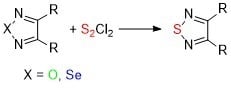
Direct Exchange of Oxygen and Selenium Atoms in the 1,2,5-Oxadiazoles and 1,2,5-Selenadiazoles by Action of Sulfur Monochloride
Abstract
:1. Introduction
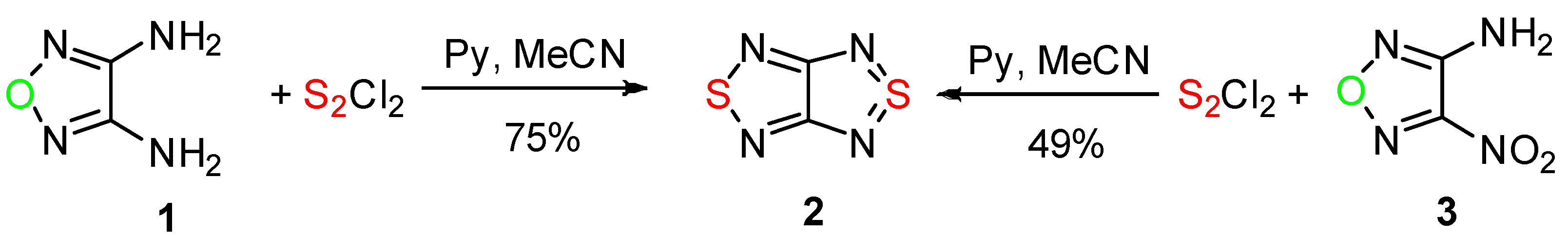
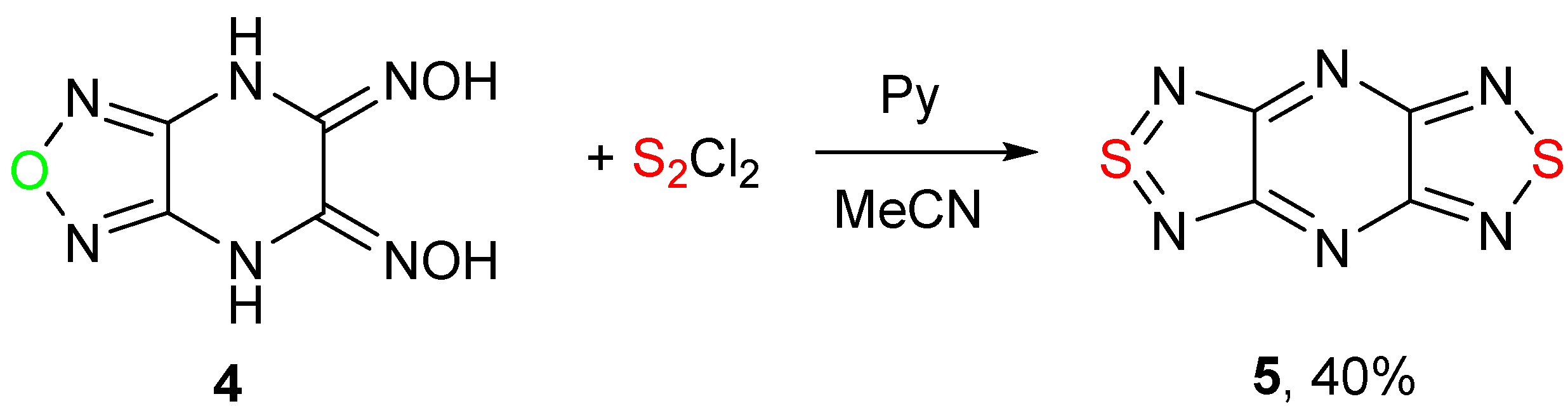
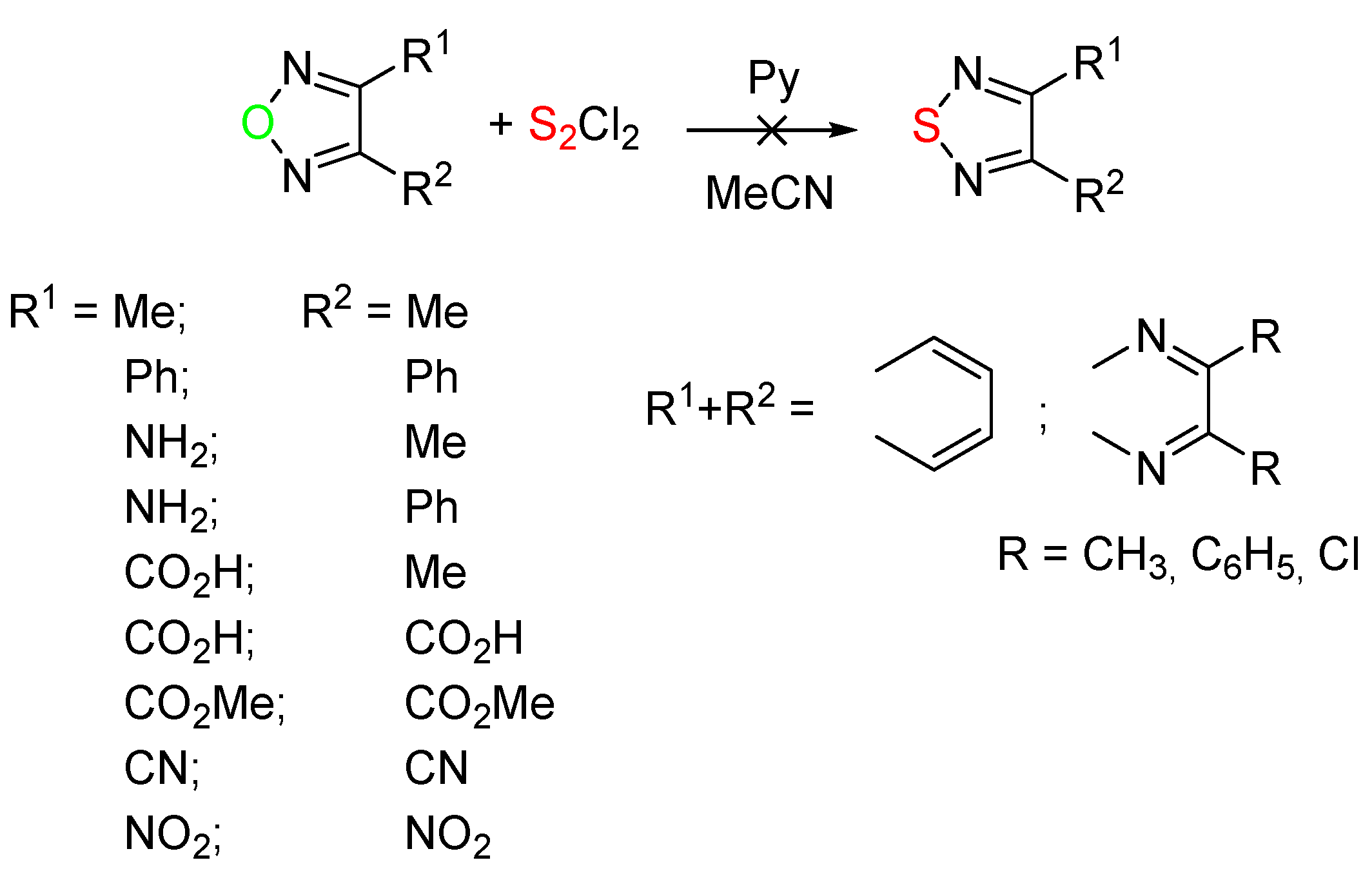
2. Results and Discussion
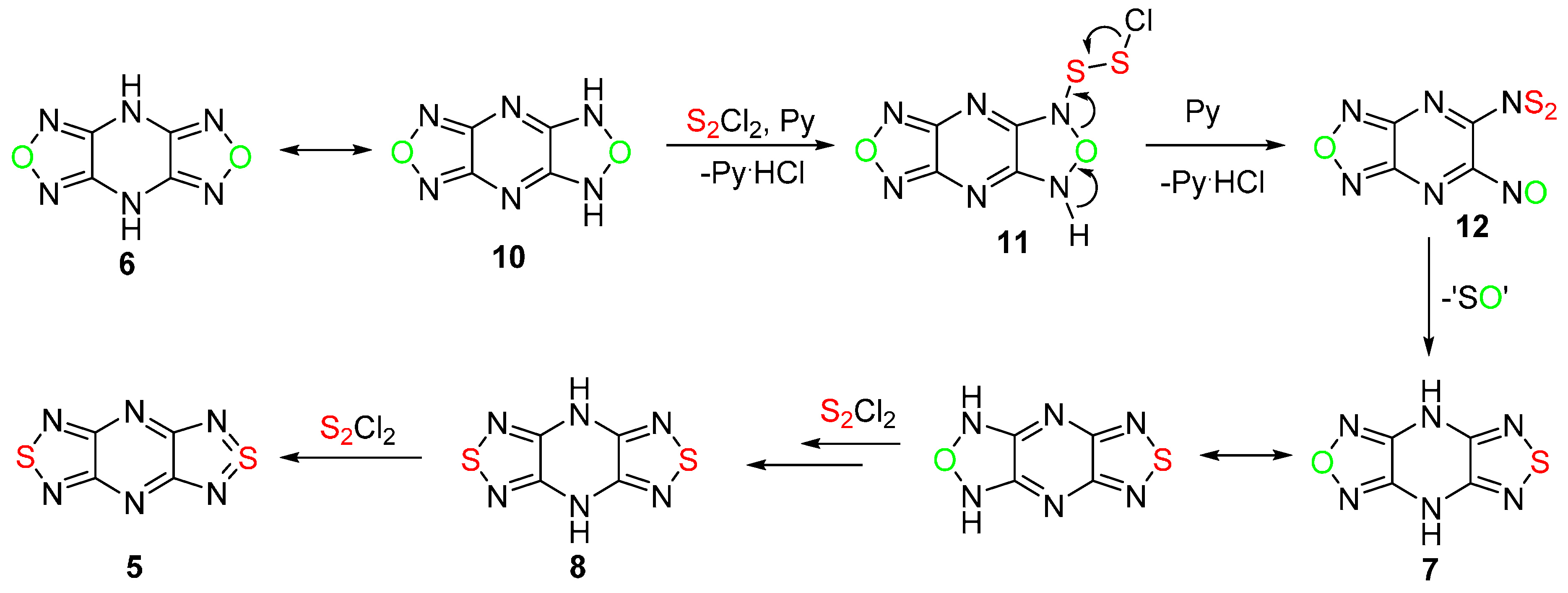
2.1. Conversion of 1,2,5-Oxadiazoles into 1,2,5-Thiadiazoles
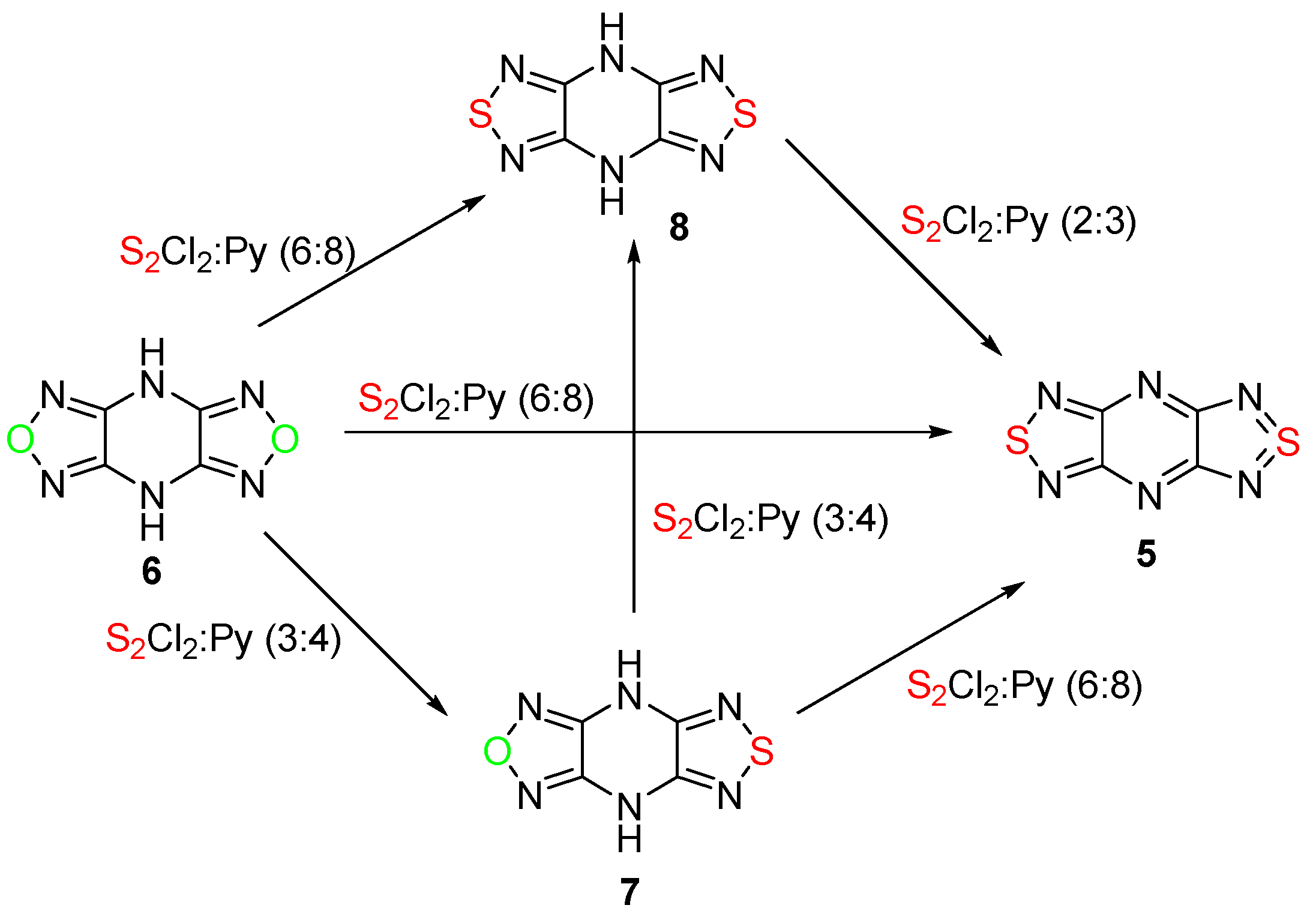
| No. | Starting Compound | Quantity of Reagents for 1 Equiv of Starting Compound | Temperature, °С | Reaction Time, h | Reaction Product, Yield % | |
|---|---|---|---|---|---|---|
| S2Cl2 | Pyridine | |||||
| 1 | 6 | 6 | 8 | 82 | 4 | 5, 76 |
| 2 | 6 | 3 | 4 | 20 | 20 | 7, 90 |
| 3 | 6 | 6 | 8 | 20 | 20 | 8, 83 |
| 4 | 7 | 3 | 4 | 20 | 20 | 8, 66 |
| 5 | 7 | 6 | 8 | 82 | 5 | 5, 56 |
| 6 | 8 | 2 | 3 | 82 | 2 | 5, 85 |
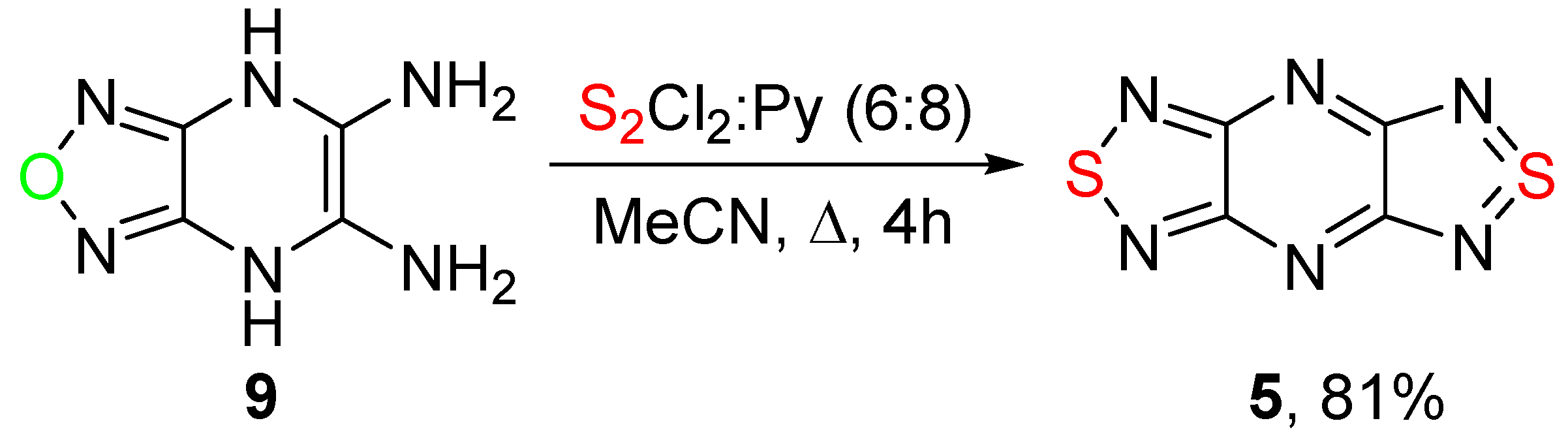
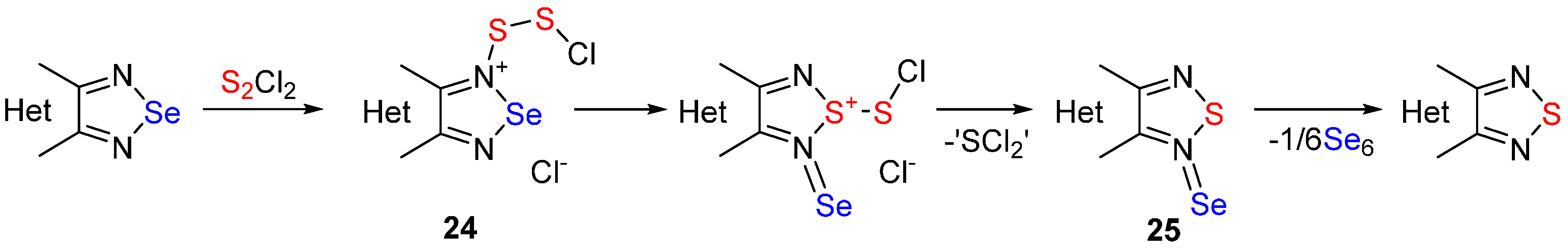
2.2. Conversion of 1,2,5-Selenadiazoles into 1,2,5-Thiadiazoles

| No. | Starting Compound | Quantity of S2Cl2 for 1 Equiv of Starting Compound | Тemperature, °С | Reaction Time, h | Reaction Product, Yield % |
|---|---|---|---|---|---|
| 1 | 13 | 3 | 20 | 3 | 7, 91 |
| 2 | 13 | 6 | 100 | 6 | 8, 78 |
| 3 | 14 | 3 | 20 | 20 | 8, 83 |
| 4 | 15 | 6 | 100 | 6 | 8, 85 |
| 5 | 16 | 3 | 20 | 20 | 2, 93 |
| 6 | 17 | 6 | 100 | 6 | 2, 73 |
| 7 | 18 | 2 | 70 | 3 | 21, 75 |
| 8 | 19 | 2 | 20 | 6 | 22, 47 |
| 9 | 20 | 2 | 70 | 2 | 23, 57 |

3. Experimental Section
3.1. General Information
3.2. General Procedure for the Reaction of 1,2,5-Oxadiazoles with S2Cl2 and Pyridine in Acetonitrile
3.3. General Procedure for the Reaction of 1,2,5-Selenadiazoles with S2Cl2 in DMF
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Todres, Z.V. Chalcogenadiazoles: Chemistry and Applications; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012. [Google Scholar]
- Koutentis, P.A. 1,2,5-Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, pp. 516–564. [Google Scholar]
- Koutentis, P.A. 1,2,5-Thiadiazoles and related compounds. In Science of Synthesis; Storr, R.C., Gilchrist, T.L., Eds.; Thieme: Stuttgart, Germany, 2003; Volume 13, pp. 297–348. [Google Scholar]
- Neto, B.A.D.; Lapis, A.A.M.; da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and derivatives: Synthesis, Properties, Reactions, and applications in light technology of small molecules. Eur. J. Org. Chem. 2013, 2013, 228–235. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Knyazeva, E.A.; Rakitin, O.A. Recent developments in the synthesis and applications of 1,2,5-thia- and selenadiazoles. A Review. Org. Prep. Proc. Int. 2014, 46, 475–544. [Google Scholar] [CrossRef]
- Lonchakov, A.V.; Rakitin, O.A.; Gritsan, N.P.; Zibarev, A.V. Breathing some new life into an old topic: Chalcogen-nitrogen π-heterocycles as electron acceptors. Molecules 2013, 18, 9850–9990. [Google Scholar] [CrossRef] [PubMed]
- Zibarev, A.V.; Mews, R. A new class of paramagnetics: 1,2,5-Chalcogenadiazolydil salts as potential building blocks for molecular magnets and conductors. In Selenium and Tellurium Chemistry: From Small Molecules to Biomolecules and Materials; Woollins, J.D., Laitinen, R.S., Eds.; Springer: Berlin, Germany, 2011; pp. 123–149. [Google Scholar]
- Gritsan, N.P.; Zibarev, A.V. Chalcogen-nitrogen π-heterocyclic radical anion salts: The synthesis and properties. Russ. Chem. Bull. 2011, 60, 2131–2140. [Google Scholar] [CrossRef]
- Semenov, N.A.; Lonchakov, A.V.; Pushkarevsky, N.A.; Suturina, E.A.; Korolev, V.V.; Lork, E.; Vasiliev, V.G.; Konchenko, S.N.; Beckmann, J.; Gritsan, N.P.; et al. Coordination of halide and chalcogenolate anions to heavier 1,2,5-chalcogenadiazoles: Experiment and theory. Organometallics 2014, 33, 4302–4314. [Google Scholar] [CrossRef]
- Pushkarevsky, N.A.; Lonchakov, A.V.; Semenov, N.A.; Lork, E.; Buravov, L.I.; Konstantinova, L.S.; Silber, T.G.; Robertson, N.; Gritsan, N.P.; Rakitin, O.A.; et al. First charge-transfer complexes between tetrathiafulvalene and 1,2,5-chalcogenadiazole derivatives: Design, synthesis, crystal structures, electronic and electrical properties. Synth. Met. 2012, 162, 2267–2276. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Knyazeva, E.A.; Nefyodov, A.A.; Camacho, P.S.; Ashbrook, S.E.M.; Woollins, J.D.; Zibarev, A.V.; Rakitin, O.A. Direct synthesis of fused 1,2,5-selenadiazoles from 1,2,5-thiadiazoles. Tetrahedron Lett. 2015, 56, 1107–1110. [Google Scholar] [CrossRef]
- Semenov, N.A.; Pushkarevsky, N.A.; Suturina, E.A.; Chulanova, E.A.; Kuratieva, N.V.; Bogomyakov, A.S.; Irtegova, I.G.; Vasilieva, N.V.; Konstantinova, L.S.; Gritsan, N.P.; et al. Bis(toluene)chromium(I) [1,2,5]thiadiazolo[3,4-c][1,2,5]thiadiazolidyl and [1,2,5]thiadiazolo[3,4-b]pyrazinidyl: New heterospin (S1 = S2 = 1/2) radical-ion salts. Inorg. Chem. 2013, 52, 6654–6663. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Knyazeva, E.A.; Obruchnikova, N.V.; Gatilov, Y.V.; Zibarev, A.V.; Rakitin, O.A. Reactions of vicinal nitroamines with sulfur monochloride—A short and convenient route to fused 1,2,5-thiadiazoles and their 1-oxides. Tetrahedron Lett. 2013, 54, 3075–3078. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Knyazeva, E.A.; Obruchnikova, N.V.; Vasilieva, N.V.; Irtegova, I.G.; Nelyubina, Y.V.; Bagryanskaya, I.Y.; Shundrin, L.A.; Sosnovskaya, Z.Y.; Zibarev, A.V.; et al. 1,2,5-Thiadiazole 2-oxides: Selective synthesis, structural characterization and electrochemical properties. Tetrahedron 2014, 70, 5558–5568. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Konstantinova, L.S. Sulfur monochloride in the synthesis of heterocyclic compounds. Adv. Heterocycl. Chem. 2008, 96, 175–229. [Google Scholar]
- Rakitin, O.A. One-pot synthesis of sulfur heterocycles from simple organic substrates. Arkivoc 2009, 1, 129–149. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Konstantinova, L.S. Design of sulfur heterocycles with sulfur monochloride: Retrosynthetic analysis and prospects. Mendeleev Commun. 2009, 19, 55–61. [Google Scholar]
- Rakitin, O.A.; Konstantinova, L.S. Sulfur monochloride in organic synthesis. Russ. Chem. Rev. 2014, 83, 225–250. [Google Scholar]
- Elderfield, R.C. Heterocyclic Compounds; Wiley: New York, NY, USA, 1950; Volume 1. [Google Scholar]
- Li, Q.F.; Xu, Y.Q.; Liu, C.G.; Kim, J. Catalytic Synthesis of Thiophene from the Reaction of Furan and Hydrogen Sulfide. Catal. Lett. 2008, 122, 354–358. [Google Scholar] [CrossRef]
- Mashkina, A.V. Investigation of the mechanism of catalytic recyclization of furan to thiophene. Chem. Heterocycl. Comp. 2010, 46, 1063–1067. [Google Scholar] [CrossRef]
- Pedersen, C.L.; Lohse, C.; Polyakoff, M. Photolysis of Benzo[c]-1,2,5-thiadiazole 2-Oxide. Spectroscopic Evidence for the Reversible Formation of 2-Thionitrosonitrosobenzene. Acta Chem. Scand. B 1978, 32, 625–631. [Google Scholar] [CrossRef]
- Ul-Haq, A.; Pia Donzello, M.; Stuzhin, P.A. Iron(II) complexes of hexaphenyl(1,2,5-thia/selenadiazolo)-porphyrazine: The direct replacement of Se by S in the 1,2,5-selenadiazole ring. Mendeleev Commun. 2007, 17, 337–339. [Google Scholar] [CrossRef]
- Starchenkov, I.B.; Andrianov, V.G. 4H,8H-bis(1,2,5-oxadiazolo)[3,4-b:3′,4′-e]Pyrazine. Chem. Heterocycl. Comp. 1996, 32, 717. [Google Scholar] [CrossRef]
- Starchenkov, I.B.; Andrianov, V.G.; Mishnev, A.V. The chemistry of furazano-[3,4-b]pyrazine. 7. Properties of 5,6-diamino- and 5,6-dihydrazino-furazano[3,4-b]pyrazine. Chem. Heterocycl. Comp. 1999, 35, 499–508. [Google Scholar] [CrossRef]
- Bagryanskaya, I.Y.; Gatilov, Y.V.; Gritsan, N.P.; Ikorskii, V.N.; Irtegova, I.G.; Lonchakov, A.V.; Lork, E.; Mews, R.; Ovcharenko, V.I.; Semenov, N.A.; et al. [1,2,5]Selenadiazolo[3,4-c][1,2,5]thiadiazole and [1,2,5] selenadiazolo-[3,4-c][1,2,5]thiadiazolidyl—A synthetic, structural, and theoretical study. Eur. J. Inorg. Chem. 2007, 4751–4761. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bobkova, I.E.; Nelyubina, Y.V.; Chulanova, E.A.; Irtegova, I.G.; Vasilieva, N.V.; Camacho, P.S.; Ashbrook, S.E.M.; Hua, G.; Slawin, A.M.Z.; et al. [1,2,5]Selenadiazolo[3,4-b]pyrazines: Synthesis from 3,4-Diamino-1,2,5-selenadiazole and Generation of Persistent Radical Anions. J. Org. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Komin, A.P.; Carmack, M. The chemistry of 1,2,5-thiadiazoles V. Synthesis of 3,4-diamino-1,2,5-thiadiazole and [1,2,5] thiadiazolo[3,4-b]pyrazines. J. Heterocycl. Chem. 1976, 13, 13–22. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinova, L.S.; Knyazeva, E.A.; Rakitin, O.A. Direct Exchange of Oxygen and Selenium Atoms in the 1,2,5-Oxadiazoles and 1,2,5-Selenadiazoles by Action of Sulfur Monochloride. Molecules 2015, 20, 14522-14532. https://doi.org/10.3390/molecules200814522
Konstantinova LS, Knyazeva EA, Rakitin OA. Direct Exchange of Oxygen and Selenium Atoms in the 1,2,5-Oxadiazoles and 1,2,5-Selenadiazoles by Action of Sulfur Monochloride. Molecules. 2015; 20(8):14522-14532. https://doi.org/10.3390/molecules200814522
Chicago/Turabian StyleKonstantinova, Lidia S., Ekaterina A. Knyazeva, and Oleg A. Rakitin. 2015. "Direct Exchange of Oxygen and Selenium Atoms in the 1,2,5-Oxadiazoles and 1,2,5-Selenadiazoles by Action of Sulfur Monochloride" Molecules 20, no. 8: 14522-14532. https://doi.org/10.3390/molecules200814522