Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives
Abstract
:1. Introduction
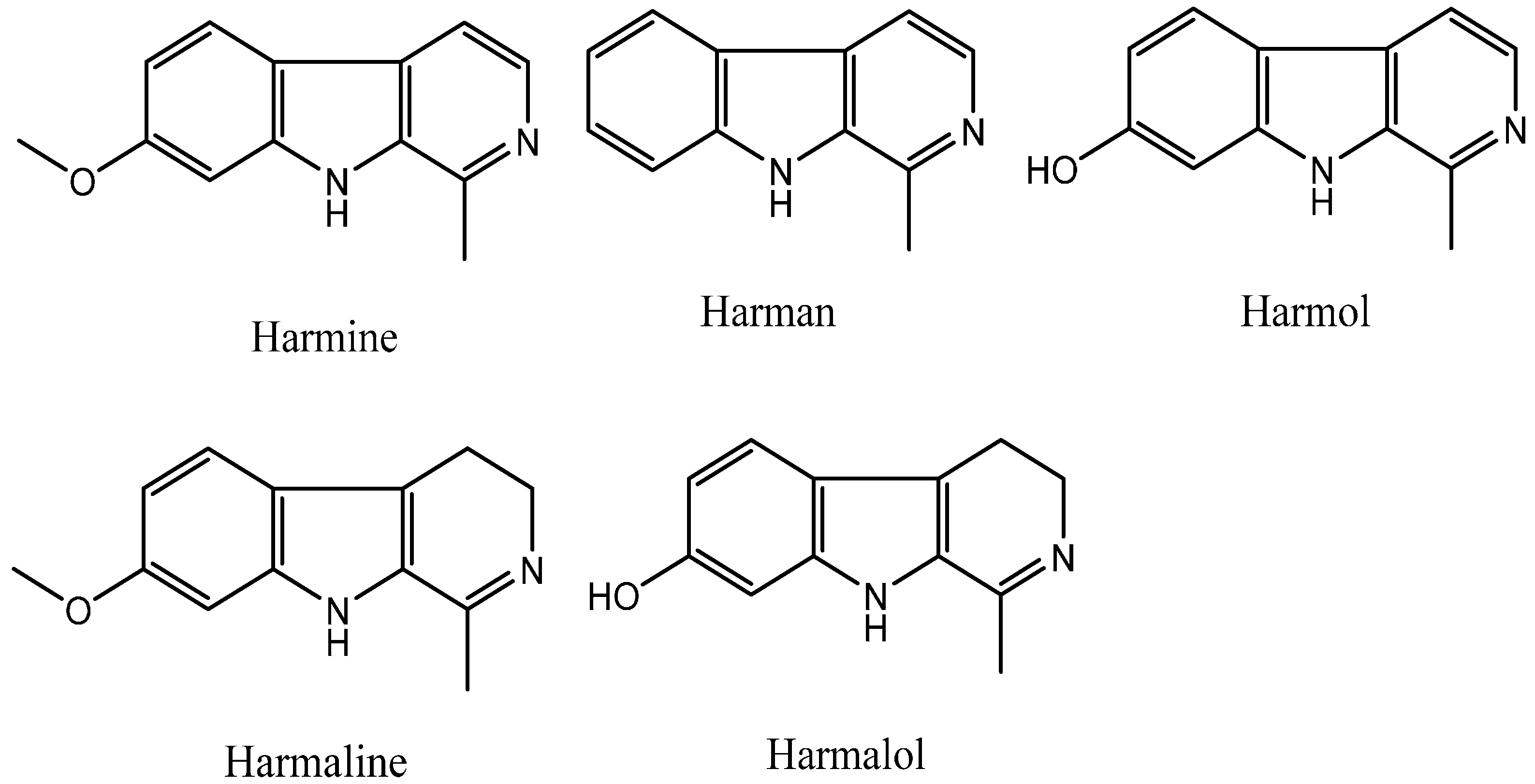
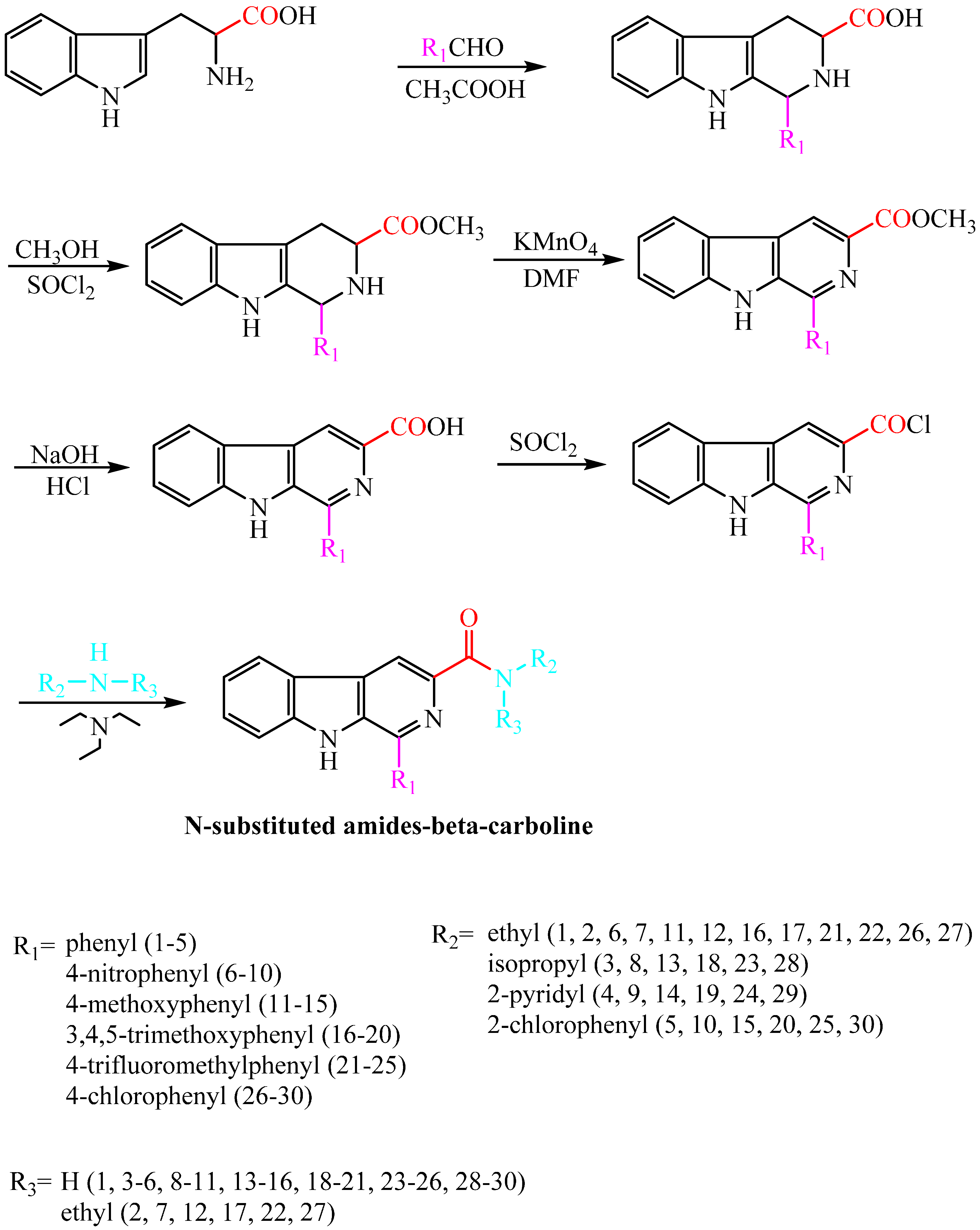
2. Results and Discussion
2.1. Synthesis

2.2. Fungicidal Activities
| Compounds | FO | CG | RS | PL | PN | OC |
|---|---|---|---|---|---|---|
| Harmaline | 32.9 | 19.7 | 38.4 | 49.4 | 30.2 | 10.7 |
| Harmalol | 12.3 | 21 | 25 | 30.4 | 20.4 | 5.8 |
| Harmine | 41.6 | 27.3 | 71.9 | 78.8 | 9.3 | 10.4 |
| Harmol | 33.3 | 13.8 | 27.8 | 46.7 | 38.4 | 10.2 |
| F1 | 23 | 31.5 | 52.9 | 27.4 | 19.5 | 13.5 |
| F2 | 0.2 | 4.6 | 9.9 | - d | - | 0.3 |
| F3 | - | 3.2 | 14.9 | - | 18.6 | - |
| F4 | 30.2 | 32 | 43.2 | 95.9 | 63.3 | 8.5 |
| F5 | 19.9 | 30.5 | 53.4 | 76.3 | 31.7 | 6.8 |
| F6 | 0.5 | 1 | 15.7 | 32.1 | 19 | - |
| F7 | 3.1 | 10.2 | 15.7 | 2.1 | 9.8 | 0.5 |
| F8 | - | 16.5 | 23.8 | 32.4 | 23.6 | - |
| F9 | - | 19.6 | 15.1 | 53 | 36.4 | - |
| F10 | - | 2.8 | 9.1 | 57.7 | 42.4 | 3.4 |
| F11 | 2.8 | 20.1 | 9.4 | 35.6 | 23.4 | 6.8 |
| F12 | 3.4 | 11.4 | 7.3 | 8.2 | 9.6 | - |
| F13 | 9.9 | 11 | 36.6 | / e | / | / |
| F14 | 0.5 | 14 | 18.6 | 50.1 | 11.1 | 2.9 |
| F16 | 7.2 | 7.9 | 31.9 | 3.7 | - | 43.3 |
| F17 | 13.1 | 12.9 | 18.1 | 62.3 | 46.3 | 17.9 |
| F18 | 10.2 | 16.4 | 38.7 | 23 | 2.8 | 6.6 |
| F19 | - | 5.1 | 2.2 | 17.5 | - | 2.4 |
| F20 | 1.2 | 12.3 | 7 | 68.4 | 55.4 | 15.3 |
| F21 | 2.5 | 13.6 | 0.3 | 6.9 | - | 4.3 |
| F22 | 6.1 | 7.8 | 2 | 40.4 | - | 3.3 |
| F23 | 0.5 | 12.4 | 3.4 | 25.9 | - | 2.1 |
| F24 | 20.8 | 39.6 | 14.5 | 76.6 | 53.7 | 8.7 |
| F25 | 24.6 | 43.1 | 26 | 82.5 | 52.1 | 0.3 |
| F26 | 3.4 | 15.1 | 11.8 | 20. 3 | 13.9 | - |
| F27 | 17.1 | 28.4 | 30.7 | 75.8 | 54.4 | 8.5 |
| F28 | 10.3 | 14.4 | 22.1 | 37.1 | 14.4 | - |
| F29 | 6.1 | 34.8 | 26.4 | / | / | / |
| F30 | - | 16.7 | 34 | 69.8 | 44 | 2.6 |
| a Metalaxyl | / | / | / | 93.4 | 84.1 | / |
| b Validamycin | / | / | 36.4 | / | / | / |
| c Carbendazim | 89 | 100 | / | / | / | 88.1 |
2.3. Leaf-Piece Assays
| Compound | Preventative | Curative |
|---|---|---|
| Harmaline | 0 | 44.4 |
| Harmalol | 0 | 7.4 |
| Harmine | 22.2 | 7.4 |
| Harmol | 7.4 | 51.6 |
| F1 | 7.4 | 37 |
| F2 | 14.8 | 29.6 |
| F3 | 14.8 | 29.6 |
| F4 | 92.6 | 59.3 |
| F5 | 44.4 | 44.4 |
| F6 | 0 | 51.9 |
| F7 | 29.6 | 37 |
| F8 | 7.4 | 37 |
| F9 | 66.7 | 29.6 |
| F10 | 51.9 | 0 |
| F11 | 22.2 | 63 |
| F12 | 22.2 | 33.3 |
| F13 | / b | / |
| F14 | 0 | 22.2 |
| F16 | 14.8 | 44.4 |
| F17 | 37 | 0 |
| F18 | 7.4 | 51.9 |
| F19 | 0 | 44.4 |
| F20 | 22.2 | 22.2 |
| F21 | 7.4 | 29.6 |
| F22 | 22.2 | 22.2 |
| F23 | 0 | 29.6 |
| F24 | 7.4 | 29.6 |
| F25 | 22.2 | 14.8 |
| F26 | 0 | 29.6 |
| F27 | 29.6 | 7.4 |
| F28 | 14.8 | 37 |
| F29 | / | / |
| F30 | 22.2 | 14.8 |
| a Metalaxyl | 59.3 | 66.7 |
3. Experimental Section
3.1. General Synthesis
Synthetic Procedure for the Precursors of Target Compounds F1–30
3.2. Spectral Data
3.3. Fungicidal Activities
3.4. Leaf-Piece Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, R.H.; Peng, W.L.; Wang, Z.H. β-Carboline Alkaloids: Biochemical and Pharmacological Functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, R.A.; Spencer, I.D. The carbolines. Adv. Heterocycl. Chem. 1964, 3, 79–207. [Google Scholar] [PubMed]
- Allen, J.R.F.; Holmstedt, B.R. The Simple β-Carboline Alkaloids. Phytochemistry 1980, 19, 1573–1582. [Google Scholar] [CrossRef]
- De Meester, C. Genotoxic potential of β-carbolines: A review. Mutat. Res. 1995, 339, 139–153. [Google Scholar] [CrossRef]
- Jahaniani, F.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Mahmoudian, M. Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochemistry 2005, 66, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nagao, M.; Sugimura, T. Interactions of norharman and harman with DNA. Nucleic Acids. Res. 1977, 4, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.; Ebrahimi, S.; Mahmoudian, M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its beta-carboline alkaloids. J. Pharm. Pharm. Sci. 2002, 5, 19–23. [Google Scholar] [PubMed]
- Yamashita, K.; Ohgaki, H.; Wakabayashi, K.; Nagao, M.; Sugimura, T. DNA adducts formed by the comutagens harman and norharman in various tissues of mice. Cancer Lett. 1988, 42, 179–183. [Google Scholar] [CrossRef]
- Funayama, Y.; Nishio, K.; Wakabayashi, K.; Nagao, M.; Shimoi, K.; Ohira, T.; Hasegawa, S.; Saijo, N. Effects of beta- and gamma-carboline derivatives of DNA topoisomerase activities. Mutat. Res. 1996, 349, 183–191. [Google Scholar] [CrossRef]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee. Life Sci. 2006, 78, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase is inhibited by tobacco smoke: Beta-carboline alkaloids act as potent and reversible inhibitors. Biochem. Biophys. Res. Commun. 2005, 326, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Kokuba, H. Harmol induces autophagy and subsequent apoptosis in U251MG human glioma cells through the downregulation of survivin. Oncol. Rep. 2013, 29, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Yamada, H.; Moriya, S.; Miyazawa, K. The beta-Carboline Alkaloid Harmol Induces Cell Death via Autophagy but Not Apoptosis in Human Non-small Cell Lung Cancer A549 Cells. Biol. Pharm. Bull. 2011, 34, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- El Gendy, M.A.M.; Soshilov, A.A.; Denison, M.S.; El-Kadi, A.O.S. Transcriptional and posttranslational inhibition of dioxin-mediated induction of CYP1A1 by harmine and harmol. Toxicol. Lett. 2012, 208, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Kawabata, H.; Tomita, I. Enhancing effect of heterocyclic amines and beta-carbolines on UV or chemically induced mutagenesis in E. coli. Mutat. Res. 1992, 268, 287–95. [Google Scholar] [CrossRef]
- Di Giorgio, C.; Delmas, F.; Ollivier, E.; Elias, R.; Balansard, G.; Timon-David, P. In vitro activity of the beta-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp. Parasitol. 2004, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Liu, Y.X.; Liu, Y.; Wang, L.Z.; Wang, Q.M. Synthesis and Antiviral and Fungicidal Activity Evaluation of β-Carboline, Dihydro-β-carboline, Tetrahydro-β-carboline Alkaloids, and Their Derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Kim, Y.H.; Park, G.T.; Lee, S.J. Beta-carboline alkaloids harmaline and harmalol induce melanogenesis through p38 mitogen-activated protein kinase in B16F10 mouse melanoma cells. J. Biochem. Mol. Biol. Ens. 2010, 43, 824–829. [Google Scholar] [CrossRef] [PubMed]
- El Gendy, M.A.M.; Soshilov, A.A.; Denisonb, M.S.; El-Kadi, A.O.S. Harmaline and harmalol inhibit the carcinogen-activating enzyme CYP1A1 via transcriptional and posttranslational mechanisms. Food Chem. Toxicol. 2012, 50, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.; Liu, Y.; Huang, Y.; Li, Y.; Wang, Q. Design, synthesis, anti-TMV, fungicidal, and insecticidal activity evaluation of 1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid derivatives based on virus inhibitors of plant sources. Bioorg. Med. Chem. Lett. 2014, 24, 5228–5233. [Google Scholar] [CrossRef] [PubMed]
- Love, B.E. Synthesis of β-carbolines. A review. Org. Prep. Proced. Int. 1996, 28, 1–64. [Google Scholar] [CrossRef]
- Silverman, B.D.; Daniel, E.P.; Mike, P.; Isidore, R. Comparative molecular moment analysis (CoMMA). Perspect. Drug Discov. Des. 1998, 12, 183–196. [Google Scholar] [CrossRef]
- Guan, H.J.; Chen, H.S.; Peng, W.L.; Ma, Y.; Cao, R.H.; Liu, X.D.; Xu, A.L. Design of β-carboline derivatives as DNA-targeting antitumor agents. Eur. J. Med. Chem. 2006, 41, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.H.; Guan, X.D.; Shi, B.X.; Chen, Z.Y.; Ren, Z.H.; Peng, W.L.; Song, H.C. Design, synthesis and 3D-QSAR of β-carboline derivatives as potent antitumor agents. Eur. J. Med. Chem. 2010, 45, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Milen, M.; Hazai, L.; Kolonits, P.; Kalaus, G.; Szabo, L.; GoMoRy, A.; SzaNtay, C. Studies on stereoselective approaches to β-carboline derivatives. Cent. Eur. J. Chem. 2005, 3, 118–136. [Google Scholar] [CrossRef]
- Dai, W.M.; Zhu, H.J.; Hao, X.J. Chiral ligands Dderived from abrine-3-Asymmetric pictet-spengler reaction of abrine methyl ester and synthesis of chiral 1,2,3,4-tetrahydro-β-carbolines as promoters in addition of diethylzinc toward aromatic aldehydes. Tetrahedron Lett. 1996, 37, 5971–5974. [Google Scholar] [CrossRef]
- Kuo, F.M.; Tseng, M.C.; Yen, Y.H.; Chu, Y.H. Microwave accelerated Pictet-Spengler reactions of tryptophan with ketones directed toward the preparation of 1,1-disubstituted indole alkaloids. Tetrahedron 2004, 60, 12075–12084. [Google Scholar] [CrossRef]
- Snyder, H.R.; Hansch, C.H.; Katz, L.; Parmerter, S.M.; Spaeth, E.C. The Synthesis of Derivatives of β-Carboline. II. Syntheses from dl-Tryptophan and Aldehydes. J. Am. Chem. Soc. 1948, 70, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Lippke, K.P.; Schunack, W.G.; Wenning, W. β-carbolines as benzodiazepine receptor ligands, synthesis and benzodiazepine receptor interaction of esters of β-carbolines-3-carboxylic acid. Med. Chem. 1983, 26, 499–503. [Google Scholar] [CrossRef]
- Borisovich, S.B.; Aleksandrovich, N.K.; Vadimovich, K.V. Method of production of 1- and 1,1-disubstituted-4-phenyl-2,3,4,9-tetrahydro-1H-β-carboline. R.U. Patent 2332418, 27 August 2004. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, S.; Zhu, S.; Luo, J.; Zhang, Y.; Weng, Q. Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives. Molecules 2015, 20, 13941-13957. https://doi.org/10.3390/molecules200813941
Li Z, Chen S, Zhu S, Luo J, Zhang Y, Weng Q. Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives. Molecules. 2015; 20(8):13941-13957. https://doi.org/10.3390/molecules200813941
Chicago/Turabian StyleLi, Zhibin, Shaohua Chen, Shaowen Zhu, Jianjun Luo, Yaomou Zhang, and Qunfang Weng. 2015. "Synthesis and Fungicidal Activity of β-Carboline Alkaloids and Their Derivatives" Molecules 20, no. 8: 13941-13957. https://doi.org/10.3390/molecules200813941





