Antioxidant, Antimicrobial Effects and Phenolic Profile of Lycium barbarum L. Flowers
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC/MS Analysis of Phenolic Compounds
| Polyphenolic Compounds | m/z Value | RT ± SD (min) | Extract | Hydrolyzed Extract |
|---|---|---|---|---|
| Caffeic acid | 179 | 6.52 ± 0.04 | - | ˂0.2 |
| Chlorogenic acid | 353 | 6.43 ± 0.05 | 216.91 ± 2.19 | ˂0.2 |
| p-Coumaric acid | 163 | 9.48 ± 0.08 | 12.84 ± 0.03 | 6.21 ± 0.01 |
| Ferulic acid | 193 | 12.8 ± 0.10 | 42.48 ± 0.03 | 42.98 ± 0.52 |
| Sinapic acid | 223 | 15.00 ± 0.10 | - | 2.82 ± 0.02 |
| Isoquercitrin | 463 | 20.29 ± 0.10 | 20.46 ± 0.01 | - |
| Rutin | 609 | 20.76 ± 0.15 | 60.53 ± 0.04 | - |
| Quercitrin | 447 | 23.64 ± 0.13 | 11.13 ± 0.01 | - |
| Quercetin | 301 | 27.55 ± 0.15 | - | 75.51 ± 0.07 |
| Kaempferol | 285 | 32.48 ± 0.17 | - | 89.73 ± 0.05 |
2.2. Total Phenolic and Flavonoids Content
| Total Bioactive Compounds | Lycium barbarum Flower Extract |
|---|---|
| TPC (mg/g fresh weight GAE) | 3.75 ± 0.13 |
| Total flavonoids (mg/g fresh weight QE) | 0.61 ± 0.02 |
2.3. Free Radical Scavenging Activity
| Samples | TEAC (mg TE/g f.w.) | HAPX (%) | EPR (mg FSE/g f.w.) |
|---|---|---|---|
| L. barbarum flowers | 7.10 ± 0.28 | 9.45 ± 2.66 | 29.84 ± 2.56 |
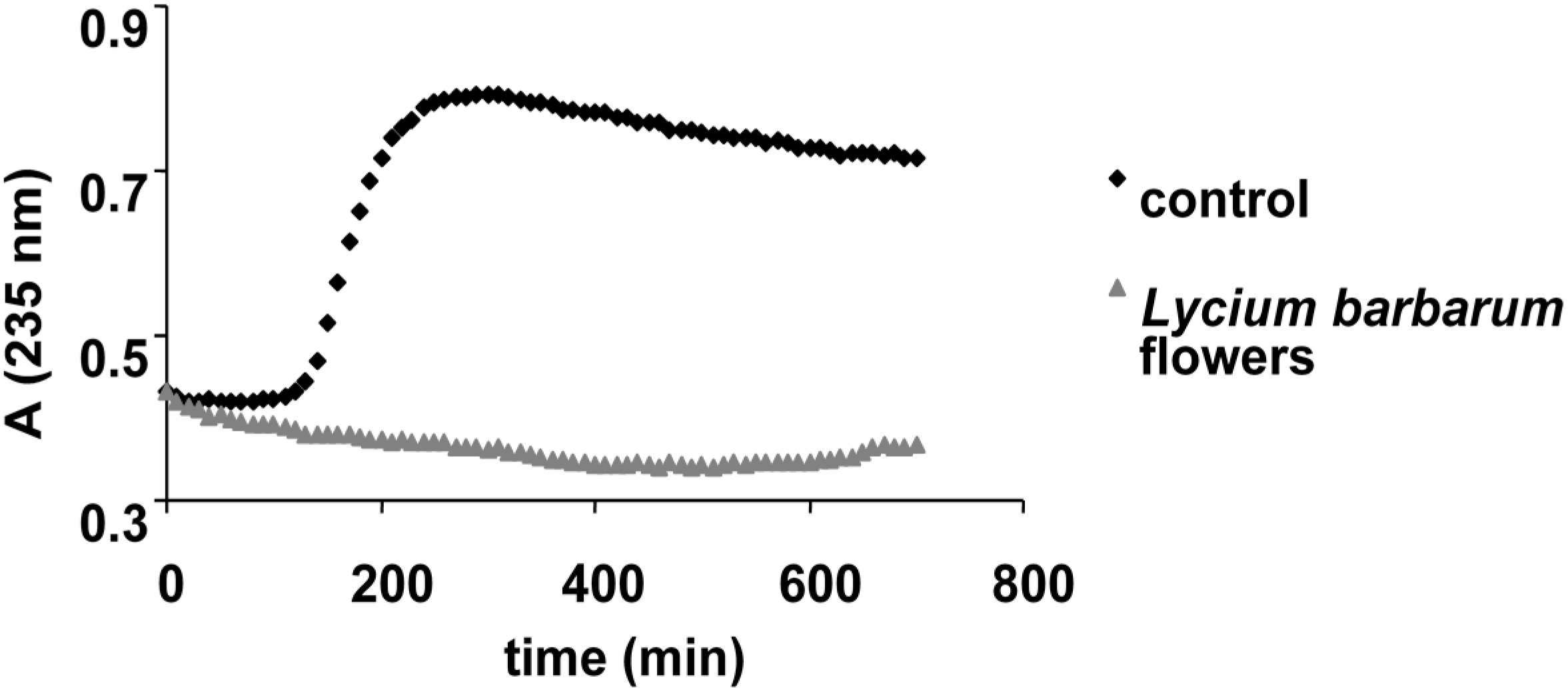
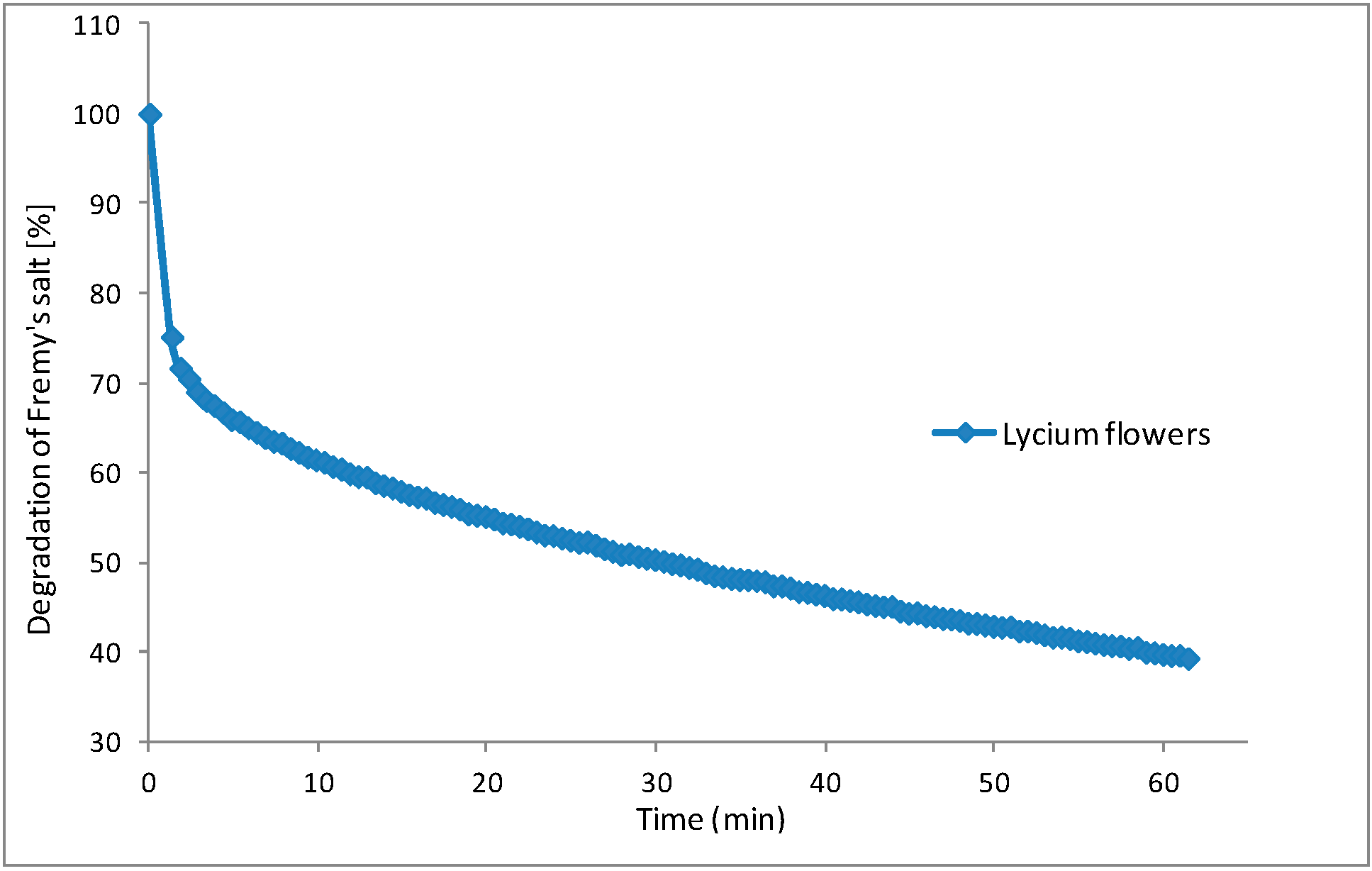
2.4. Assay of Antimicrobial Activity
| Bacterial Strains | Standard Antibiotic | Inhibition Zone (mm) | MIC (µg/mL) | |
|---|---|---|---|---|
| Gentamicin | Ciprofloxacin | L. barbarum Flowers | ||
| Staphylococcus aureus | 5.1 ± 0.2 | 5.2 ± 0.3 | 3.5 ± 0.1 | 75 |
| Bacillus subtilis | 4.2 ± 0.3 | 4.4 ± 0.1 | 2.7 ± 0.2 | 100 |
| Listeria monocytogenes | 7.3 ± 0.3 | 6.2 ± 0.2 | 1 ± 0.4 | 100 |
| Escherichia coli | 4.1 ± 0.2 | 5.1 ± 0.4 | 0 | >100 |
| Salmonella typhimurium | 4.2 ± 0.1 | 4.3 ± 0.1 | 1 ± 0.1 | 100 |
3. Experimental Section
3.1. Plant Collection and Sample Preparation
3.2. Chemicals
3.3. HPLC/MS Analysis
| Peak No. | Phenolic Compound | m/z | RT ± SD | Peak No. | Phenolic Compound | m/z | RT ± SD |
|---|---|---|---|---|---|---|---|
| 1 | Caftaric acid | 311 | 3.54 ± 0.05 | 10 | Rutin | 609 | 20.76 ± 0.15 |
| 2 | Gentisic acid | 153 | 3.69 ± 0.03 | 11 | Myricetin | 317 | 21.13 ± 0.12 |
| 3 | Caffeic acid | 179 | 6.52 ± 0.04 | 12 | Fisetin | 285 | 22.91 ± 0.15 |
| 4 | Chlorogenic acid | 353 | 6.43 ± 0.05 | 13 | Quercitrin | 447 | 23.64 ± 0.13 |
| 5 | p-Coumaric acid | 163 | 9.48 ± 0.08 | 14 | Quercetin | 301 | 27.55 ± 0.15 |
| 6 | Ferulic acid | 193 | 12.8 ± 0.10 | 15 | Patuletin | 331 | 29.41 ± 0.12 |
| 7 | Sinapic acid | 223 | 15.00 ± 0.10 | 16 | Luteolin | 285 | 29.64 ± 0.19 |
| 8 | Hyperoside | 463 | 19.32 ± 0.12 | 17 | Kaempferol | 285 | 32.48 ± 0.17 |
| 9 | Isoquercitrin | 463 | 20.29 ± 0.10 | 18 | Apigenin | 279 | 33.10 ± 0.15 |
3.4. Determination of Phenolic Compounds
3.4.1. Determination of Total Phenolic Content
3.4.2. Determination of Flavonoids Content
3.5. Free Radical Scavenging Activity
3.5.1. TEAC Assay
3.5.2. Hemoglobin/Ascorbate Peroxidase Activity Inhibition Assay
3.5.3. Inhibition of Lipid Peroxidation Catalyzed by Cytochrome c
3.5.4. EPR Spectroscopy Measurements
3.6. Antimicrobial Activity Test
3.6.1. Antimicrobial Activity Assay
3.6.2. Minimum Inhibitory Concentration
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, Y.; Cobb, R.E.; Zhao, H. Recent advances in natural product discovery. Curr. Opin. Biotechnol. 2014, 30, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Neblea, M.; Marian, M.; Duta, M. Medicinal plant diversity in the Flora of the west part of Bucegi mountains (Romania). Acta. Hortic. 2011, 955, 41–49. [Google Scholar]
- Fukuda, T.; Yokoyama, J.; Ohashi, H. Phylogeny and biogeography of the genus Lycium (Solanaceae): Inferences from chloroplast DNA sequences. Mol. Phylogenet. Evol. 2001, 19, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Ciocârlan, V. Flora ilustrată a României. Pteridophyta et Spermatophyta. In Illustrated Flora of Romania Pteridophyta et Spermatophyta; Ceres Publishing House: Bucharest, Romania, 2009; p. 709. [Google Scholar]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Harsh, M.L.; Nag, T.N. Diosgenin and phytosterols from Lycium barbarum Linn. Curr. Sci. 1981, 50, 235. [Google Scholar]
- Parvu, M.; Toiu, A.; Vlase, L.; Parvu, E.A. Determination of some polyphenolic compounds from Allium species by HPLC-UV-MS. Nat. Prod. Res. 2010, 24, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Andriamadio, J.H.; Rasoanaivo, L.H.; Benedec, D.; Vlase, L.; Gheldiu, A.M.; Duma, M.; Toiu, A.; Raharisololalao, A.; Oniga, I. HPLC/MS analysis of polyphenols, antioxidant and antimicrobial activities of Artabotrys hildebrandtii O. Hffm. extracts. Nat. Prod. Res. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Meda, R.N.T.; Vlase, L.; Lamien-Meda, A.; Lamien, C.E.; Muntean, D.; Tiperciuc, B.; Oniga, I.; Nacoulma, O.G. Identification and quantification of phenolic compounds from Balanites aegyptiaca (L.) Del (Balanitaceae) galls and leaves by HPLC-MS. Nat. Prod. Res. 2011, 25, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Chemical constituents of three Allium species from Romania. Molecules 2013, 18, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, Ș.; Bădărău, A.S.; Bischin, C.; Vodnar, D.C.; Silaghi-Dumitrescu, R.; Gheldiu, A.M.; Mocan, A.; Vlase, L. Antimicrobial and antioxidant activities and phenolic profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.) K.D. Hill & L.A.S. Johnson leaves. Molecules 2015, 20, 4720–4734. [Google Scholar]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Damian, G.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R. Polyphenolic composition, antioxidant and antibacterial activities for two Romanian subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 2013, 18, 8725–8739. [Google Scholar] [CrossRef] [PubMed]
- Bischin, C.; Deac, F.; Silaghi-Dumitrescu, R.; Worrall, J.A.; Rajagopal, B.S.; Damian, G.; Cooper, C.E. Ascorbate peroxidase activity of cytochrome c. Free Radic. Res. 2011, 45, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Crișan, G.; Vlase, L.; Crișan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative studies on polyphenolic composition, antioxidant and antimicrobial activities of Schisandra chinensis leaves and fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean Peumo (Cryptocarya alba) fruits and comparison with German Peumo (Crataegus monogyna) from southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, P.; Rathinaswamy, G.; Asirvatahm, D. Antimicrobial properties and phytochemical constituents of Rheo discolor Hance. Ethnobot. Leafl. 2008, 12, 841–845. [Google Scholar]
- Dahech, I.; Farah, W.; Trigui, M.; Hssouna, A.B.; Belghith, H.; Belghith, K.S.; Abdallah, F.B. Antioxidant and antimicrobial activities of Lycium shawii fruits extract. Int. J. Biol. Macromol. 2013, 60, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Jung, H.J.; Woo, E.R. Antimicrobial property of (+)-Lyoniresinol-3α-O-β-d glucopyranoside isolated from the root bark of Lycium chinense Miller against human pathogenic microorganisms. Arch. Pharmacal. Res. 2005, 28, 1031–1036. [Google Scholar] [CrossRef]
- Lee, D.G.; Park, Y.; Kim, M.R.; Jung, H.J.; Seu, Y.B.; Hahm, K.S.; Woo, E.R. Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol. Lett. 2004, 26, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Tămaș, M.; Toiu, A.; Oniga, I.; Deliu, C.; Oltean, B.; Coldea, G. Quantitative determination of total polyphenols and flavonoids from indigenous species of Epilobium of wild origin and in vitro regenerates plantlets. Contrib. Bot. 2009, 44, 119–123. [Google Scholar]
- Toma, C.C.; Olah, N.K.; Vlase, L.; Mogoșan, C.; Mocan, A. Comparative studies on polyphenolic composition, antioxidant and diuretic effects of Nigella sativa L. (black cumin) and Nigella damascena L. (lady-in-a-mist) seeds. Molecules 2015, 20, 9560–9574. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.C.; Bischin, C.; Damian, G.; Silaghi-Dumitrescu, R. Antioxidant activity evaluation involving hemoglobin-related free radical reactivity. In Advanced Protocols in Oxidative Stress III. Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1208, pp. 247–255. [Google Scholar]
- Sample Availability: Samples of the vegetal material are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocan, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.-M.; Oprean, R.; Crișan, G. Antioxidant, Antimicrobial Effects and Phenolic Profile of Lycium barbarum L. Flowers. Molecules 2015, 20, 15060-15071. https://doi.org/10.3390/molecules200815060
Mocan A, Vlase L, Vodnar DC, Gheldiu A-M, Oprean R, Crișan G. Antioxidant, Antimicrobial Effects and Phenolic Profile of Lycium barbarum L. Flowers. Molecules. 2015; 20(8):15060-15071. https://doi.org/10.3390/molecules200815060
Chicago/Turabian StyleMocan, Andrei, Laurian Vlase, Dan Cristian Vodnar, Ana-Maria Gheldiu, Radu Oprean, and Gianina Crișan. 2015. "Antioxidant, Antimicrobial Effects and Phenolic Profile of Lycium barbarum L. Flowers" Molecules 20, no. 8: 15060-15071. https://doi.org/10.3390/molecules200815060