Preformulation Studies of Bee Venom for the Preparation of Bee Venom-Loaded PLGA Particles
Abstract
:1. Introduction
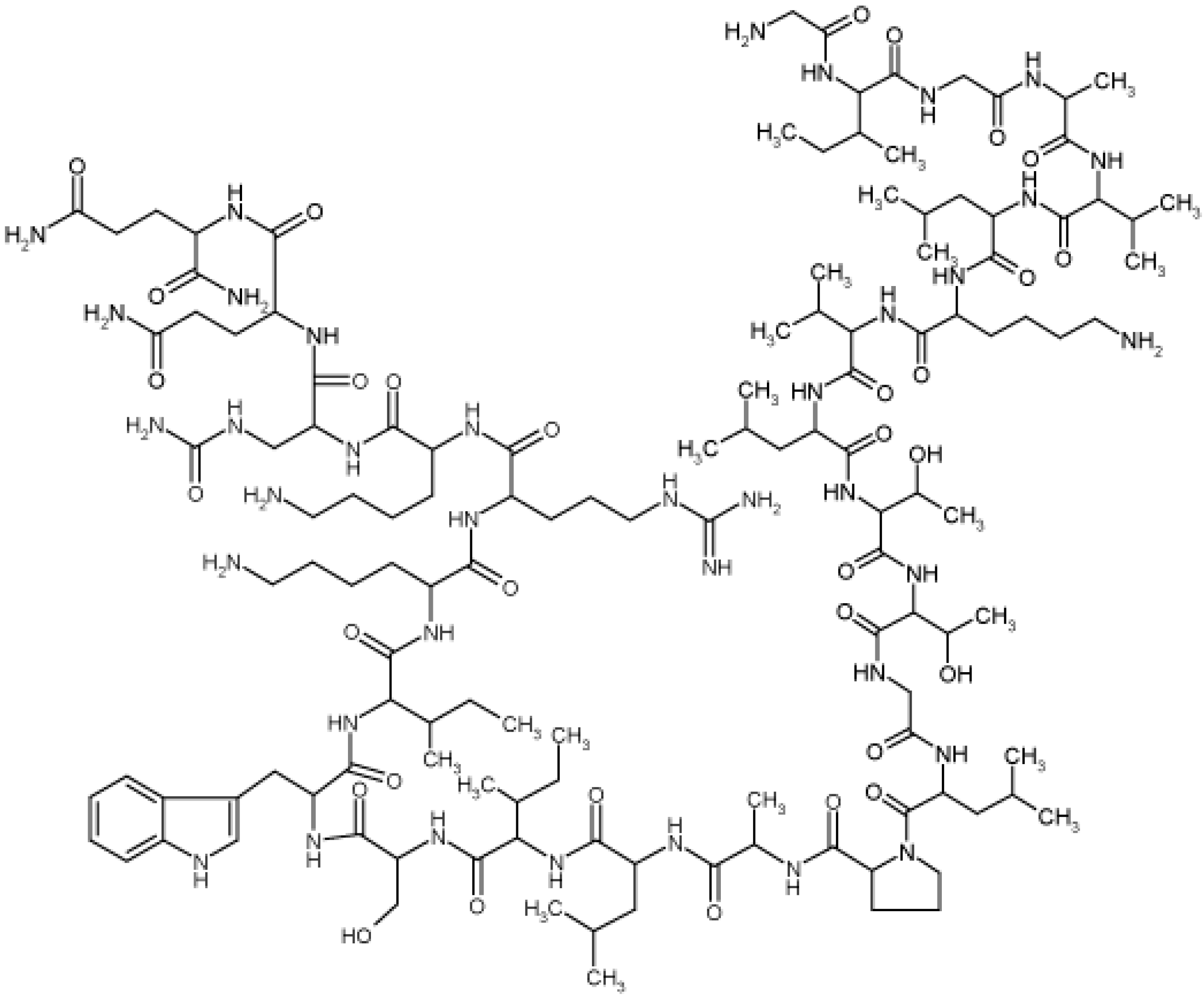
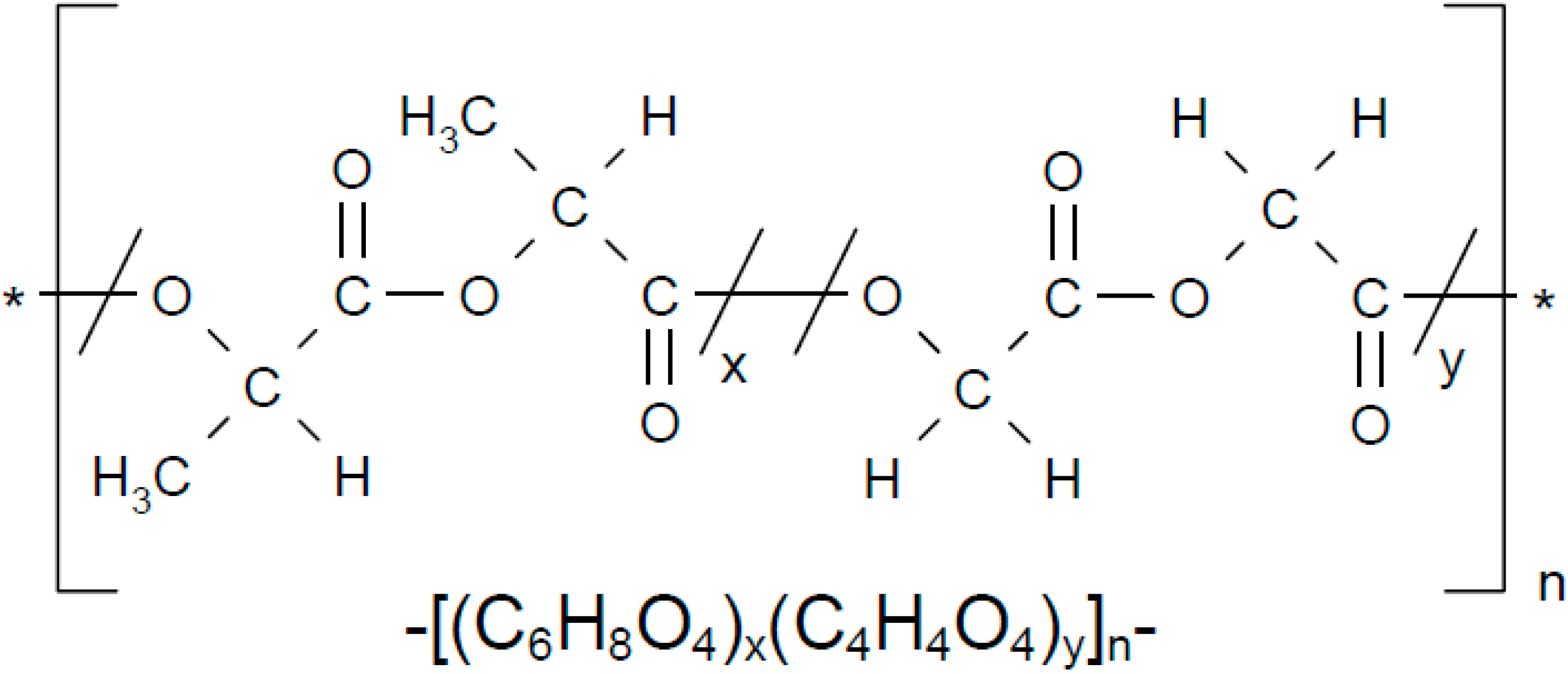
2. Results and Discussion
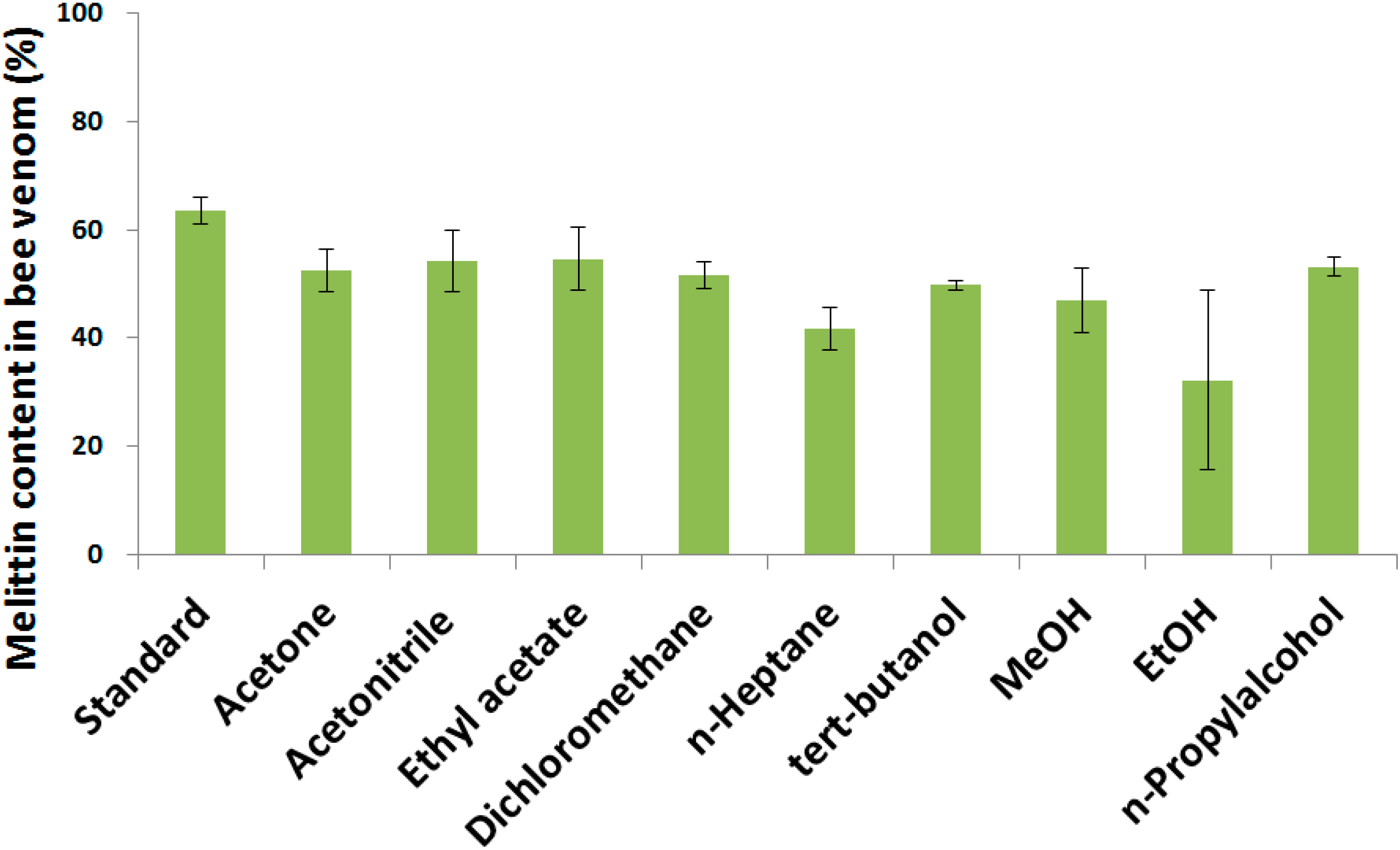
2.1. Determination of Melittin Content in the BV
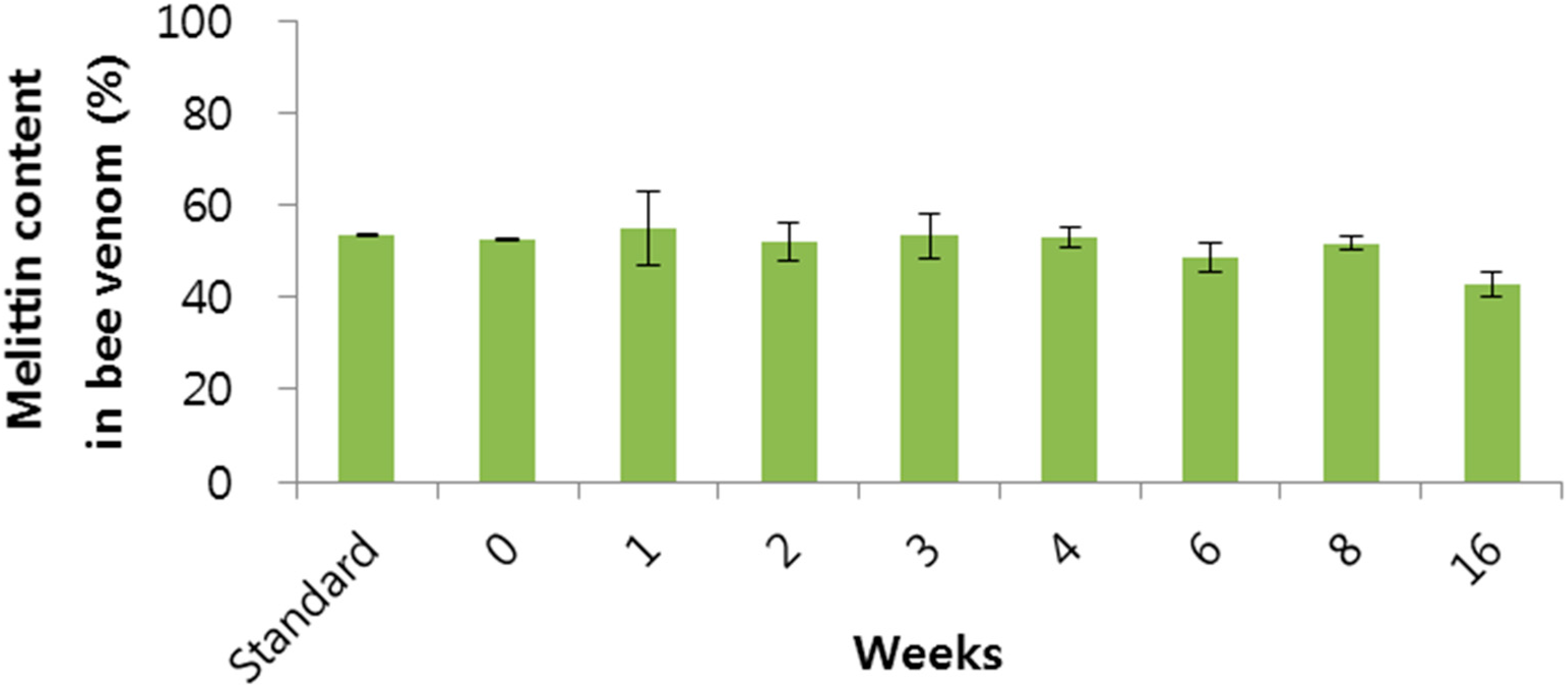
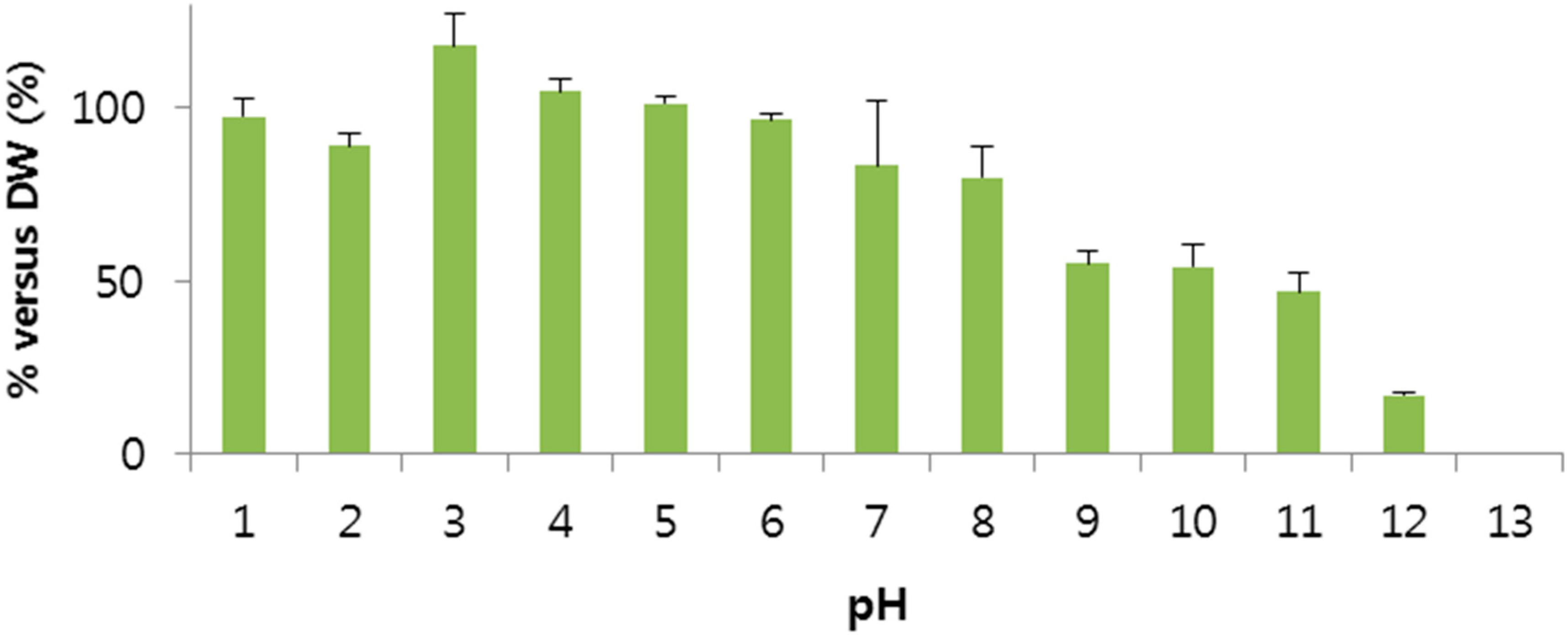
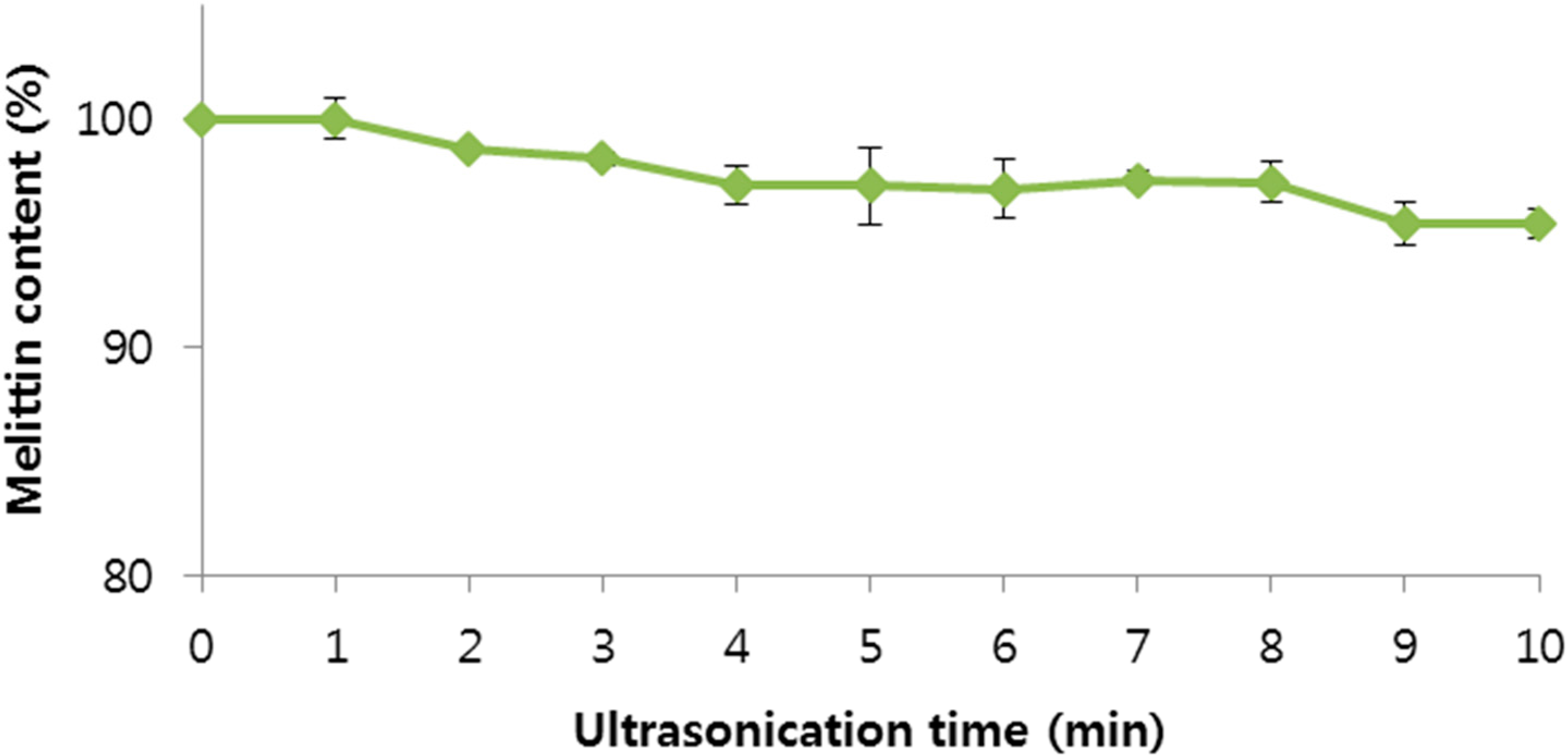
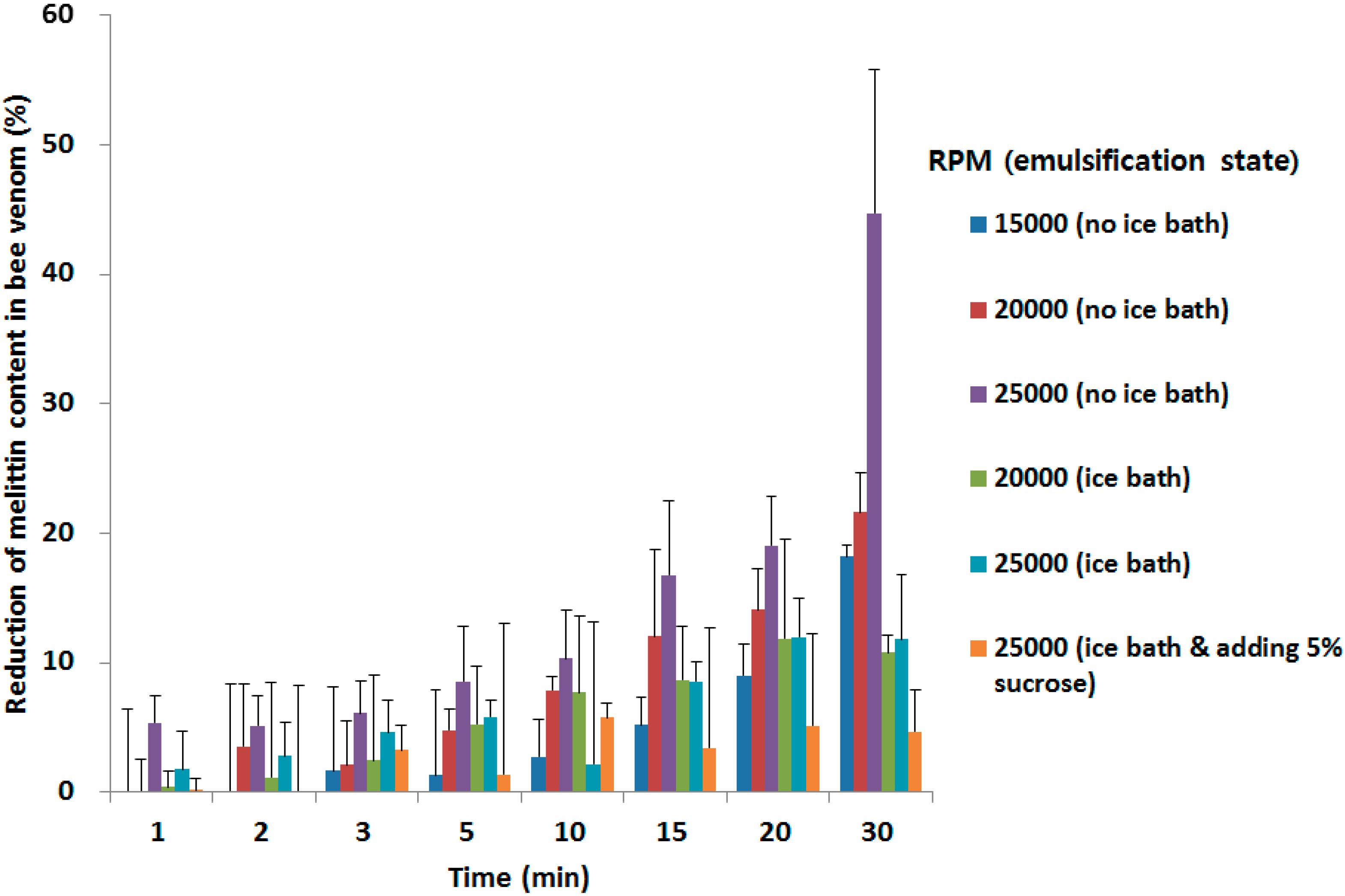
2.2. Stability of BV
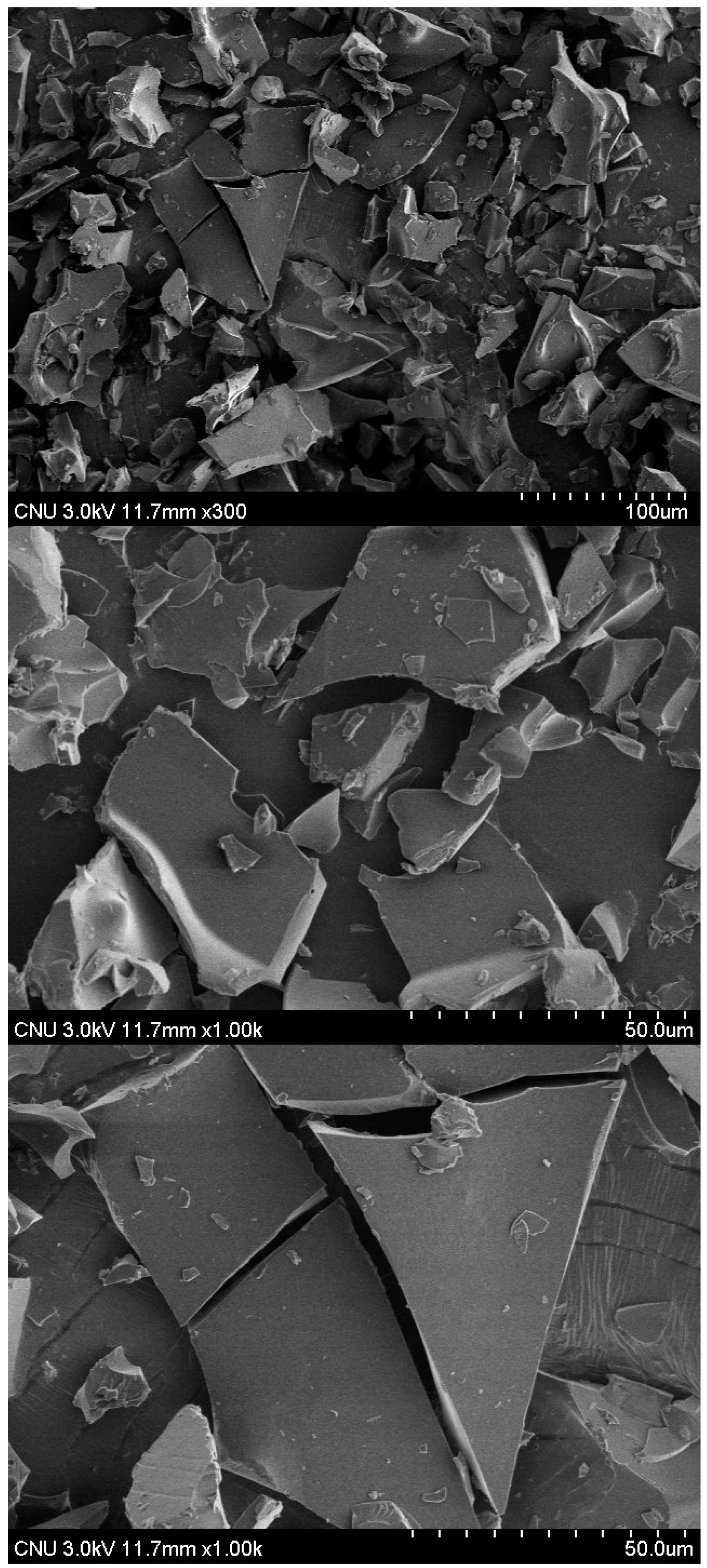
2.3. Surface Morphology of BV
3. Experimental Section
3.1. Materials
3.2. High Performance Liquid Chromatography (HPLC)
3.3. Characterizations of BV
3.3.1. Determination of Melittin Content in the BV
3.3.2. Stability Test of BV
3.3.3. Observation of BV Surface Morphology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Niemz, A.; Tirrell, D.A. Self-association and membrane-binding behavior of melittins containing trifluoroleucine. J. Am. Chem. Soc. 2001, 123, 7407–7413. [Google Scholar] [CrossRef] [PubMed]
- Jutel, M.; Pichler, W.J.; Skrbic, D.; Urwyler, A.; Dahinden, C.; Muller, U.R. Bee Venom Immunotherapy Results in Decrease of IL-4 and IL-5 and Increase of Ifn-Gamma Secretion in Specific Allergen-Stimulated T-Cell Cultures. J. Immunol. 1995, 154, 4187–4194. [Google Scholar] [PubMed]
- Thomas, G.R.; Hiley, C.R. Cardiovascular Effects of Intracerebro-Ventricular Bradykinin and Melittin in the Rat. J. Pharm. Pharmacol. 1988, 40, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Pogliani, E.M.; Cofrancesco, E. Thrombotic thrombocytopenic purpura: A review. Haematologica 1983, 68, 546–557. [Google Scholar] [PubMed]
- Liu, X.; Chen, D.; Xie, L.; Zhang, R. Effect of honey bee venom on proliferation of K1735M2 mouse melanoma cells in vitro and growth of murine B16 melanomas in vivo. J. Pharm. Pharmacol. 2002, 54, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.E.; Morley, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. Letter: An anti-inflammatory peptide from bee venom. Nature 1973, 245, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C. Honeybee venom: A rich source of pharmacologically active peptides. Endeavour 1988, 12, 60–65. [Google Scholar] [CrossRef]
- Liu, H.; Tong, F. Advances in the study of bee venom and its clinical uses. Zhong Yao Cai 2003, 26, 456–458. [Google Scholar] [PubMed]
- Eiseman, J.L.; von Bredow, J.; Alvares, A.P. Effect of honeybee (Apis mellifera) venom on the course of adjuvant-induced arthritis and depression of drug metabolism in the rat. Biochem. Pharmacol. 1982, 31, 1139–1146. [Google Scholar] [CrossRef]
- Hadjipetroukourounakis, L.; Yiangou, M. Bee Venom, Adjuvant Induced Disease and Interleukin Production. J. Rheumatol. 1988, 15, 1126–1128. [Google Scholar]
- Lee, J.Y.; Kang, S.S.; Kim, J.H.; Bae, C.S.; Choi, S.H. Inhibitory effect of whole bee venom in adjuvant-induced arthritis. In Vivo 2005, 19, 801–805. [Google Scholar] [PubMed]
- Durham, S.R.; Walker, S.M.; Varga, E.M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Chen, D.; Hao, T.; Zhao, X.; Hu, H.; Ma, X. Effect of bee venom peptide-copolymer interactions on thermosensitive hydrogel delivery systems. Int. J. Pharm. 2007, 345, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.; Hariharan, S.; Kumar, M.N. PLGA nanoparticles in drug delivery: The state of the art. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 387–422. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.J.; Putney, S.; Johnson, O.L.; Cleland, J.L. Recombinant human growth hormone poly(lactic-co-glycolic acid) microsphere formulation development. Adv. Drug Deliv. Rev. 1997, 28, 71–84. [Google Scholar] [PubMed]
- Ogawa, Y.; Yamamoto, M.; Okada, H.; Yashiki, T.; Shimamoto, T. A New Technique to Efficiently Entrap Leuprolide Acetate into Microcapsules of Polylactic Acid or Copoly(Lactic Glycolic) Acid. Chem. Pharm. Bull. 1988, 36, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Tuncay, M.; Calis, S.; Kas, H.S.; Ercan, M.T.; Peksoy, I.; Hincal, A.A. Diclofenac sodium incorporated PLGA (50:50) microspheres: Formulation considerations and in vitro/in vivo evaluation. Int. J. Pharm. 2000, 195, 179–188. [Google Scholar] [CrossRef]
- Peiren, N.; Vanrobaeys, F.; de Graaf, D.C.; Devreese, B.; van Beeumen, J.; Jacobs, F.J. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim. Biophys. Acta 2005, 1752, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.L.; Powell, M.F.; Shire, S.J. The Development of Stable Protein Formulations: A Close Look at Protein Aggregation, Deamidation, and Oxidation. Crit. Rev. Ther. Drug Carrier Syst. 1994, 10, 307–377. [Google Scholar]
- Van de Weert, M.; Hennink, W.E.; Jiskoot, W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 2000, 17, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, A.; Osterberg, T.; Mikaelsson, M. Recombinant factor VIII SQ—Influence of oxygen, metal ions, pH and ionic strength on its stability in aqueous solution. Int. J. Pharm. 1997, 155, 121–131. [Google Scholar] [CrossRef]
- Kerwin, B.A.; Akers, M.J.; Apostol, I.; Moore-Einsel, C.; Etter, J.E.; Hess, E.; Lippincott, J.; Levine, J.; Mathews, A.J.; Revilla-Sharp, P.; et al. Acute and long-term stability studies of deoxy hemoglobin and characterization of ascorbate-induced modifications. J. Pharm. Sci. 1999, 88, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.; Khurana, R.; Coats, A.; Frokjaer, S.; Brange, J.; Vyas, S.; Uversky, V.N.; Fink, A.L. Effect of environmental factors on the kinetics of insulin fibril formation: Elucidation of the molecular mechanism. Biochemistry 2001, 40, 6036–6046. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.W.; DeLuca, P.P. Stability of ribonuclease A in solution and the freeze-dried state. J. Pharm. Sci. 1990, 79, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Bello, J.; Bello, H.R.; Granados, E. Conformation and aggregation of melittin: Dependence on pH and concentration. Biochemistry 1982, 21, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Hagihara, Y. Mechanism of the conformational transition of melittin. Biochemistry 1992, 31, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, Y.; Kataoka, M.; Aimoto, S.; Goto, Y. Charge repulsion in the conformational stability of melittin. Biochemistry 1992, 31, 11908–11914. [Google Scholar] [CrossRef] [PubMed]
- Aubert-Pouessel, A.; Venier-Julienne, M.C.; Saulnier, P.; Sergent, M.; Benoit, J.P. Preparation of PLGA microparticles by an emulsion-extraction process using glycofurol as polymer solvent. Pharm. Res. 2004, 21, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Chi, E.Y.; Krishnan, S.; Randolph, T.W.; Carpenter, J.F. Physical stability of proteins in aqueous solution: Mechanism and driving forces in nonnative protein aggregation. Pharm. Res. 2003, 20, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Trehan, A. Biodegradable microspheres for protein delivery. J. Control. Release 2003, 90, 261–280. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Hao, J.Y.; Davies, O.R.; Serhatkulu, G.; Stolnik-Trenkic, S.; Howdle, S.M.; Shakesheff, K.M. The production of protein-loaded microparticles by supercritical fluid enhanced mixing and spraying. J. Control. Release 2005, 101, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Timasheff, S.N. Stabilization of protein structure by sugars. Biochemistry 1982, 21, 6536–6544. [Google Scholar] [CrossRef] [PubMed]
- Maa, Y.F.; Hsu, C.C. Performance of Sonication and microfluidization for liquid-liquid emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Salvado, A.; del Pozo, A. Use of ultrasound to prepare lipid emulsions of lorazepam for intravenous injection. Int. J. Pharm. 2001, 216, 1–8. [Google Scholar] [CrossRef]
- Abismail, B.; Canselier, J.P.; Wilhelm, A.M.; Delmas, H.; Gourdon, C. Emulsification by ultrasound: Drop size distribution and stability. Ultrason. Sonochem. 1999, 6, 75–83. [Google Scholar] [CrossRef]
- Adami, R.C.; Collard, W.T.; Gupta, S.A.; Kwok, K.Y.; Bonadio, J.; Rice, K.G. Stability of peptide condensed plasmid DNA formulations. J. Pharm. Sci. 1998, 87, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choi, S.G.; Davaa, E.; Park, J.S. Encapsulation enhancement and stabilization of insulin in cationic liposomes. Int. J. Pharm. 2011, 415, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-H.; Kim, J.-H.; Jeon, J.-W.; Park, J.-K.; Lee, B.-J.; Suh, G.-H.; Cho, C.-W. Preformulation Studies of Bee Venom for the Preparation of Bee Venom-Loaded PLGA Particles. Molecules 2015, 20, 15072-15083. https://doi.org/10.3390/molecules200815072
Park M-H, Kim J-H, Jeon J-W, Park J-K, Lee B-J, Suh G-H, Cho C-W. Preformulation Studies of Bee Venom for the Preparation of Bee Venom-Loaded PLGA Particles. Molecules. 2015; 20(8):15072-15083. https://doi.org/10.3390/molecules200815072
Chicago/Turabian StylePark, Min-Ho, Ju-Heon Kim, Jong-Woon Jeon, Jin-Kyu Park, Bong-Joo Lee, Guk-Hyun Suh, and Cheong-Weon Cho. 2015. "Preformulation Studies of Bee Venom for the Preparation of Bee Venom-Loaded PLGA Particles" Molecules 20, no. 8: 15072-15083. https://doi.org/10.3390/molecules200815072






