Rapid Estimation of Tocopherol Content in Linseed and Sunflower Oils-Reactivity and Assay
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reaction Rates of α-Tocopherol, γ-Tocopherol and Antioxidants Present in Sunflower Oil and in Linseed Oil with DPPH in Protic and Aprotic Solvents
| Compound | Ethyl Acetate | 2-Propanol | MP-AB * | |||
|---|---|---|---|---|---|---|
| kobs (min−1) | t95% (min) | kobs (min−1) | t95% (min) | kobs (min−1) | t95% (min) | |
| α-tocopherol | 0.42 | 7.2 | 1.3 | 2.4 | 7.4 | 0.41 |
| sunflower oil | 0.37 | 8.0 | 1.2 | 2.5 | 7.4 | 0.41 |
| γ-tocopherol | 0.24 | 12 | 0.52 | 5.8 | 2.1 | 1.4 |
| linseed oil | 0.29 | 10 | 0.55 | 5.4 | 2.2 | 1.3 |
2.2. The Degree of Oxidation of Tocopherols by DPPH in Ethyl Acetate is Concentration Dependent
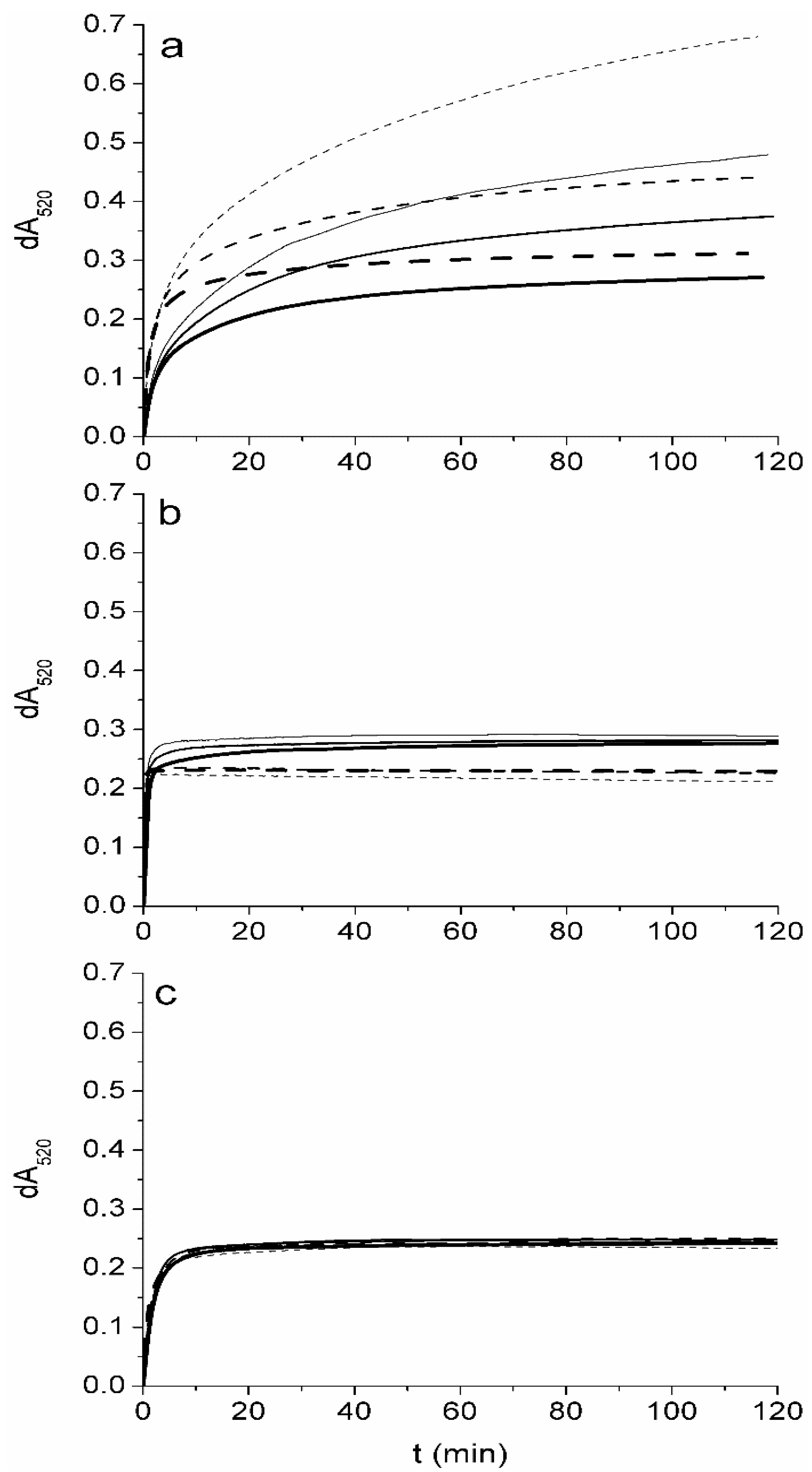
2.3. A DPPH Assay in 2-Propanol can be Used for Rapid Estimation of the Content of Tocopherols in Plant Oils
| Vit. E HPLC (mmol/kg) | AOP (mmol Vit. E/kg) | Contrib. of Vit. E to AOP (%) | |
|---|---|---|---|
| Sunflower Oil | |||
| oil1 | 1.17 ± 0.04 | 1.13 ± 0.04 | 104 |
| oil2 | 1.64 ± 0.07 | 1.58 ± 0.06 | 104 |
| oil3 | 1.58 ± 0.10 | 1.52 ± 0.06 | 104 |
| oil4 | 1.31 ± 0.09 | 1.32 ± 0.06 | 99 |
| oil5 | 1.46 ± 0.09 | 1.43 ± 0.06 | 102 |
| oil6 | 1.47 ± 0.07 | 1.43 ± 0.05 | 103 |
| average | 1.44 ± 0.18 | 1.40 ± 0.16 | 102 ± 2 |
| correlation | 0.99; p = 0.0005 | ||
| Linseed Oil | |||
| oil1 | 0.98 ± 0.05 | 1.30 ± 0.07 | 75 |
| oil2 | 1.02 ± 0.03 | 1.36 ± 0.05 | 75 |
| oil3 | 0.82 ± 0.04 | 1.13 ± 0.03 | 73 |
| oil4 | 1.05 ± 0.04 | 1.33 ± 0.05 | 79 |
| oil5 | 1.08 ± 0.05 | 1.39 ± 0.06 | 78 |
| oil6 | 1.19 ± 0.07 | 1.60 ± 0.07 | 74 |
| oil7 | 1.12 ± 0.05 | 1.46 ± 0.07 | 77 |
| average | 1.04 ± 0.12 | 1.37 ± 0.15 | 76 ± 2 |
| correlation | 0.97; p = 0.0005 | ||
2.4. The Relative Contribution of Tocopherols to AOP in Linseed Oil

3. Experimental Section
3.1. Reagents and Materials
3.2. Reaction Rate Constants
3.3. AOP of Sunflower and Linseed Oils
3.4. Concentration of Hydroperoxides in Plant Oils
3.5. Preparation of Peroxidized Linseed Oil and Oil Stripped of Tocopherols
3.6. Determination of Vitamin E Content in Sunflower and Linseed Oils
3.7. Determination of Fatty Acid Composition of Sunflower and Linseed Oils
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
- Schwartz, H.; Ilainen, V.; Pfironen, V.; Lampi, A.M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Rossi, M.; Alamprese, C.; Ratti, S. Tocopherols and tocotrienols as free radical-scavengers in refined vegetable oils and their stability during deep-fat frying. Food Chem. 2007, 102, 812–817. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Yanishlieva, N.V.; Kamal-Eldin, A.; Marinova, E.M.; Toneva, A.G. Kinetics of antioxidant action of α- and γ-tocopherols in sunflower and soybean triacylglycerols. Eur. J. Lipid Sci. Technol. 2002, 104, 262–270. [Google Scholar] [CrossRef]
- Mukai, K.; Noborio, S.; Nagaoka, S.-I. Why is the order reversed? Peroxyl-scavenging activity and fats-and-oils protecting activity of vitamin E. Int. J. Chem. Kinet. 2005, 37, 605–610. [Google Scholar] [CrossRef]
- Fuster, M.D.; Lampi, A.M.; Hopia, A.; Kamal-Eldin, A. Effects of α- and γ-tocopherols on the autooxidation of purified sunflower triacylglycerols. Lipids 1998, 33, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Lampi, A.M.; Kataja, L.; Kamal-Eldin, A.; Vieno, P. Antioxidant activities of α- and γ-tocopherols in the oxidation of rapeseed oil triacylglycerols. J. Am. Oil Chem. Soc. 1999, 76, 749–755. [Google Scholar] [CrossRef]
- Robledo, S.N.; Tesio, A.Y.; Ceballos, C.D.; Zon, M.A.; Fernandez, H. Electrochemical ultra-micro sensors for the determination of synthetic and natural antioxidants in edible vegetable oils. Sens. Actuators B Chem. 2014, 192, 467–473. [Google Scholar] [CrossRef]
- Robledo, S.N.; Lourdes Zachetti, V.G.; Alicia Zon, M.; Fernandez, H. Quantitative determination of tocopherols in edible vegetable oils using electrochemical ultra-microsensors combined with chemometric tools. Talanta 2013, 116, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphellyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS assays to assess the antioxidant profile of untreated oils. Food Anal. Methods 2015, 8, 1294–1302. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Juhņeviča, K.; Lācis, G.; Šnē, E.; Segliņa, D. Cold-pressed Japanese quince (Chaenomeles japonica (Thunb.) Lindl. ex Spach) seed oil as a rich source of α-tocopherol, carotenoids and phenolics: A comparison of the composition and antioxidant activity with nine other plant oils. Eur. J. Lipid Sci. Technol. 2014, 116, 563–570. [Google Scholar] [CrossRef]
- Górnaś, P. Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: Rapid and simple determination of all four homologues (α, β, γ and δ) by RP-HPLC/FLD. Food Chem. 2015, 172, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Soliven, A.; Segliņa, D. Seed oils recovered from industrial fruit by-products are a rich source of tocopherols and tocotrienols: Rapid separation of α/β/γ/δ homologues by RP-HPLC/FLD. Eur. J. Lipid Sci. Technol. 2015, 116, 773–777. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Wandan, K.M.M. Blending of corn oil with black cumin (Nigella sativa) and coriander (Coriandrum sativum) seed oils: Impact on functionality, stability and radical scavenging activity. Food Chem. 2012, 132, 873–879. [Google Scholar] [CrossRef]
- Arranz, S.; Cert, R.; Perez-Jimenez, J.; Cert, A.; Saura-Calixto, F. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008, 110, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Hrncirik, K.; Fritsche, S. Comparability and reliability of different techniques for the determination of phenolic compounds in virgin olive. Eur. J. Lipid Sci. Technol. 2004, 106, 540–549. [Google Scholar] [CrossRef]
- Tasioula-Margari, M.; Okogeri, O. Simultaneous determination of phenolic compounds and tocopherols in virgin olive oil using HPLC and UV detection. Food Chem. 2001, 74, 377–383. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef] [PubMed]
- Prevc, T.; Šegatin, N.; Ulrih, N.P.; Cigić, B. DPPH assay of vegetable oils and model antioxidants in protic and aprotic solvents. Talanta 2013, 109, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Dufour, C.; Loonis, M.; Dangles, O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Friaa, O.; Brault, D. Kinetics of the reaction between the antioxidant Trolox® and the free radical DPPH• in semi-aqueous solution. Org. Biomol. Chem. 2006, 4, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Litwinienko, G. Scavenging of DPPH• radicals by vitamin E is accelerated by its partial ionization: The role of sequential proton loss electron transfer. Org. Lett. 2005, 7, 4951–4954. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Kawabata, J. DPPH (=2,2-diphenyl-1-picrylhydrazyl) radical-scavenging reaction of protocatechuic acid (=3,4-dihydroxybenzoic acid): Difference in reactivity between acids and their esters. Helv. Chim. Acta 2006, 89, 1395–1407. [Google Scholar] [CrossRef]
- Foti, M.C. Solvent effects on the activation parameters of the reaction between an α-tocopherol analogue and DPPH: The role of H-bonded complexes. Int. J. Chem. Kinet. 2012, 44, 524–531. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Verleyen, T.; Verhe, R.; Huyghebaert, A.; Dewettinck, K.; de Greyt, W. Identification of α-tocopherol oxidation products in triolein at elevated temperatures. J. Agric. Food Chem. 2001, 49, 1508–1511. [Google Scholar] [CrossRef] [PubMed]
- Terentis, A.C.; Thomas, S.R.; Burr, J.A.; Liebler, D.C.; Stocker, R. Vitamin E oxidation in human atherosclerotic lesions. Circ. Res. 2002, 90, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Sawai, Y.; Moon, J.H. NMR analytical approach to clarify the molecular mechanisms of the antioxidative and radical-scavenging activities of antioxidants in tea using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 6247–6253. [Google Scholar] [CrossRef] [PubMed]
- Brandwilliams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH• assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Kawabata, J.; Okamoto, Y.; Kodama, A.; Makimoto, T.; Kasai, T. Oxidative dimers produced from protocatechuic and gallic esters in the DPPH radical scavenging reaction. J. Agric. Food Chem. 2002, 50, 5468–5471. [Google Scholar] [CrossRef] [PubMed]
- Marteau, C.F.; Favier, D.; Nardello-Rataj, V.; Aubry, J.M. Dramatic solvent effect on the synergy between α-tocopherol and BHT antioxidants. Food Chem. 2014, 160, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 2007, 71, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, I.H.; Lee, C.; Choi, H.-D.; Kim, B.H.; Akoh, C.C. Discrimination of origin of sesame oils using fatty acid and lignan profiles in combination with canonical discriminant analysis. J. Am. Oil Chem. Soc. 2013, 90, 337–347. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Birlouez-Aragon, I.; Wolff, S.P. Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay. J. Agric. Food Chem. 1995, 43, 17–21. [Google Scholar] [CrossRef]
- Park, P.W.; Goins, R.E. In-situ preparation of fatty-acid methyl-estrs for analysis of fatty-acid composition in foods. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Samples Availability: Samples of the oils are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prevc, T.; Levart, A.; Cigić, I.K.; Salobir, J.; Ulrih, N.P.; Cigić, B. Rapid Estimation of Tocopherol Content in Linseed and Sunflower Oils-Reactivity and Assay. Molecules 2015, 20, 14777-14790. https://doi.org/10.3390/molecules200814777
Prevc T, Levart A, Cigić IK, Salobir J, Ulrih NP, Cigić B. Rapid Estimation of Tocopherol Content in Linseed and Sunflower Oils-Reactivity and Assay. Molecules. 2015; 20(8):14777-14790. https://doi.org/10.3390/molecules200814777
Chicago/Turabian StylePrevc, Tjaša, Alenka Levart, Irena Kralj Cigić, Janez Salobir, Nataša Poklar Ulrih, and Blaž Cigić. 2015. "Rapid Estimation of Tocopherol Content in Linseed and Sunflower Oils-Reactivity and Assay" Molecules 20, no. 8: 14777-14790. https://doi.org/10.3390/molecules200814777