Porphyrins as Corrosion Inhibitors for N80 Steel in 3.5% NaCl Solution: Electrochemical, Quantum Chemical, QSAR and Monte Carlo Simulations Studies
Abstract
:1. Introduction
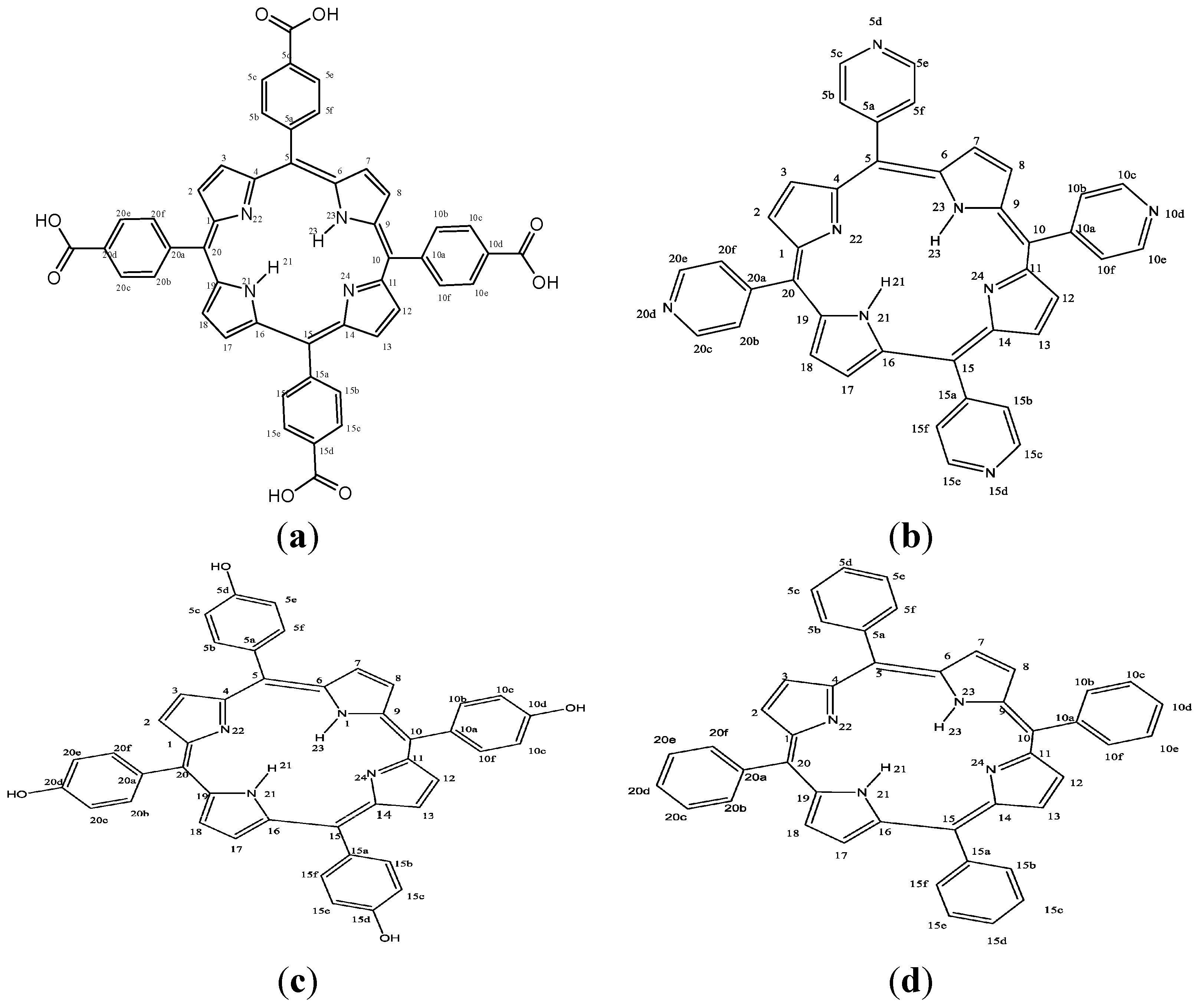
2. Results and Discussion
2.1. EIS Measurements
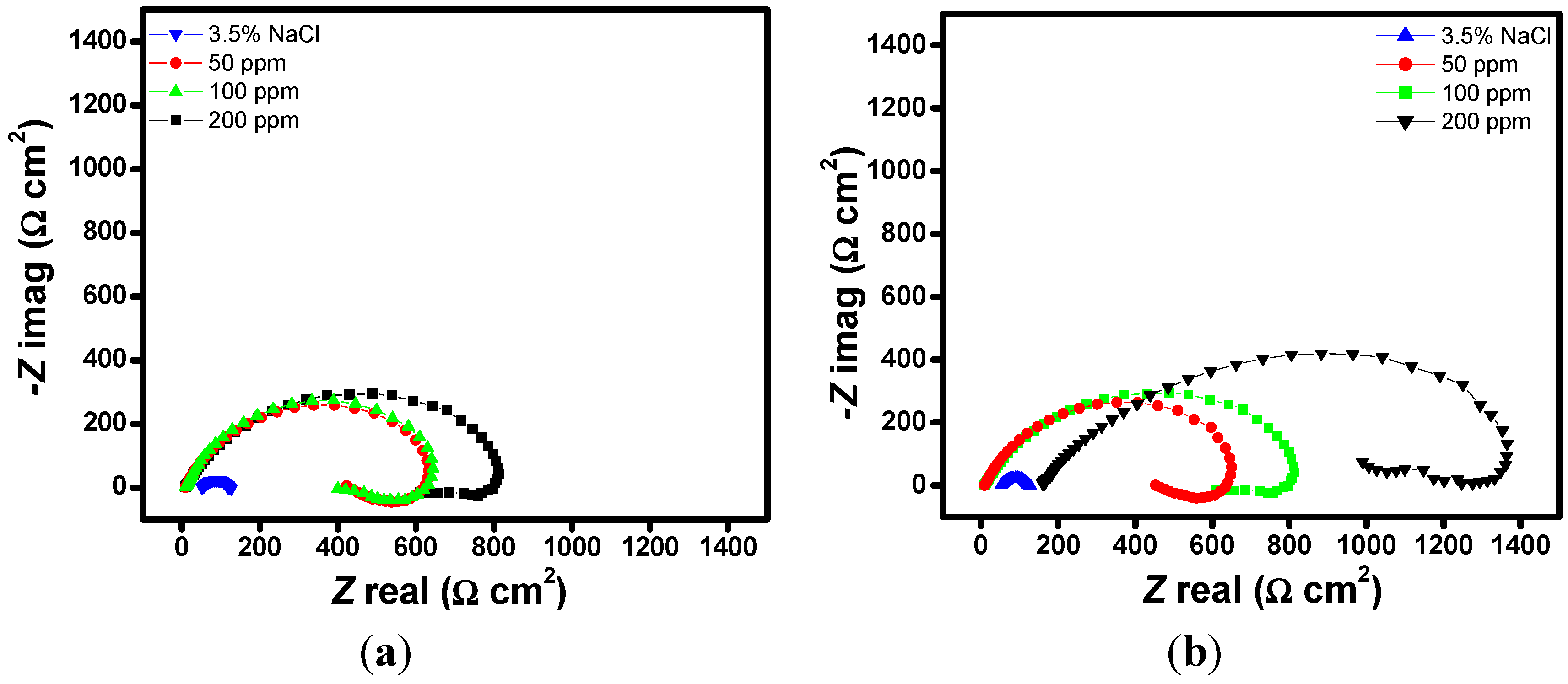
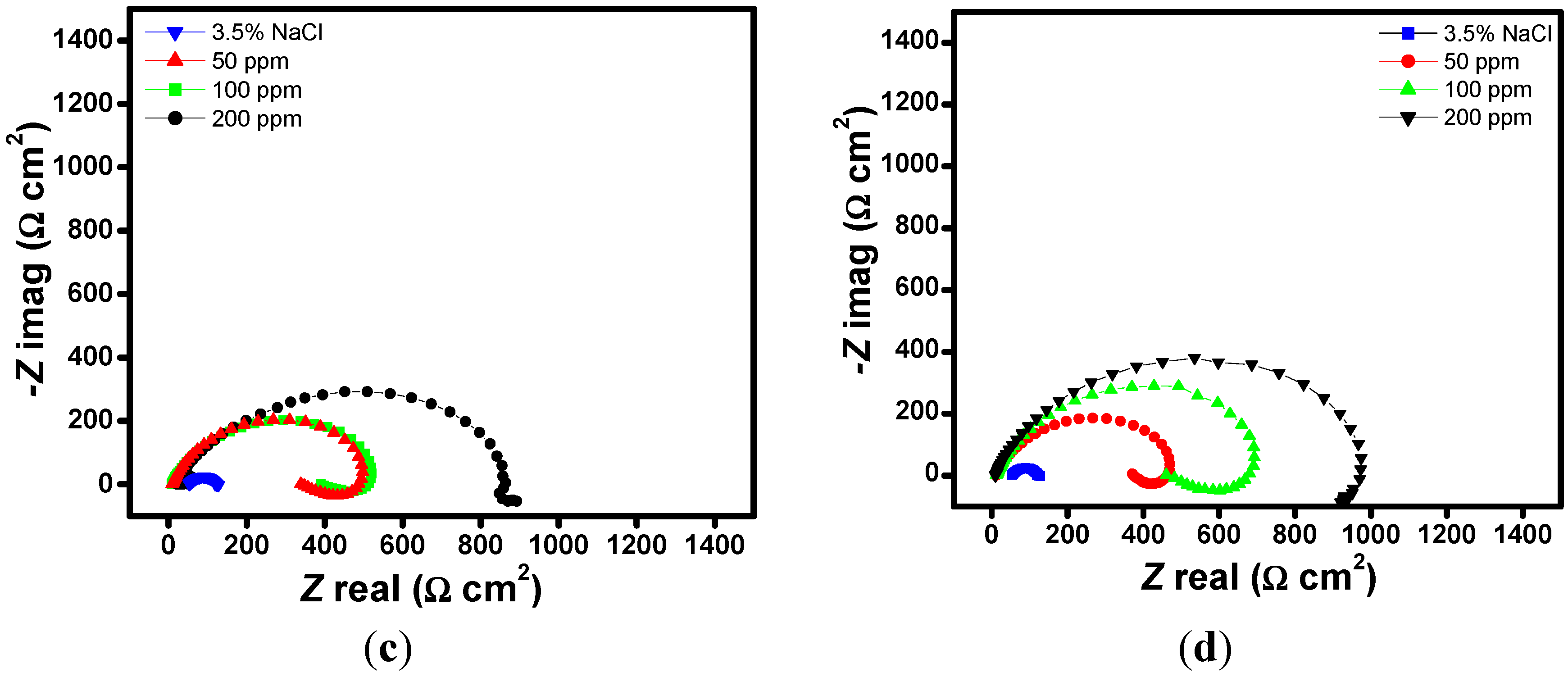
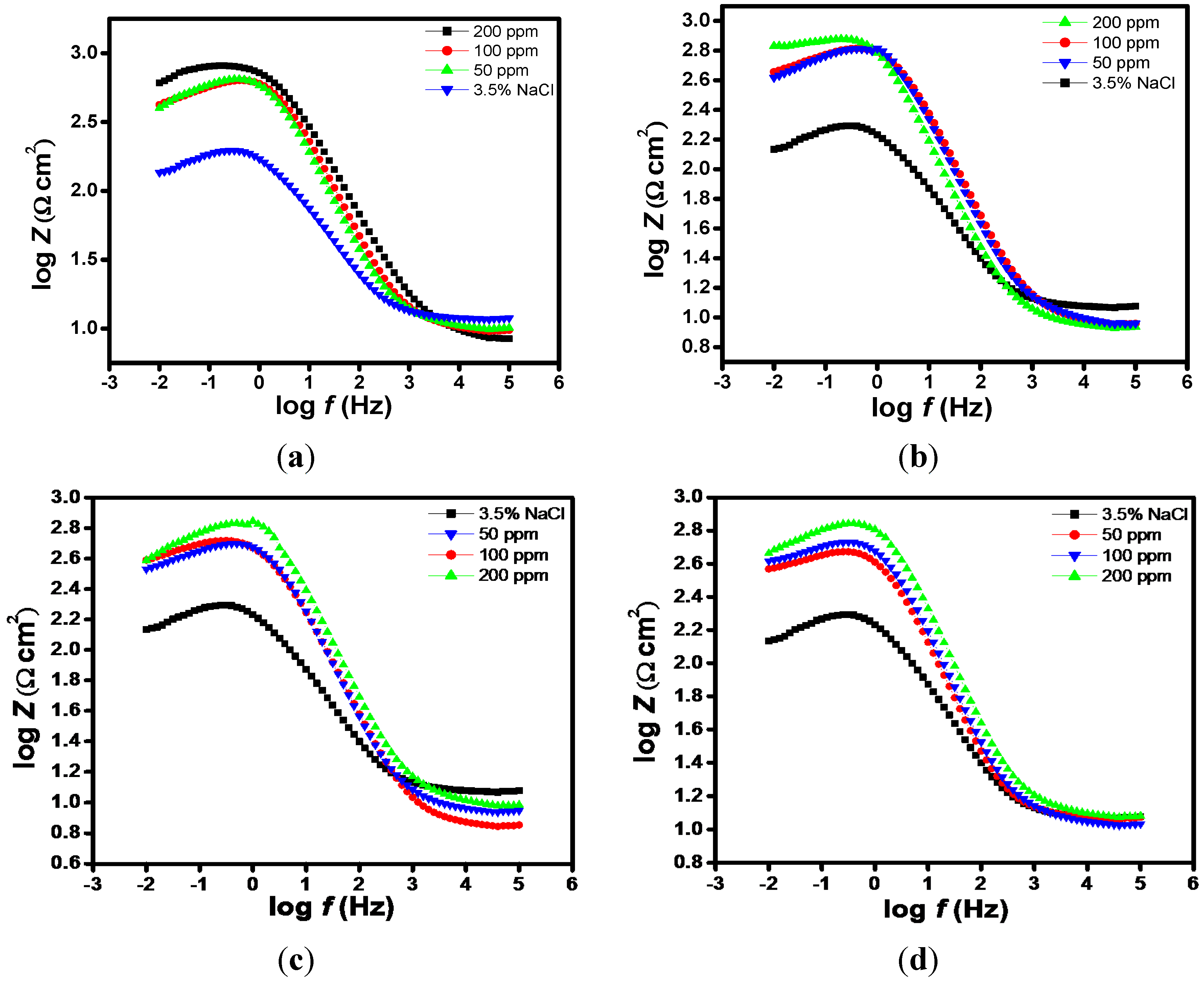

| Aggressive Solutions | Rs (Ω·cm2) | Rct (Ω·cm2) | n | Yo (Ω−1·sn·cm-2) | L (H·cm2) | Chi | η% |
|---|---|---|---|---|---|---|---|
| 3.5% NaCl | 10.0 | 121 | 0.616 | 129 | 33 | 0.0006 | - |
| HPTB 50 ppm | 1.4 | 600 | 0.728 | 95 | 20 | 0.0011 | 80 |
| HPTB 100 ppm | 1.1 | 622 | 0.818 | 99 | 28 | 0.0003 | 81 |
| HPTB 200 ppm | 1.8 | 818 | 0.858 | 102 | 22 | 0.0007 | 85 |
| T4PP 50 ppm | 1.9 | 612 | 0.722 | 116 | 22 | 0.0009 | 80 |
| T4PP 100 ppm | 1.3 | 743 | 0.827 | 78 | 19 | 0.0013 | 84 |
| T4PP 200 ppm | 1.7 | 1356 | 0.864 | 43 | 24 | 0.0007 | 91 |
| THP 50 ppm | 1.7 | 495 | 0.788 | 88 | 13 | 0.0008 | 76 |
| THP 100 ppm | 1.0 | 525 | 0.834 | 101 | 14 | 0.0006 | 77 |
| THP 200 ppm | 1.9 | 879 | 0.838 | 94 | 0 | 0.0012 | 86 |
| TPP 50 ppm | 1.8 | 429 | 0.816 | 102 | 14 | 0.0008 | 72 |
| TPP 100 ppm | 1.2 | 634 | 0.820 | 93 | 27 | 0.0008 | 81 |
| TPP 200 ppm | 1.4 | 986 | 0.825 | 99 | 21 | 0.0014 | 88 |
| Aggressive Solution | −S | −α (°) |
|---|---|---|
| 3.5% NaCl | 0.487 | 31.4 |
| HPTB (200 ppm) | 0.808 | 58.4 |
| T4PP (200 ppm) | 0.745 | 57.3 |
| THP (200 ppm) | 0.723 | 59.6 |
| TPP (200 ppm) | 0.812 | 60.5 |
2.2. Polarization Measurements

| Aggressive Solutions | −Ecorr (mV vs. SCE) | Icorr (µA·cm−2) | ba (mV/dec) | bc (mV/dec) | η% |
|---|---|---|---|---|---|
| 3.5% NaCl | 730 | 60.77 | 69 | 214 | - |
| HPTB 50 ppm | 740 | 49.63 | 76 | 403 | 18 |
| HPTB 100 ppm | 742 | 31.19 | 90 | 583 | 49 |
| HPTB 200 ppm | 732 | 11.08 | 105 | 854 | 82 |
| T4PP 50 ppm | 738 | 44.68 | 67 | 285 | 26 |
| T4PP 100 ppm | 737 | 33.02 | 68 | 319 | 46 |
| T4PP 200 ppm | 750 | 9.13 | 69 | 282 | 85 |
| THP 50 ppm | 739 | 39.28 | 66 | 211 | 35 |
| THP 100 ppm | 745 | 28.77 | 65 | 203 | 53 |
| THP 200 ppm | 746 | 12.17 | 72 | 307 | 80 |
| TPP 50 ppm | 749 | 46.24 | 71 | 224 | 24 |
| TPP 100 ppm | 749 | 38.49 | 90 | 483 | 37 |
| TPP 200 ppm | 750 | 10.03 | 113 | 908 | 84 |
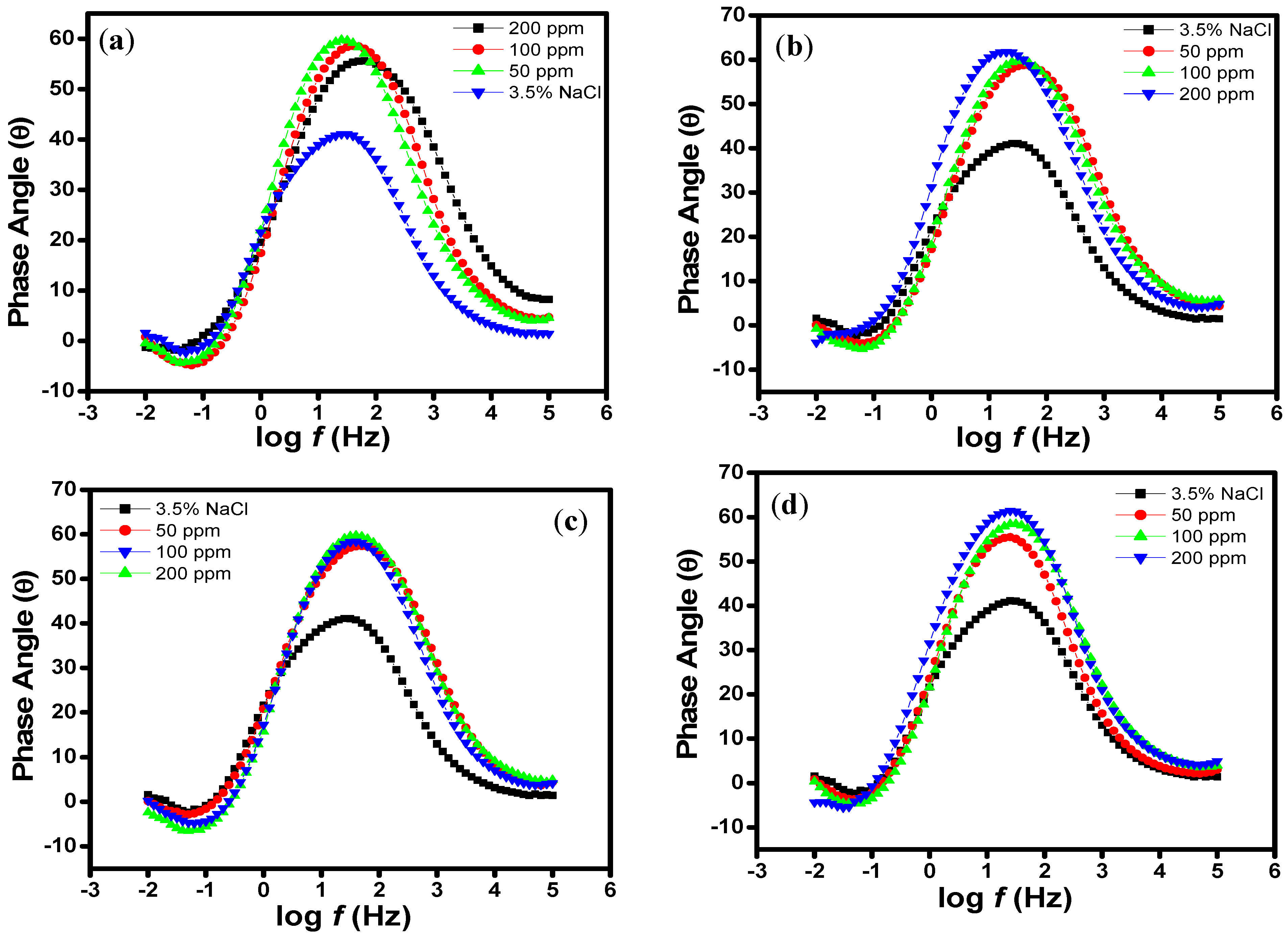
2.3. Scanning Electrochemical Microscopy (SECM)
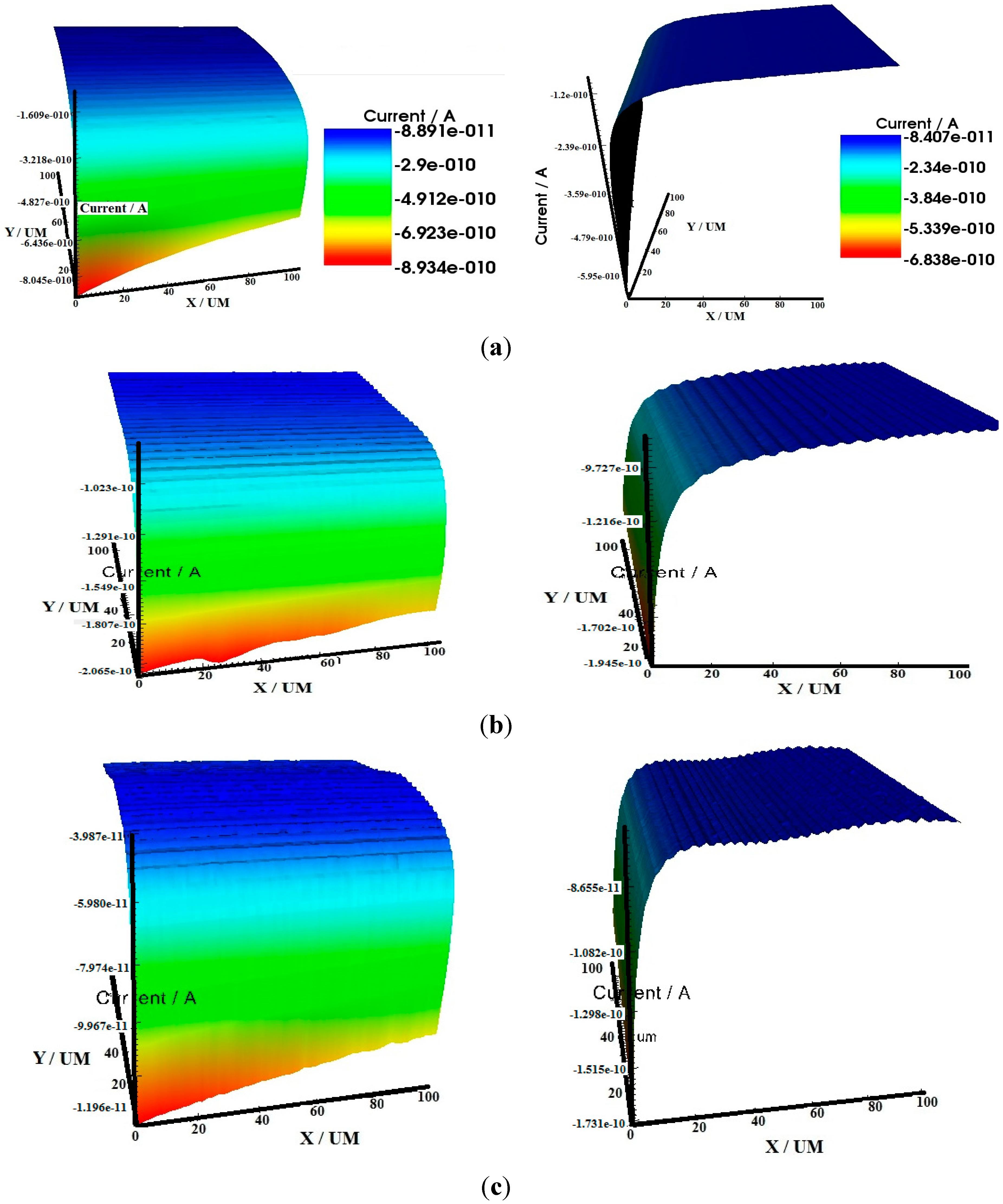
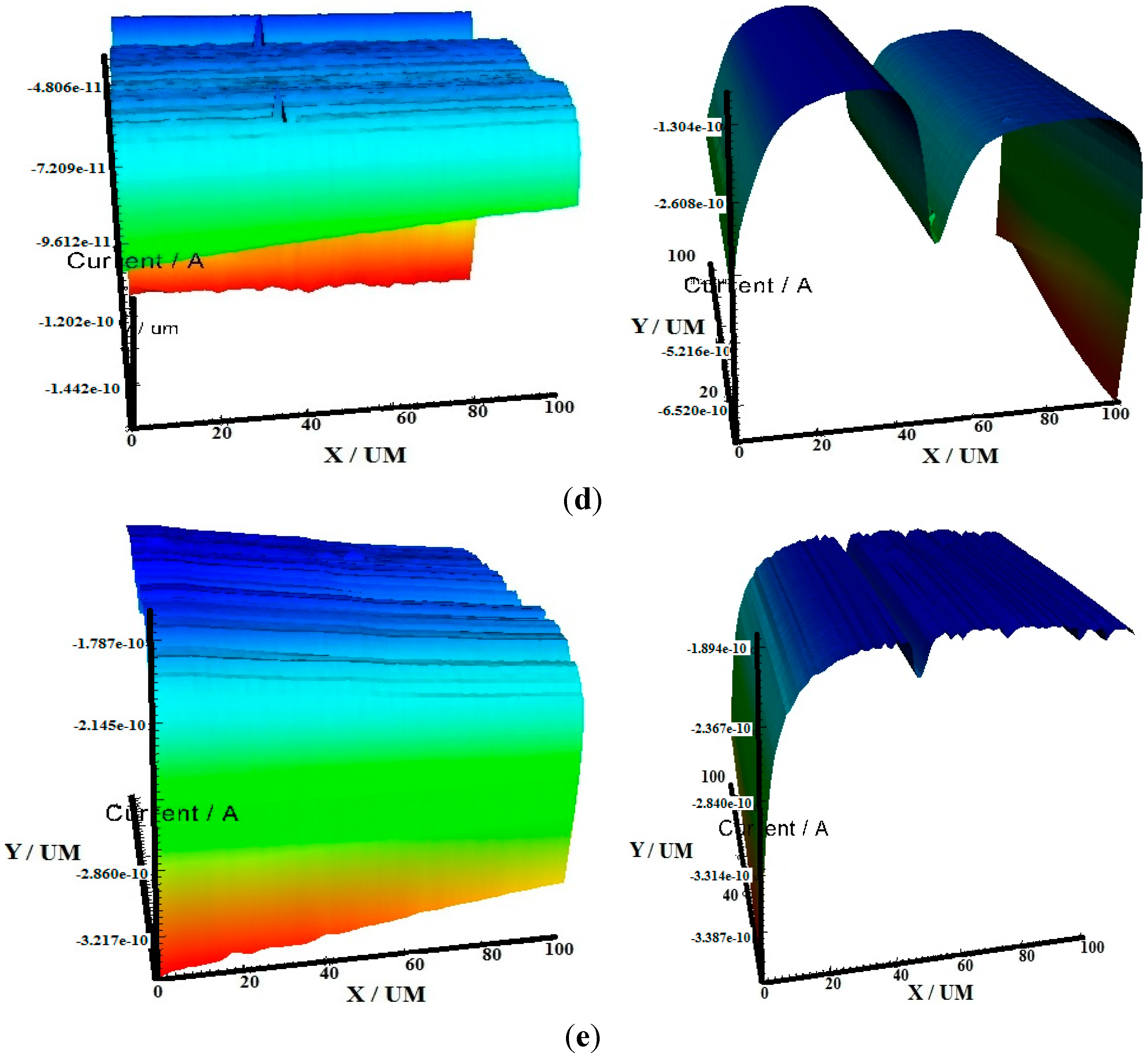
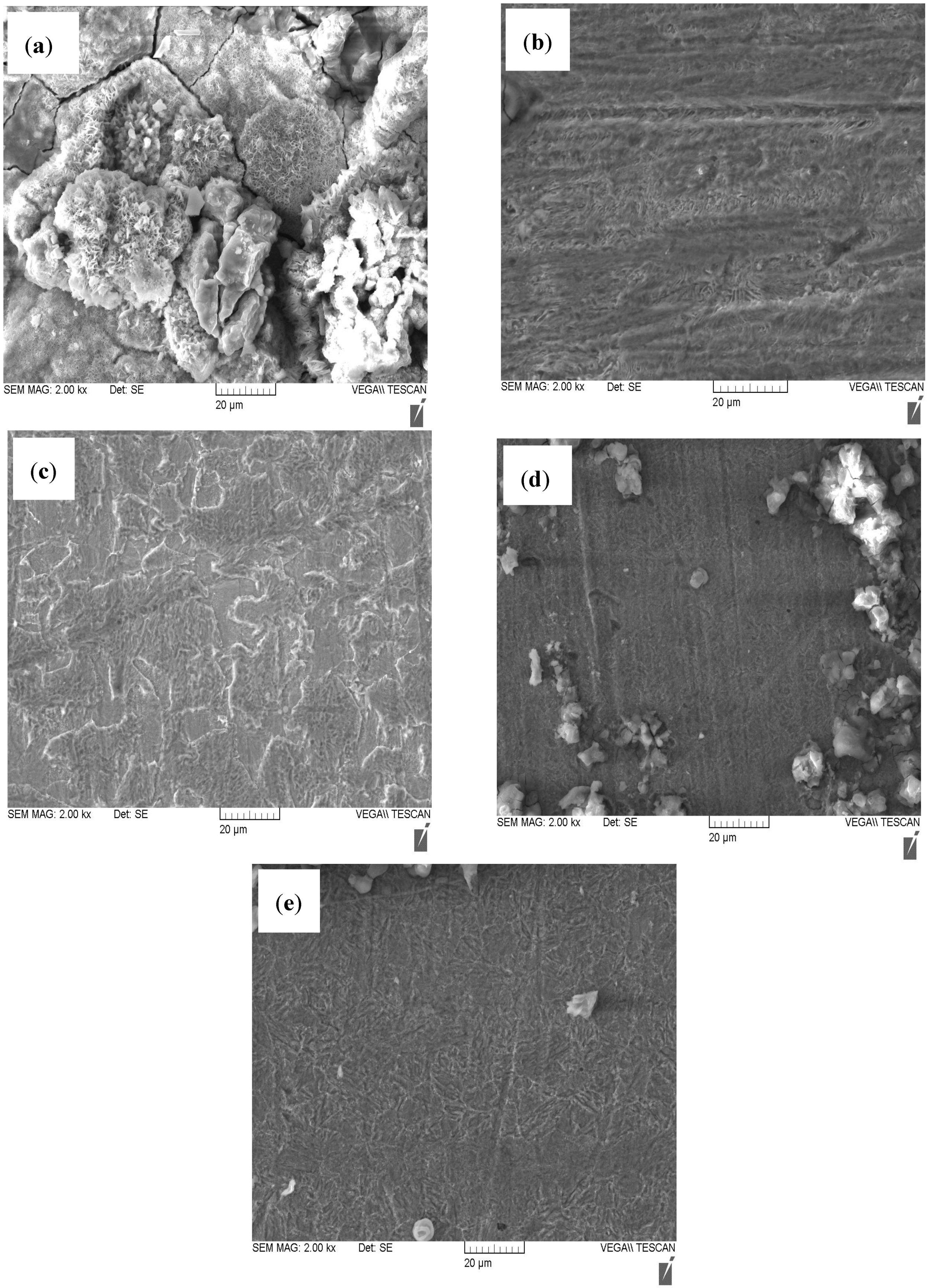
2.4. Scanning Electron Microscopy (SEM)
2.5. Quantum Chemical Studies
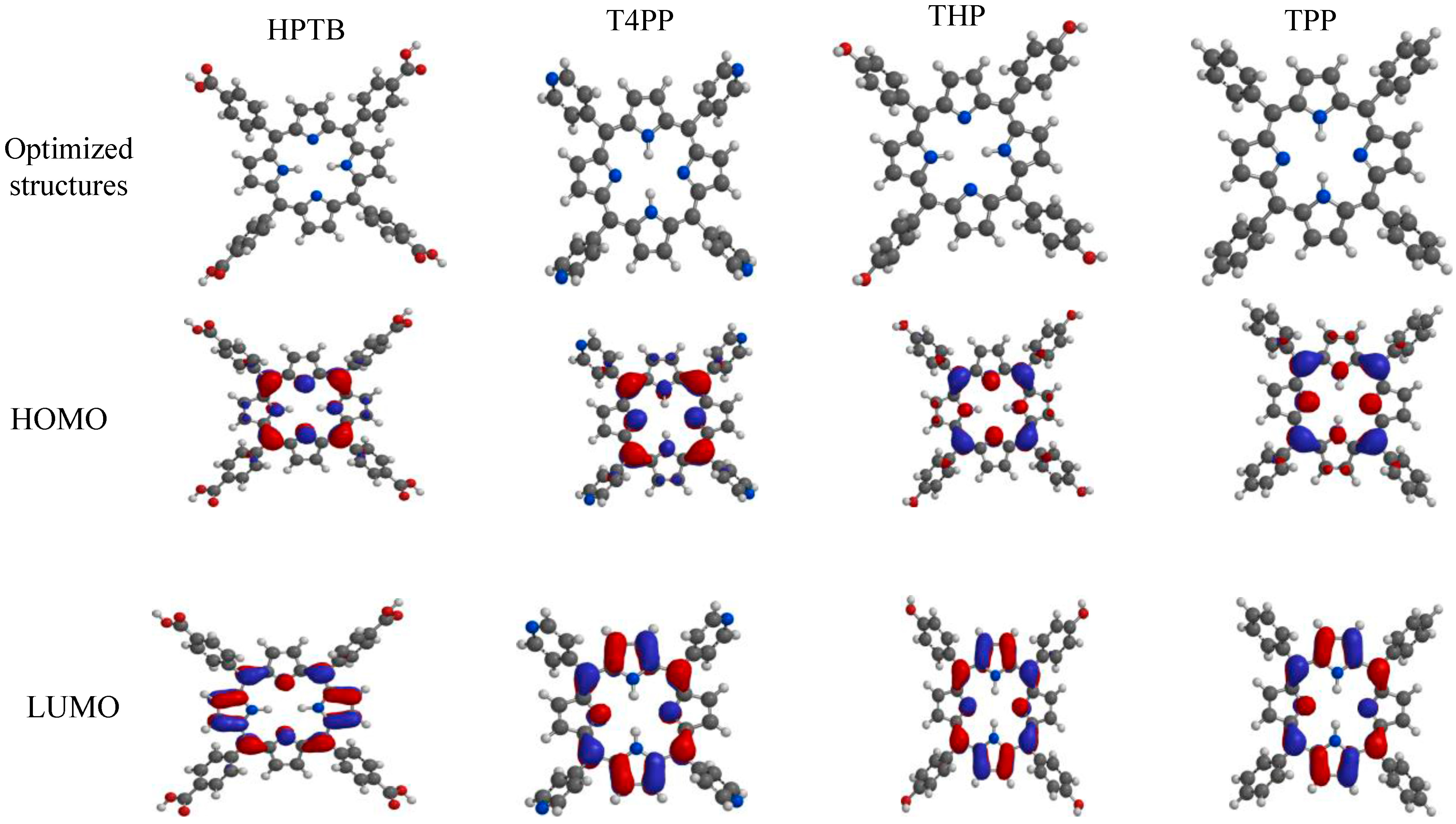
| Inhibitors | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | η (eV) | σ (eV)−1 | ω (eV) | μ (Debye) | χ (eV) |
|---|---|---|---|---|---|---|---|---|
| HPTB | −5.31 | −2.64 | 2.67 | 1.34 | 0.75 | 6.00 | 2.69 | 3.98 |
| T4PP | −5.47 | −2.74 | 2.73 | 1.37 | 0.73 | 6.17 | 0.11 | 4.11 |
| THP | −4.74 | −2.11 | 2.63 | 1.32 | 0.76 | 4.50 | 4.75 | 3.43 |
| TPP | −4.90 | −2.20 | 2.70 | 1.35 | 0.74 | 4.67 | 0.08 | 3.55 |
2.6. Monte Carlo Simulations


| Inhibitors | Total Energy | Adsorption Energy | Rigid Adsorption Energy | Deformation Energy | dEad/dNi |
|---|---|---|---|---|---|
| THP | 259.07 | −368.11 | −395.81 | 27.69 | −368.11 |
| HPTB | 312.78 | −301.66 | −330.92 | 29.25 | −301.66 |
| TPP | 395.21 | −276.84 | −295.42 | 18.57 | −276.84 |
| T4PP | 393.89 | −274.01 | −294.15 | 20.14 | −274.01 |
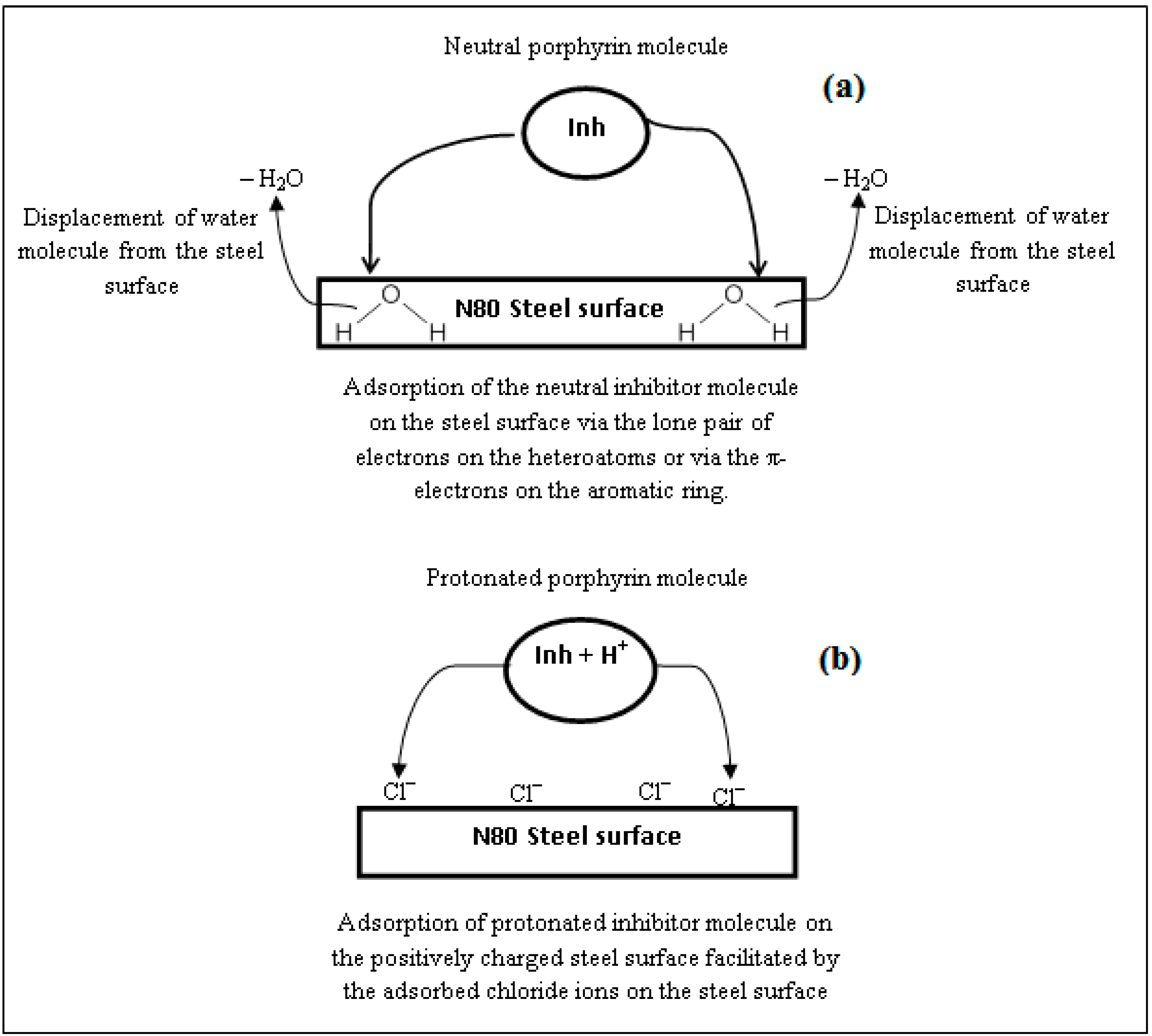
2.7. Mechanism of Corrosion Inhibition
3. Experimental Section
3.1. Materials and Aggressive Solutions
3.2. Electrochemical Measurements
3.3. Scanning Electrochemical Microscopy (SECM)
3.4. Scanning Electron Microscopy (SEM)
3.5. Quantum Chemical Calculations
3.6. Monte Carlo Simulation Studies
4. Conclusions
- (1)
- The studied porphyrins are good inhibitors for N80 steel corrosion in the studied media showing increasing η% with increase in concentration.
- (2)
- The shift in Ecorr (<85 mV) from polarization measurements indicated that the studied porphyrins acted as mixed type inhibitors.
- (3)
- EIS data revealed that the porphyrins adsorbed on N80 steel surface and exhibit non-ideal capacitive behaviour.
- (4)
- The SECM and SEM analyses showed that the porphyrins inhibit N80 steel corrosion by forming protective film on the steel surface.
- (5)
- Quantum chemical parameters and QSAR analyses showed that the corrosion inhibition performances of the porphyrins could be related to their EHOMO, ELUMO, ω, and μ values.
- (6)
- Monte Carlo simulation studies showed that THP has the highest adsorption energy, while T4PP has the least adsorption energy in agreement with the values of σ from quantum chemical calculations.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rajeev, P.; Surendranathan, A.O.; Murthy, C.S.N. Corrosion mitigation of the oil well steels using organic inhibitors—A review. J. Mater. Environ. Sci. 2012, 3, 856–869. [Google Scholar]
- Bai, W.; Yu, J.; Yang, Y.; Ye, Y.; Guo, J.; Zhang, Y. Effect of CO2 saturation on the corrosion behaviour of AZ31B magnesium alloy in Na3PO4 solutions. Int. J. Electrochem. Sci. 2013, 8, 3441–3453. [Google Scholar]
- Han, J.; William, C.J.; Zhang, J. Effect of sodium chloride on corrosion of mild steel in CO2-saturated brines. J. Appl. Electrochem. 2011, 41, 741–749. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Aliyeva, L.I.; Abbasov, V.M.; Ismayilov, T.I. Corrosion inhibition of low carbon steel in CO2-saturated solution using anionic surfactant. Adv. Appl. Sci. Res. 2012, 3, 1185–1201. [Google Scholar]
- Lopez, D.A.; Perez, T.; Simison, S.N. The influence of microstructure and chemical composition of carbon and low alloy steels in CO2 corrosion- A state of art appraisal. Mater. Des. 2003, 24, 561–575. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions. Corros. Sci. 2010, 52, 933–942. [Google Scholar] [CrossRef]
- Mashuga, M.E.; Olasunkanmi, L.O.; Adekunle, A.S.; Yesudass, S.; Kabanda, M.M.; Ebenso, E.E. Adsorption, thermodynamics and quantum chemical studies of 1-hexyl-3-methylimidazolium based ionic liquids as corrosion inhibitors for mild steel in HCl. Materials 2015, 8, 3607–3632. [Google Scholar] [CrossRef]
- Longo, F.R. Corrosion and anti-corrosives. In Porphyrins as Corrosion Inhibitors; Naval Air Development Center: Warminster, PA, USA, 1984. [Google Scholar]
- Lokesh, K.S.; de Keersmaecker, M.; Adriaens, A. Self-assembled films of porphyrins with amine groups at different positions: Influence of their orientation on the corrosion inhibition and the electrocatalytic activity. Molecules 2012, 17, 7824–7842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesaga, M.; Pyrzyńska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Xiao, J.; Meyerhoff, M.E. Retention behavior of amino acids and peptides on protoporphyrinsilica stationary phases with varying metal ion centers. Anal. Chem. 1996, 68, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Fu, C. Porphyrins as ligands for trace metal analysis by high-performance liquid chromatography. Talanta 1997, 44, 593–604. [Google Scholar] [CrossRef]
- Hettiarachichi, S.; Chan, Y.W.; Wilson, R.B., Jr.; Agarwala, V.S. Macrocyclic corrosion inhibitors for steel in acid chloride environments. Corrosion 1989, 45, 30–34. [Google Scholar] [CrossRef]
- Maranhao, S.L.A.; Guedes, I.C.; Anaissi, F.J.; Toma, H.E.; Aoki, I.V. Electrochemical and corrosion studies of poly(nickel-tetraaminophthalocyanine) on carbon steel. Electrochim. Acta 2006, 52, 519–526. [Google Scholar] [CrossRef]
- Lokesh, K.S.; de Keersmaecker, M.; Elia, A.; Depla, D.; Dubruel, P.; Vandenabeele, P.; van Vlierberghe, S.; Adriaens, A. Adsorption of cobalt (II) 5,10,15,20-tetrakis(2-aminophenyl)-porphyrin onto copper substrates: Characterization and impedance studies for corrosion inhibition. Corros. Sci. 2012, 62, 73–82. [Google Scholar] [CrossRef]
- Bessone, J.; Meyer, C.; Tuttner, K.; Lorenz, W.J. AC-impedance measurements on aluminium barrier type oxide films. Electrochim. Acta 1983, 28, 171–175. [Google Scholar] [CrossRef]
- Singh, A.; Ahamad, I.; Singh, V.K.; Quraishi, M.A. Inhibition effect of environmentally benign Karanj (Pongamia pinnata) seed extract on corrosion of mild steel in hydrochloric acid solution. J. Solid State Electrochem. 2011, 15, 1087–1097. [Google Scholar] [CrossRef]
- Yadav, D.K.; Chauhan, D.S.; Ahamad, I.; Quraishi, M.A. Electrochemical behavior of steel/acid interface: Adsorption and inhibition effect of oligomeric aniline. RSC. Adv. 2013, 3, 632–646. [Google Scholar] [CrossRef]
- Yuce, A.O.; Solmaz, R.; Kardas, G. Investigation of inhibition effect of rhodanine-N-acetic acid on mild steel corrosion in HCl solution. Mater. Chem. Phys. 2012, 131, 615–620. [Google Scholar] [CrossRef]
- Perez-Navarrete, J.B.; Olivares-Xometl, C.O.; Likhanova, N.V. Adsorption and corrosion inhibition of amphiphilic compounds on steel pipeline grade API 5L X52 in sulphuric acid 1 M. J. Appl. Electrochem. 2010, 40, 1605–1617. [Google Scholar] [CrossRef]
- Bustamante, R.A.; Silve, G.N.; Quijano, M.A.; Hernandez, H.H.; Romo, M.R.; Cuan, A.; Pardave, M.P. Electrochemical study of 2-mercaptoimidazole as a novel corrosion inhibitor for steels. Electrochim. Acta 2009, 54, 5393–5399. [Google Scholar] [CrossRef]
- Yadav, D.K.; Quraishi, M.A. Application of some condensed uracils as corrosion inhibitors for mild steel: Gravimetric, electrochemical, surface morphological, UV-visible, and theoretical investigations. Ind. Eng. Chem. Res. 2012, 51, 14966–14979. [Google Scholar] [CrossRef]
- Mallaiya, K.; Subramaniam, R.; Srikandan, S.S.; Gowri, S.; Rajasekaran, N.; Selvaraj, A. Electrochemical characterization of the protective film formed by the unsymmetrical Schiff’s base on the mild steel surface in acid media. Electrochim. Acta 2011, 56, 3857–3863. [Google Scholar] [CrossRef]
- Musa, A.Y.; Jalgham, R.T.T.; Mohamad, A.B. Molecular dynamic and quantum chemical calculations for phthalazine derivatives as corrosion inhibitors of mild steel in 1 M HCl. Corros. Sci. 2012, 56, 176–183. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Adsorption and inhibitive properties of some new Mannich bases of isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472–1481. [Google Scholar] [CrossRef]
- Liao, Q.Q.; Yue, Z.W.; Yang, D.; Wang, Z.H.; Li, Z.H.; Ge, H.H.; Li, Y.J. Self-assembled monolayer of ammonium pyrrolidine dithiocarbamate on copper detected using electrochemical methods, surface enhanced Raman scattering and quantum chemistry calculations. Thin Solid Films 2011, 519, 6492–6498. [Google Scholar] [CrossRef]
- Raja, P.B.; Fadaeinasab, M.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Litaudon, M.; Awang, K. Evaluation of green corrosion inhibition by alkaloid extracts of Ochrosia oppositifolia and Isoreserpiline against mild steel in 1 M HCl medium. Ind. Eng. Chem. Res. 2013, 52, 10582–10593. [Google Scholar] [CrossRef]
- Lebrini, M.; Lagrenée, M.; Vezin, H.; Traisnel, M.; Bentiss, F. Experimental and theoretical study for corrosion inhibition of mild steel in normal hydrochloric acid solution by some new macrocyclic polyether compounds. Corros. Sci. 2007, 49, 2254–2269. [Google Scholar] [CrossRef]
- Khaled, K.F.; Amin, M.A. Corrosion monitoring of mild steel in sulphuric acid solutions in presence of some thiazole derivatives-Molecular dynamics, chemical and electrochemical studies. Corros. Sci. 2009, 51, 1964–1975. [Google Scholar] [CrossRef]
- Hassan, H.H. Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives: Part II: Time and temperature effects and thermodynamic treatments. Electrochim. Acta 2007, 53, 1722–1730. [Google Scholar] [CrossRef]
- Yadav, D.K.; Quraishi, M.A.; Maiti, B. Inhibition effect of some benzylidenes on mild steel in 1 M HCl: An experimental and theoretical correlation. Corros. Sci. 2012, 55, 254–266. [Google Scholar] [CrossRef]
- Naderi, E.; Jafari, A.H.; Ehteshamzadeha, M.; Hosseini, M.G. Effect of carbon steel microstructures and molecular structure of two new Schiff base compounds on inhibition performance in 1 M HCl solution by EIS. Mater. Chem. Phys. 2009, 115, 852–858. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some quinoxalin-6-yl derivatives as corrosion inhibitors for mild steel in hydrochloric acid: Experimental and theoretical studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Murulana, L.C.; Kabanda, M.M.; Ebenso, E.E. Experimental and theoretical studies on the corrosion inhibition of mild steel by some sulphonamides in aqueous HCl. RSC Adv. 2015, 5, 28743–28761. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr. Corrosion Inhibitors, 2nd ed.; NACE International: Houston, TX, USA, 1973. [Google Scholar]
- Ji, G.; Dwivedi, P.; Sundaram, S.; Prakash, R. Inhibitive Effect of Chlorophytum borivilianum root extract on mild steel corrosion in HCl and H2SO4 solutions. Ind. Eng. Chem. Res. 2013, 52, 10673–10681. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Liu, W.; Kuanhai, D.; Pan, J.; Huang, B.; Ren, C.; Zeng, D. A study on the inhibition of N80 steel in 3.5% NaCl solution saturated with CO2 by fruit extract of Gingko biloba. J. Taiwan Inst. Chem. Eng. 2014, 45, 1918–1926. [Google Scholar] [CrossRef]
- Obi-Egbedi, N.O.; Obot, I.B.; El-Khaiary, M.I.; Umoren, S.A.; Ebenso, E.E. Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some phenanthroline derivatives on mild steel surface. Int. J. Electrochem. Sci. 2 2011, 6, 5649–5675. [Google Scholar]
- Ebenso, E.E.; Kabanda, M.M.; Murulana, L.C.; Singh, A.K.; Shukla, S.K. Electrochemical and quantum chemical investigation of some azine and thiazine dyes as potential corrosion inhibitors for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2012, 51, 12940–12958. [Google Scholar] [CrossRef]
- Kabanda, M.M.; Shukla, S.K.; Singh, A.K.; Murulana, L.C.; Ebenso, E.E. Electrochemical andquantum chemical studies on calmagite and fast sulphone black F dyes as corrosion inhibitors for mild steel in hydrochloric medium. Int. J. Electrochem. Sci. 2012, 7, 8813–8831. [Google Scholar]
- Kabanda, M.M.; Ebenso, E.E. Density functional theory and quantitative structure-activity relationship studies of some quinoxaline derivatives as potential corrosion inhibitors for copper in acidic medium. Int. J. Electrochem. Sci. 2012, 7, 8713–8733. [Google Scholar]
- Fu, J.; Zang, H.; Wang, Y.; Li, S.N.; Chen, T.; Liu, X.D. Experimental and theoretical study on the inhibition performance of quinoxaline and its derivatives for the corrosion of mild steel in hydrochloric acid. Ind. Eng. Chem. Res. 2012, 51, 6377–6386. [Google Scholar] [CrossRef]
- Koopmans, T. Über die zuordnung von wellenfunktionen und Eigenwerten zu den einzelnen elektronen eines atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA 1986, 83, 8440–8441. [Google Scholar] [CrossRef] [PubMed]
- Kabanda, M.M.; Murulana, L.C.; Ozcan, M.; Karadag, F.; Dehri, I.; Obot, I.B.; Ebenso, E.E. Quantum chemical studies on the corrosion inhibition of mild steel by some triazoles and benzimidazole derivatives in acidic medium. Int. J. Electrochem. Sci. 2012, 7, 5035–5056. [Google Scholar]
- Malyszko, J.; Scendo, M. Redox equilibria in the Cu2+-Cu+-Cu system in water-dimethylsulphoxide (DMSO) mixtures. Monatshefte fùr Chem. 1987, 118, 435–443. [Google Scholar] [CrossRef]
- Mert, B.D.; Mert, M.E.; Kardaş, G.; Yazici, B. Experimental and theoretical investigation of 3-amino-1,2,4-triazole-5-thiol as a corrosion inhibitor for carbon steel in HCl medium. Corros. Sci. 2011, 53, 4265–4272. [Google Scholar] [CrossRef]
- Arslan, T.; Kandemirli, F.; Ebenso, E.E.; Love, I.; Alemu, H. Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci. 2009, 51, 35–47. [Google Scholar] [CrossRef]
- Lukovits, I.; Bakó, I.; Shaban, A.; Kálmán, E. Polynomial model of the inhibition mechanism of thiourea derivatives. Electrochim. Acta 1998, 43, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Kabanda, M.M.; Obot, I.B.; Ebenso, E.E. Computational study of some amino acid derivatives as potential corrosion inhibitors for different metal surfaces and in different media. Int. J. Electrochem. Sci. 2013, 8, 10839–10850. [Google Scholar]
- Gerengi, H. Anticorrosive properties of date palm (Phoenix dactylifera L.) fruit juice on 7075 type aluminum alloy in 3.5% NaCl solution. Ind. Eng. Chem. Res. 2012, 51, 12835–12843. [Google Scholar] [CrossRef]
- Singh, A.; Ahamad, I.; Quraishi, M.A. Piper longum extract as Green Corrosion Inhibitor for Aluminium in NaOH Solution. Arab. J. Chem. 2012. Available online: http://dx.doi.org/10.1016/j.arabjc.2012.04.029 (accessed on 23 July 2015). [Google Scholar] [CrossRef]
- Kamal, C.; Sethuraman, M.G. Caulerpin-A bis-indole alkaloid as a green inhibitor for the corrosion of mild steel in 1 M HCl solution from the marine alga Caulerpa racemosa. Ind. Eng. Chem. Res. 2012, 51, 10399–10407. [Google Scholar] [CrossRef]
- Raja, P.B.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Awang, K. Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1 M HCl media. Corros. Sci. 2013, 69, 292–301. [Google Scholar] [CrossRef]
- Evgenij, B.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bard, A.J.; Fan, F.R.F.; Kwak, J.; Lev, O. Scanning electrochemical microscopy. Introduction and principles. Anal. Chem. 1989, 61, 132–138. [Google Scholar] [CrossRef]
- Lee, C.; Bard, A.J. Scanning electrochemical microscopy. Application to polymer and thin metal oxide films. Anal. Chem. 1990, 62, 1906–1913. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Lin, Y.; Quraishi, M.A.; Olasunkanmi, L.O.; Fayemi, O.E.; Sasikumar, Y.; Ramaganthan, B.; Bahadur, I.; Obot, I.B.; Adekunle, A.S.; et al. Porphyrins as Corrosion Inhibitors for N80 Steel in 3.5% NaCl Solution: Electrochemical, Quantum Chemical, QSAR and Monte Carlo Simulations Studies. Molecules 2015, 20, 15122-15146. https://doi.org/10.3390/molecules200815122
Singh A, Lin Y, Quraishi MA, Olasunkanmi LO, Fayemi OE, Sasikumar Y, Ramaganthan B, Bahadur I, Obot IB, Adekunle AS, et al. Porphyrins as Corrosion Inhibitors for N80 Steel in 3.5% NaCl Solution: Electrochemical, Quantum Chemical, QSAR and Monte Carlo Simulations Studies. Molecules. 2015; 20(8):15122-15146. https://doi.org/10.3390/molecules200815122
Chicago/Turabian StyleSingh, Ambrish, Yuanhua Lin, Mumtaz A. Quraishi, Lukman O. Olasunkanmi, Omolola E. Fayemi, Yesudass Sasikumar, Baskar Ramaganthan, Indra Bahadur, Ime B. Obot, Abolanle S. Adekunle, and et al. 2015. "Porphyrins as Corrosion Inhibitors for N80 Steel in 3.5% NaCl Solution: Electrochemical, Quantum Chemical, QSAR and Monte Carlo Simulations Studies" Molecules 20, no. 8: 15122-15146. https://doi.org/10.3390/molecules200815122





