Inhibitory Activity of Yokukansankachimpihange against Nerve Growth Factor-Induced Neurite Growth in Cultured Rat Dorsal Root Ganglion Neurons
Abstract
:1. Introduction
2. Results and Discussion
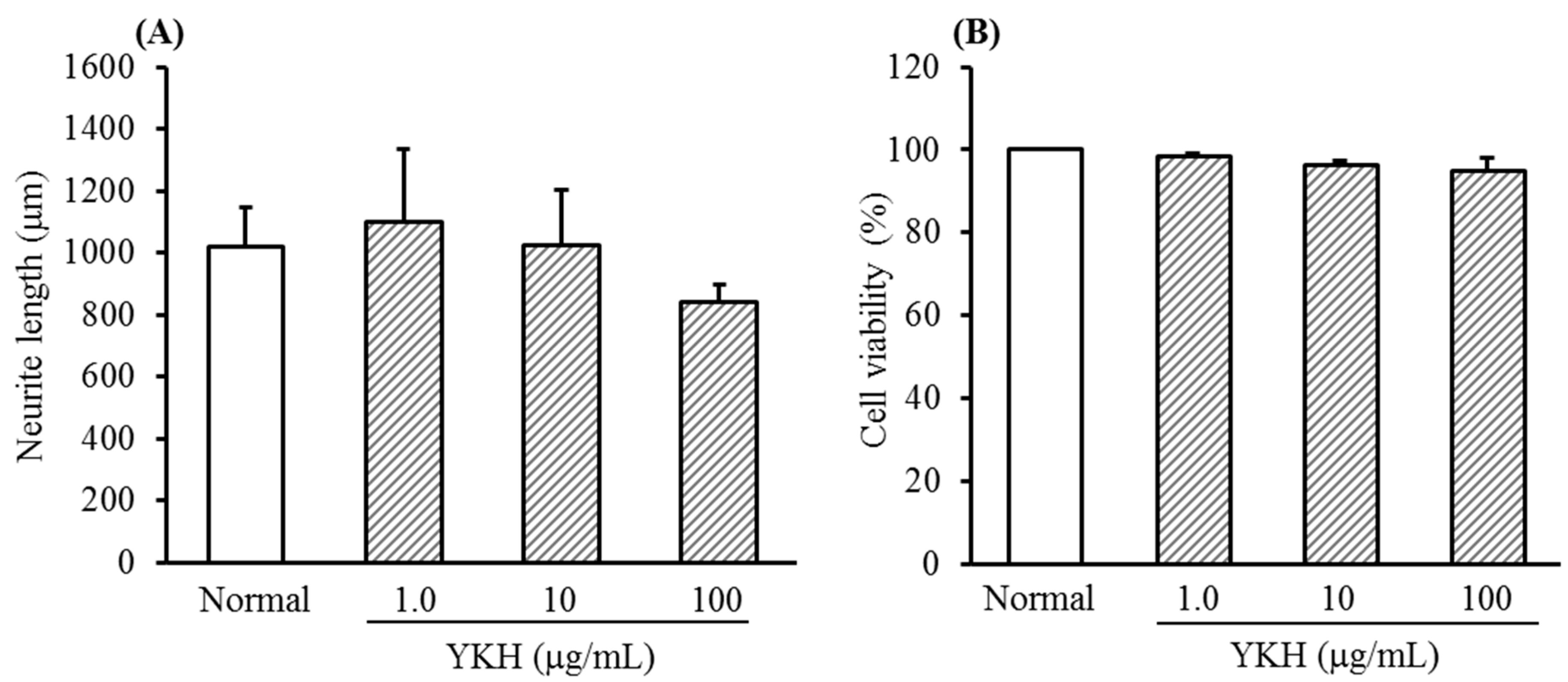
2.1. Inhibition of YKH against Neurite Length Induced with or without NGF and Cytotoxicity of Cultured DRG Neurons

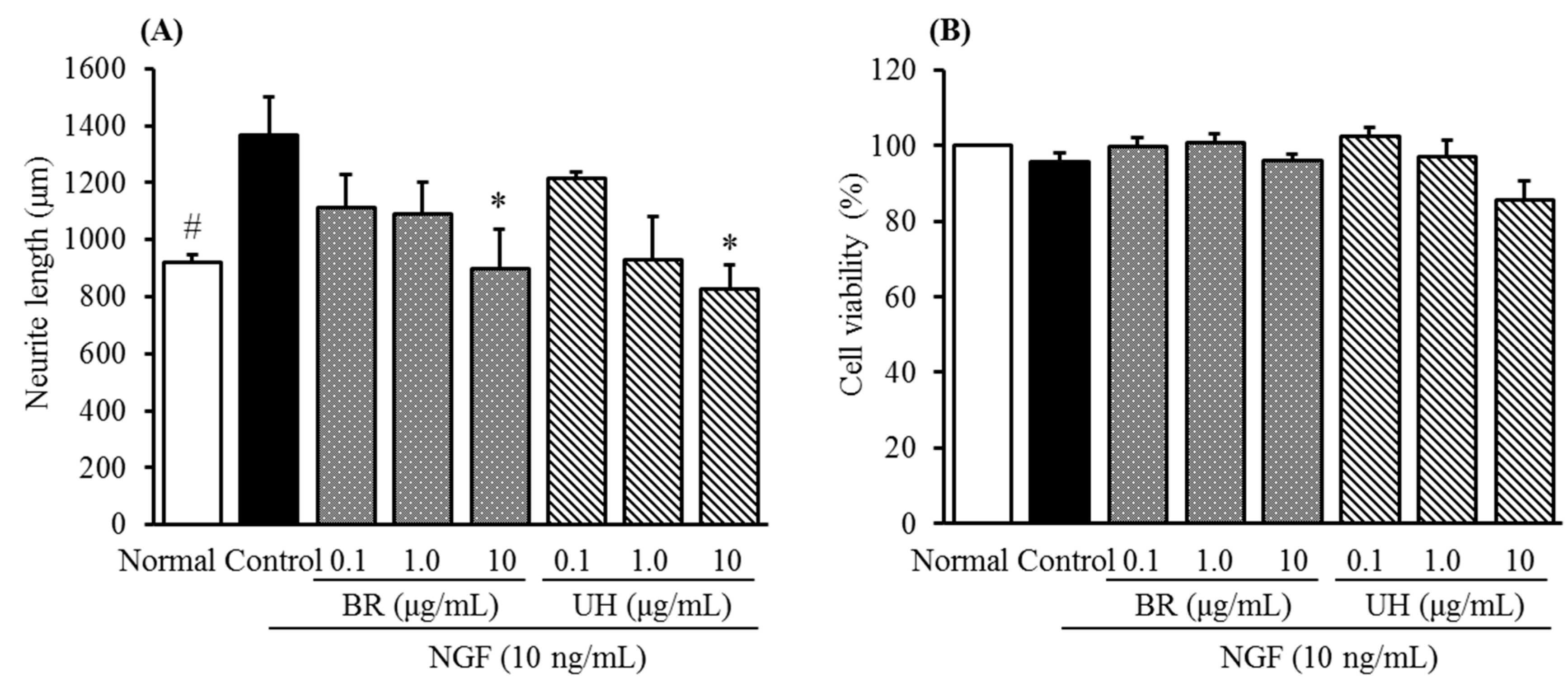
2.2. Inhibitory Activities of Uncaria Hook and Bupleurum Root Together with Their Major Chemical Constituents against NGF-Induced Neurite Growth and Cytotoxicity of Cultured DRG Neurons

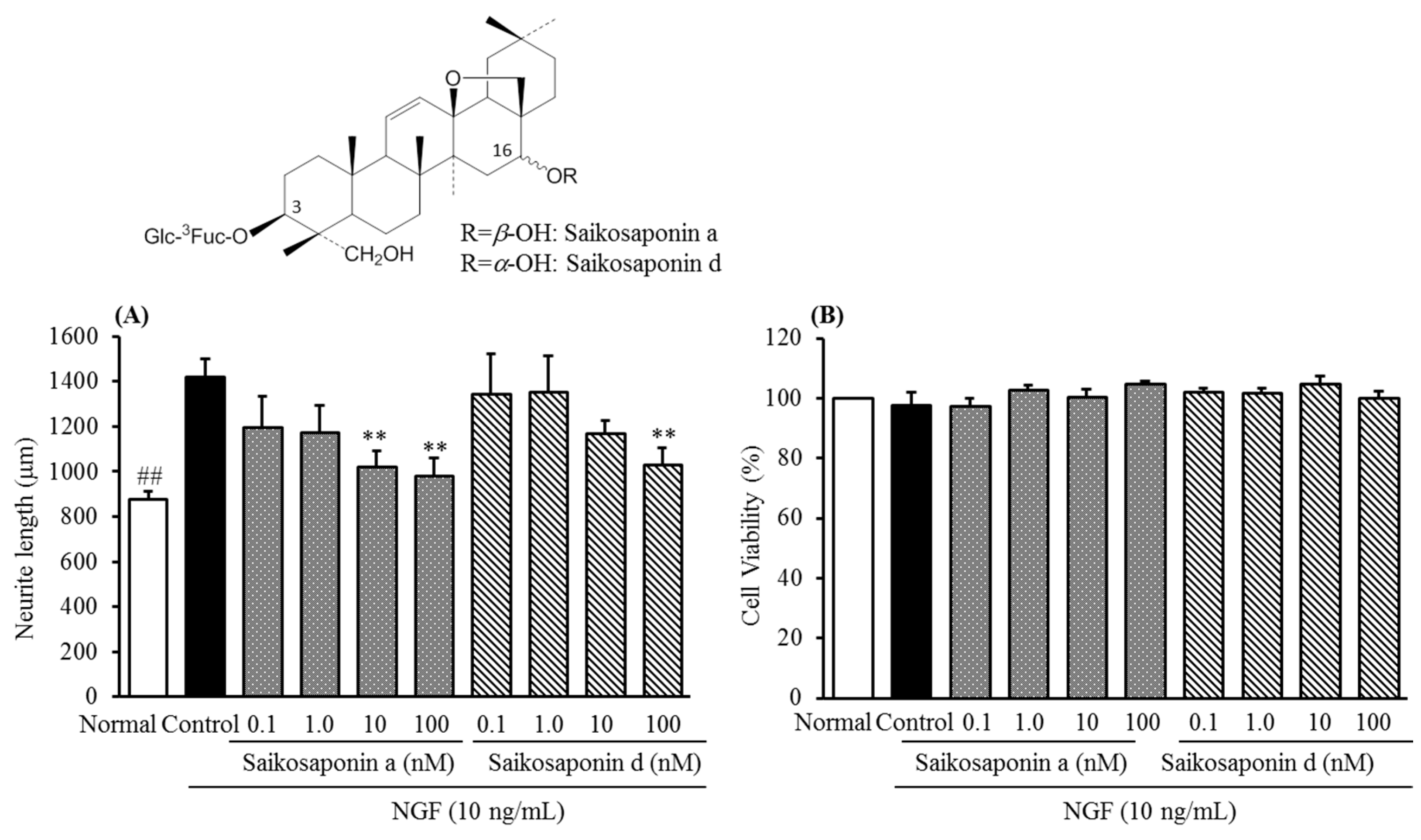
3. Experimental Section
3.1. Experimental Animals
3.2. Plant Material and Extract Preparations
3.3. Chemicals and Positive Control
3.4. Isolation and Culture of Rat DRG Neurons
3.5. Neurite Growth Assessment and Cell Survival Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Yosipovitch, G.; Ansari, N.; Goon, A.; Chan, Y.H.; Goh, C.L. Clinical characteristics of pruritus in chronic idiopathic urticaria. Br. J. Dermatol. 2002, 147, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Papoiu, A.D.; Chan, Y.H.; Rapp, S.R.; Rassette, N.; Yosipovitch, G. Itch characteristics in atopic dermatitis: Results of a web-based questionnaire. Br. J. Dermatol. 2009, 160, 642–644. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.L.; Chan, Y.H.; Rapp, S.R.; Yosipovitch, G. Differences in itch characteristics between psoriasis and atopic dermatitis patients: Results of a web-based questionnaire. Acta Derm. Venereol. 2011, 91, 537–540. [Google Scholar] [PubMed]
- Valdman-Grinshpoun, Y.; Ben-Amitai, D.; Zvulunov, A. Barrier-restoring therapies in atopic dermatitis: Current approaches and future perspectives. Dermatol. Res. Pract. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Yosipovitch, G. Therapy of pruritus. Expert. Opin. Pharmacother. 2010, 11, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Pfab, F.; Schalock, P.C.; Napadow, V.; Athanasiadis, G.I.; Yosipovitch, G.; Ring, J. Complementary integrative approach for treating pruritus. Dermatol. Ther. 2013, 26, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Yudate, T. Effects of Yokukansankachimpihange on both itching and emotional symptoms affecting atopic dermatitis patients. Skin Res. 2009, 8, 244–248. [Google Scholar]
- Yudate, T. Effects of Yokukansankachimpihange in patients with atopic dermatitis—Improvement of psychoneurotic symptoms such as irritation and insomnia. Skin Res. 2010, 9, 48–53. [Google Scholar]
- Nakamura, M. Effect of the Yokukansankachinpihange for both the intractable pruritus cutanea and the sleep disturbance. Jpn. J. Med. Pharm. Sci. 2013, 69, 131–135. [Google Scholar]
- Hsu, H.Y.; Hsu, C.S. Commonly Used Chinese Herb Formulas with Illustrations; Oriental Healing Arts Institute (OHAI) Press: Long Beach, CA, USA, 1980; pp. 343–346. [Google Scholar]
- Han, L.; Dong, X. Itch mechanisms and circuits. Annu. Rev. Biophys. 2014, 43, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Papoiu, A.D. What causes itch in atopic dermatitis? Curr. Allergy Asthma Rep. 2008, 8, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Takamori, K. An update on peripheral mechanisms and treatments of itch. Biol. Pharm. Bull. 2013, 36, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Taneda, K.; Tominaga, M.; Tengara, S.; Ogawa, H.; Takamori, K. Neurotropin inhibits both capsaicin-induced substance P release and nerve growth factor-induced neurite outgrowth in cultured rat dorsal root ganglion neurons. Clin. Exp. Dermatol. 2010, 35, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kamo, A.; Tominaga, M.; Taneda, K.; Ogawa, H.; Tkamori, K. Neurotropin inhibits the increase in intraepidermal nerve density in the acetone-treated dry-skin mouse model. Clin. Exp. Dermatol. 2013, 38, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shin, N.; Tsuchida, T.; Okubo, T.; Norimoto, H. Antianxiety-like effects of chimpi (dried citrus peels) in the elevated open-platform test. Molecules 2013, 18, 10014–10023. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.B.; Inoue, A.; Iwasa, M.; Hide, I.; Nakata, Y. Substance P release evoked by capsaicin or potassium from rat cultured dorsal root ganglion neurons is conversely modulated with bradykinin. J. Neurochem. 2006, 97, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Yanagisawa, H.; Takaku, S. Expression and histochemical localization of ciliary neurotrophic factor in cultured adult rat dorsal root ganglion neurons. Histochem. Cell Biol. 2007, 128, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Website of Erik Meijering. Available online: http://www.imagescience.org/meijering/software/neuronj/ (accessed on 6 March 2014).
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 2002, 147, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Ozawa, S.; Ogawa, H.; Takamori, K. A hypothetical mechanism of intraepidermal neurite formation in NC/Nga mice with atopic dermatitis. J. Dermatol. Sci. 2007, 46, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Ozawa, S.; Tengara, S.; Ogawa, H.; Tkamori, K. Intraepidermal nerve fibers increase in dry skin of acetone-treated mice. J. Dermatol. Sci. 2007, 48, 103–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, Y.; Sun, Y.; Chen, T.; Xu, Q. Role of four major components in the effect of Si-Ni-San, a traditional Chinese prescription, against contact sensitivity in mice. J. Pharm. Pharmacol. 2006, 58, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, J.A.; Kim, T.H.; Seo, S.W.; Jung, S.K.; Park, C.S. Oral administration of Uncariae rhynchophylla inhibits the development of DNFB-induced atopic dermatitis-like skin lesions via IFN-γ down-regulation in NC/Nga mice. J. Ethnopharmacol. 2009, 122, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Hasgimoto, S. K-252a, a potent protein kinase inhibitor, blocks nerve growth factor-induced neurite outgrowth and changes in the phosphorylation of proteins in PC12h cells. J. Cell Biol. 1988, 107, 1531–1539. [Google Scholar] [CrossRef]
- Tey, H.L.; Wallengren, J.; Yosipovitch, G. Psychosomatic factors in pruritus. Clin. Dermatol. 2013, 31, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds of rhynchophylline, hirsutine, saikosaponin a and saikosaponin d are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murayama, C.; Watanabe, S.; Nakamura, M.; Norimoto, H. Inhibitory Activity of Yokukansankachimpihange against Nerve Growth Factor-Induced Neurite Growth in Cultured Rat Dorsal Root Ganglion Neurons. Molecules 2015, 20, 14959-14969. https://doi.org/10.3390/molecules200814959
Murayama C, Watanabe S, Nakamura M, Norimoto H. Inhibitory Activity of Yokukansankachimpihange against Nerve Growth Factor-Induced Neurite Growth in Cultured Rat Dorsal Root Ganglion Neurons. Molecules. 2015; 20(8):14959-14969. https://doi.org/10.3390/molecules200814959
Chicago/Turabian StyleMurayama, Chiaki, Shimpei Watanabe, Motokazu Nakamura, and Hisayoshi Norimoto. 2015. "Inhibitory Activity of Yokukansankachimpihange against Nerve Growth Factor-Induced Neurite Growth in Cultured Rat Dorsal Root Ganglion Neurons" Molecules 20, no. 8: 14959-14969. https://doi.org/10.3390/molecules200814959




