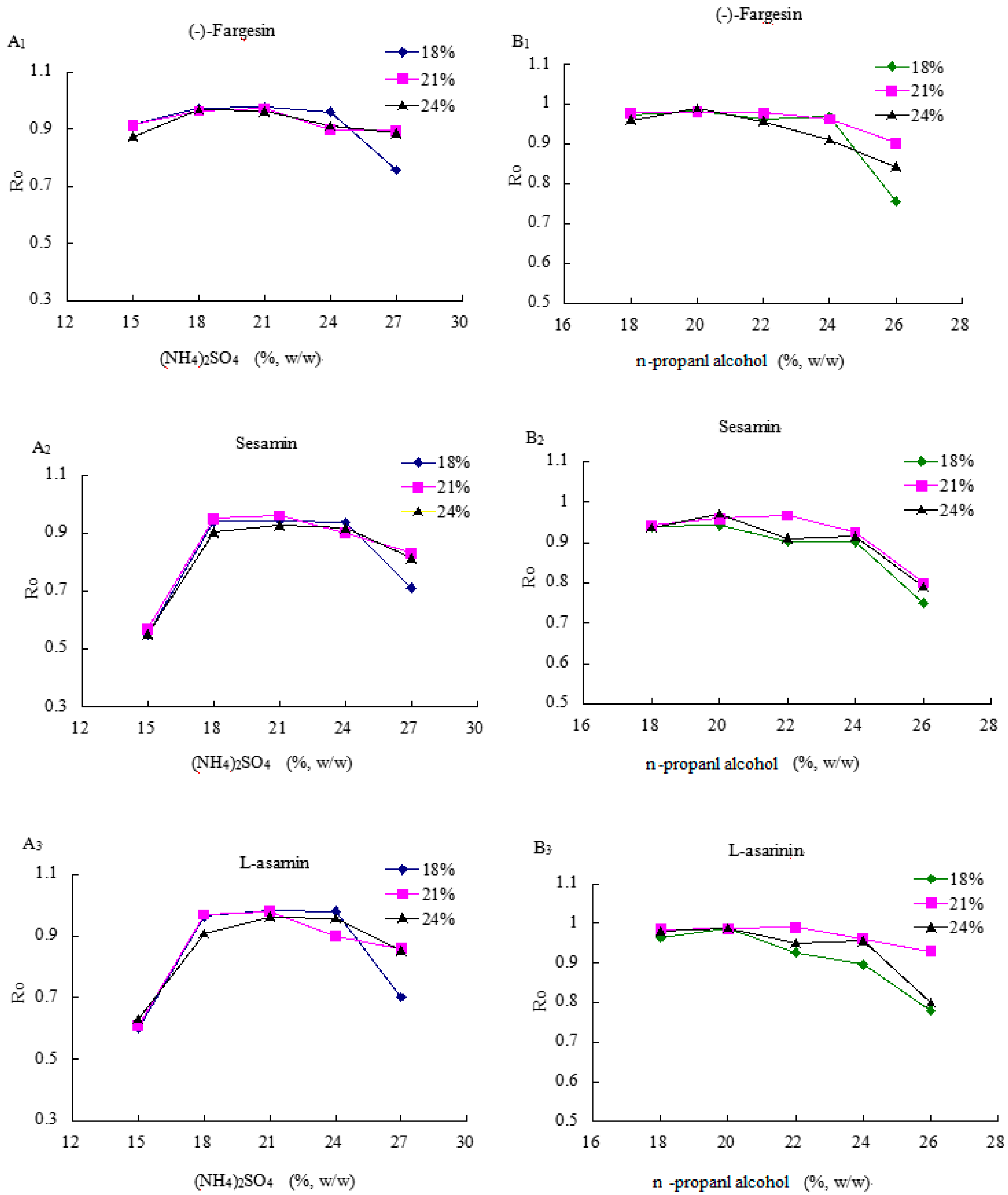
2.4. The Effects of n-Propanol on the Lignan Recovery
2.5. RSM Modeling
| Test Set | Extraction Conditions | Actual Yield (mg/g Sample) | ANN Predicted Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent to Solid (X1) | Ultrasound Temperature (X2) | Ultrasound Time (X3) | Far | Ses | Asa | Far | Ses | Asa | |
| 1 | 1 | 0 | −1 | 1.541 | 0.751 | 0.953 | 1.541 | 0.751 | 0.952 |
| 2 | 0 | 0 | 0 | 1.358 | 0.686 | 0.922 | 1.398 | 0.703 | 0.949 |
| 3 | 0 | 0 | 0 | 1.386 | 0.719 | 0.961 | 1.398 | 0.703 | 0.949 |
| 4 | 0 | 1 | 1 | 1.194 | 0.649 | 0.83 | 1.193 | 0.649 | 0.831 |
| 5 | 0 | 0 | 0 | 1.423 | 0.692 | 0.961 | 1.398 | 0.703 | 0.949 |
| 6 | 1 | 1 | 0 | 1.358 | 0.727 | 1.062 | 1.355 | 0.751 | 0.124 |
| 7 | 0 | −1 | 1 | 1.190 | 0.641 | 0.897 | 1.189 | 0.641 | 0.896 |
| 8 | 0 | 1 | −1 | 1.259 | 0.697 | 0.868 | 1.259 | 0.697 | 0.869 |
| 9 | 0 | −1 | −1 | 1.243 | 0.638 | 0.845 | 1.301 | 0.715 | 0.738 |
| 10 | 1 | 0 | 0 | 1.305 | 0.727 | 1.124 | 1.264 | 0.727 | 1.124 |
| 11 | 1 | −1 | 0 | 1.349 | 0.751 | 1.093 | 1.305 | 0.741 | 1.100 |
| 12 | −1 | 1 | 0 | 1.100 | 0.588 | 0.729 | 1.126 | 0.583 | 0.704 |
| 13 | 0 | 0 | 0 | 1.423 | 0.706 | 0.977 | 1.398 | 0.703 | 0.949 |
| 14 | −1 | 0 | −1 | 1.258 | 0.625 | 0.701 | 1.161 | 0.582 | 0.702 |
| 15 | 0 | 0 | 0 | 1.391 | 0.712 | 0.915 | 1.398 | 0.703 | 0.948 |
| 16 | −1 | −1 | 0 | 1.309 | 0.582 | 0.711 | 1.314 | 0.582 | 0.711 |
| 17 | −1 | 0 | 1 | 1.190 | 0.597 | 0.716 | 1.122 | 0.583 | 0.704 |
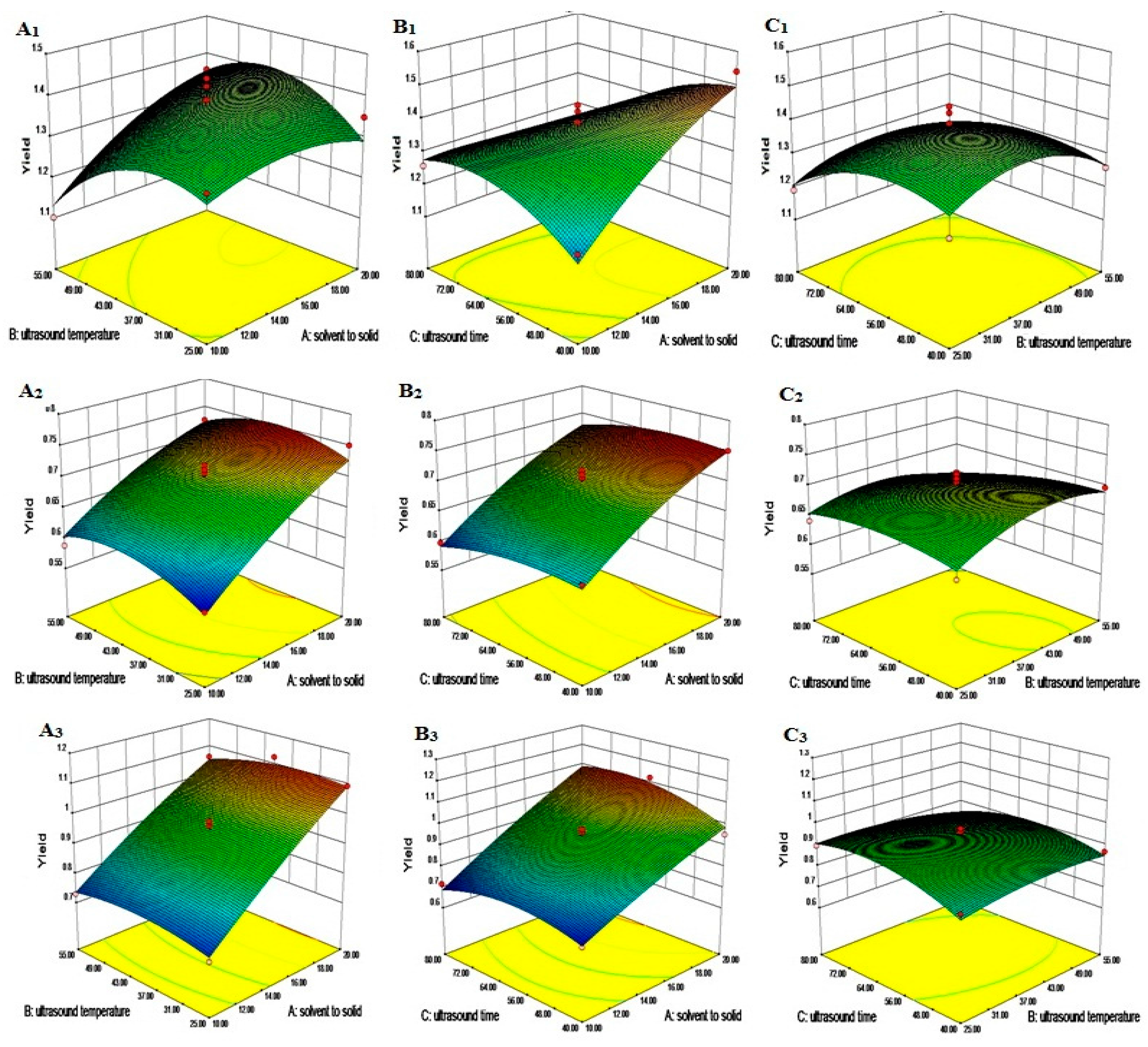
2.6. Modeling of ANN

2.7. Comparison of ATPS and Heat Reflux Extraction
| Sample | Yield of Extract | Far | Ses | Asa | |||
|---|---|---|---|---|---|---|---|
| (mg/g) | Yield (mg/g) | Purity (%) | Yield (mg/g) | Purity (%) | Yield (mg/g) | Purity (%) | |
| Extracts with ATPS | 71.57 ± 1.82 | 1.591 ± 0.16 | 2.222 | 0.763 ± 0.07 | 1.066 | 1.133 ± 0.10 | 1.583 |
| Extracts with ethanol | 89.80 ± 1.33 | 1.382 ± 0.12 | 1.539 | 0.771 ± 0.05 | 0.858 | 1.125 ± 0.09 | 1.253 |
3. Experimental Section
3.1. Materials
3.2. Preparation of the Crude Lignan Extract
3.3. Preparation of Aqueous Two-Phase Systems
3.4. Ultrasonic-Assisted ATPS (UAE-ATPS) to Extract Lignans from Z. armatum
3.5. HPLC Analysis
3.6. RSM Design
3.7. ANN Modeling
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Zhang, D.Y.; Zu, Y.G.; Fu, Y.J. Aqueous two-phase extraction and enrichment of two main flavonoids from pigeon pea roots and the antioxidant activity. Sep. Purif. Technol. 2013, 102, 26–33. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.T.; Yuan, X.F.; Zhao, Q.S. Ultrasound-assisted aqueous two-phase extraction of phenylethanoid glycosides from Cistanche deserticola Y.C. Ma stems. J. Sep. Sci. 2015, 38, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Wei, J.Q.; Guo, T.; Huang, Y.; Wang, Y.; Ma, J.P.; Zhao, P. Aqueous two phase system coupled with ultrasound for the extraction of total alkaloid from the peel of Zanthoxylum bungeanum. Sci. Technol. Food Ind. 2015, 36, 202–206. [Google Scholar]
- Yuzugullu, Y.; Duman, Y.A. Aqueous two-phase (PEG4000 = Na2SO4) extraction and characterization of an acid invertase from potato tuber (Solanum tuberosum). Prep. Biochem. Biotechnol. 2015, 45, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Hardt, S.; Hahn, T. Microfluidics with aqueous two-phase systems. Lab. Chip. 2012, 12, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.X.; Wang, H.F.; Wang, D.H.; Fang, F.; Wang, F.Z.; Wu, T. Ultrasonic extraction of antioxidants from Chinese Sumac (Rhus typhina L.) fruit using response surface methodology and their characterization. Molecules 2014, 19, 9019–9032. [Google Scholar] [PubMed]
- Ho, S.K.; Tan, C.P.; Thoo, Y.Y.; Abas, F.; Ho, C.W. Ultrasound-assisted extraction of antioxidants in Misai. Kucing (Orthosiphon stamineus). Molecules 2014, 19, 12640–12659. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, H.; Tavassoli, N.; Sadeghi, O.; Amini, M.M. Optimization of solid-phase extraction using artificial neural networks and response surface methodology in combination with experimental design for determination of gold by atomic absorption spectrometry in industrial wastewater samples. Talanta 2012, 97, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Korbahti, B.K.; Tanyolac, A. Electrochemical treatment of simulated textile wastewater with industrial components and Levafix Blue CA reactive dye: Optimization through response surface methodology. J. Hazard. Mater. 2008, 151, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Aleboyeh, A.; Daneshvar, N.; Kasiri, M.B. Optimization of C.I. Acid Red 14 azo dye removal by electrocoagulation batch process with response surface methodology. Chem. Eng. Process. 2008, 47, 827–832. [Google Scholar]
- Daneshvar, N.; Khataee, A.R.; Djafarzadeh, N. The use of artificial neural network (ANN) for modeling of decoloriation of textile dye solution containing C.I. Basic Yellow 28 by electrocoagulation process. J. Hazard. Mater. 2006, 137, 1788–1795. [Google Scholar] [PubMed]
- Aber, S.; Amani-Ghadim, A.R.; Mirzajani, V. Removal of Cr(VI) from polluted solutions by electrocoagulation: Modeling of experimental results using artificial neural network. J. Hazard. Mater. 2009, 171, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Keka, S.; Papita, D.S.; Siddhartha, D. Response surface optimization and artificial neural network modeling of microwave assisted natural dye extraction from pomegranate rind. Ind. Crop. Prod. 2012, 37, 408–414. [Google Scholar]
- Guo, T.; Deng, Y.X.; Xie, H.; Yao, C.Y.; Cai, C.C.; Pan, S.L.; Wang, Y.L. Antinociceptive and anti-inflammatory activities of ethyl acetate fraction from Zanthoxylum armatum in mice. Fitoterapia 2011, 82, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Xie, H.; Deng, Y.X.; Pan, S.L. A new lignan and other constituents from Zanthoxylum armatum DC. Nat. Prod. Res. 2012, 26, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Han, J.; Zhang, D.Y.; Wang, L.H.; Zhou, L.L. Aqueous two-phase system coupled with ultrasound for the extraction of lignans from seeds of Schisandra chinensis (turcz.) Baill. Ultrason. Sonochem. 2013, 20, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Zhang, Q.; Li, Y.F.; Dong, L.L.; Liu, S.L. Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohydr. Polym. 2015, 119, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, B.; Zhang, D.; Xiu, Z. Aqueous two-phase extraction of 1,3-propanediol from glycerol-based fermentation broths. Sep. Purif. Technol. 2009, 66, 472–478. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; Su, D.; Huang, Y.; Wang, Y.; Li, Y.-H. Ultrasound-Assisted Aqueous Two-Phase System for Extraction and Enrichment of Zanthoxylum armatum Lignans. Molecules 2015, 20, 15273-15286. https://doi.org/10.3390/molecules200815273
Guo T, Su D, Huang Y, Wang Y, Li Y-H. Ultrasound-Assisted Aqueous Two-Phase System for Extraction and Enrichment of Zanthoxylum armatum Lignans. Molecules. 2015; 20(8):15273-15286. https://doi.org/10.3390/molecules200815273
Chicago/Turabian StyleGuo, Tao, Dan Su, Yan Huang, Ya Wang, and Yong-Hui Li. 2015. "Ultrasound-Assisted Aqueous Two-Phase System for Extraction and Enrichment of Zanthoxylum armatum Lignans" Molecules 20, no. 8: 15273-15286. https://doi.org/10.3390/molecules200815273





