Hepatocyte Growth Factor Mediates the Antifibrogenic Action of Ocimum bacilicum Essential Oil against CCl4-Induced Liver Fibrosis in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of OBE
| Retention Time (Rt) (min) | Components | Relative % |
|---|---|---|
| 5.1 | 1,8-Cineole | 13.90 |
| 7.1 | trans-Sabinene | 12.00 |
| 8.2 | iso-Menthone | 38.00 |
| 8.9 | Pulegone | 6.40 |
| 9.1 | L-Carvone | 3.50 |
| 9.4 | Methyl acetate | 1.80 |
| 10.9 | Methyl eugenol | 7.50 |
| 11.1 | trans-α-Bergamotene | 3.60 |
| 11.2 | α-Farnesene | 4.10 |
| 11.4 | α-Humulene | 0.90 |
| 12.1 | α-Amorphene | 2.00 |
| 12.3 | cis-α-Bisabolene | 1.30 |
| 12.8 | trans-Caryophyllene | 0.80 |
| 13.5 | δ Cadinene | 0.90 |
| 22.1 | Dioctyl phthalate | 2.90 |
| Total identified | 99.60 |
2.2. Effect of OBE on Liver Functions in CCl4-Treated Rats
| Parameter | Group I | Group II | Group III |
|---|---|---|---|
| ALT (U/L) | 19 ± 0.45 a | 1111.2 ± 34.5 c | 349.2 ± 16.70 b |
| AST (U/L) | 41 ± 1.70 a | 1005.8 ± 47.56 c | 313.8 ± 24.05 b |
2.3. Antioxidant Effect of OBE against CCl4-Induced Oxidative Liver Damage
| Parameter | Group I | Group II | Group III |
|---|---|---|---|
| MDA (µM/g liver) | 66.8 ± 2.5 a | 112.62 ± 3.4 c | 86.3 ± 3.6 b |
| NO (mmol/L) | 4.47 ± 0.27 a | 14.11 ± 0.20 c | 5.38 ± 0.63 b |
| Parameter | Group I | Group II | Group III |
|---|---|---|---|
| SOD (U/ mg protein) | 62.6 ± 2.1 c | 18.92 ± 1.08 a | 39.6 ± 1.03 b |
| CAT (U/ mg protein) | 1.95 ± 0.06 c | 0.883 ± 0.03 a | 1.3 ± 0.062 b |
| GSH (µM/g liver) | 10.6 ± 0.23 c | 2.4 ± 0.18 a | 6.2 ± 0.31 b |
| TAC (µmol/g liver) | 4.4 ± 0.05 c | 2.1 ± 0.06 a | 3.6 ± 0.02 b |
2.4. OBE Ameliorates CCl4-Induced Liver Fibrotic Injury
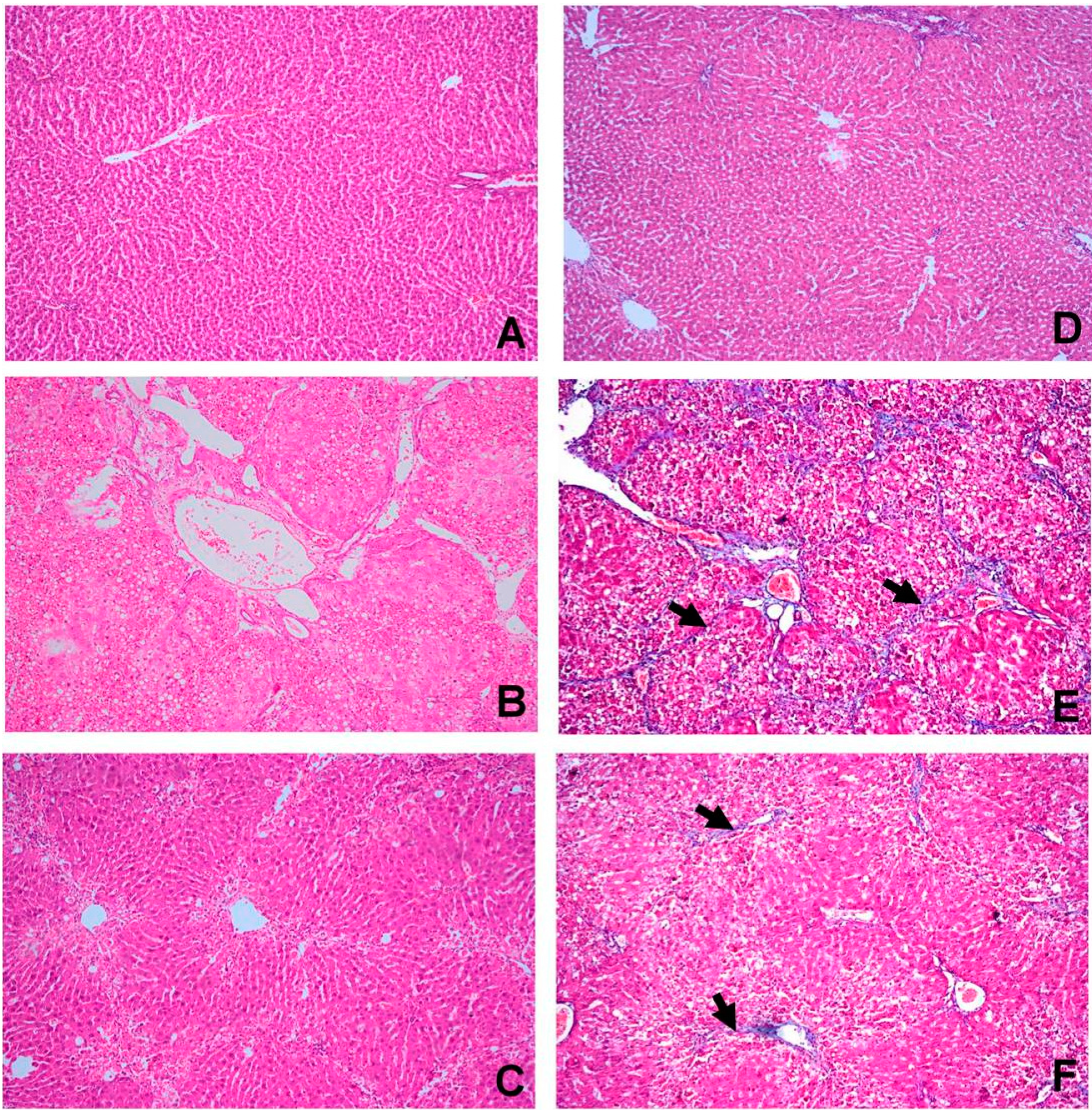
| Group | n | Pathological Grading of Hepatic Fibrosis | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | V | VI | |||
| Control | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| CCl4 model | 6 | 0 | 0 | 1 | 1 | 3 | 1 | 0 | 0.00 a |
| CCl4+OBE | 6 | 0 | 1 | 4 | 1 | 0 | 0 | 0 | 0.011 b |
2.5. OBE Inhibits α-SMA Expression in CCl4-Induced Fibrosis
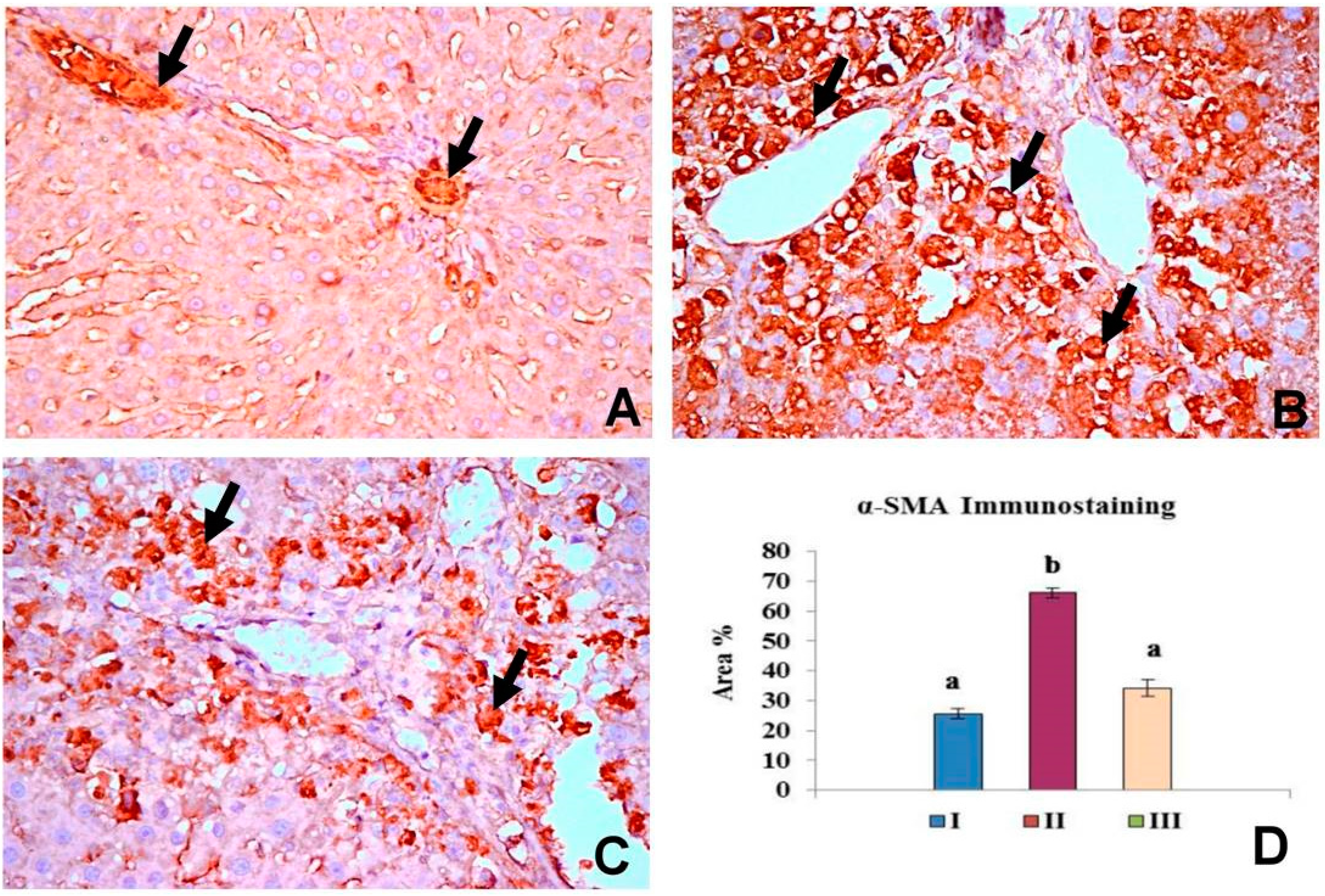
2.6. OBE Up-Regulates HGF Expression in CCl4-Induced Fibrosis

2.7. OBE Reverses the CYP2E1 Down-regulation Induced in a CCl4-Fibrosis Model

2.8. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation and Characterization of OBE
3.4. Animals and Experimental Design
3.5. Sampling
3.6. Biochemical Analyses
3.6.1. Hepatotoxicity Evaluation
3.6.2. Oxidative Stress Biomarkers and Antioxidant Profile
3.7. Histopathological Examination
3.8. Immunohistochemical Examination
3.9. Isolation of Total RNA and Real-Time PCR (qPCR)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sánchez-Valle, V.; Chávez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.D.; Kim, S.S.; Cha, H.Y.; Jang, S.H.; Chang, D.Y.; Kim, W.; Suh-Kim, H.; Lee, J.H. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp. Mol. Med. 2014, 46, e110. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Luedde, T.; Sauerbruch, T.; Scholten, D.; Streetz, K.; Tacke, F.; Tolba, R.; Trautwein, C.; Trebicka, J.; Weiskirchen, R. Experimental liver fibrosis research: Update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Weiskirchen, R. Update on hepatic stellate cells: Pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Breikaa, R.M.; Algandaby, M.M.; El-Demerdash, E.; Abdel-Naim, A.B. Multimechanistic Antifibrotic Effect of Biochanin A in Rats: Implications of Proinflammatory and Profibrogenic Mediators. PLoS ONE 2013, 8, e69276. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.T.; Gholami, H.; Kavoosi, G.; Rowshan, V.; Tafsiry, A. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Sci. Nutr. 2014, 2, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.A.; El-Abd, S.F.; Osman, M.; Kandil, A.M.; Helmy, M.S. Ameliorative Effect of Aqueous Leave Extract of Ocimum basilicum on CCl4-Induced Hepatotoxicity and Apoptosis in Albino Rats. J. Am. Sci. 2011, 7, 116–127. [Google Scholar]
- Meera, R.; Devi, P.; Kameswari, B.; Madhumitha, B.; Merlin, N.J. Antioxidant and hepatoprotective activities of Ocimum basilicum Linn. and Trigonella foenumgraecum Linn. against H2O2 and CCl4 induced hepatotoxicity in goat liver. Indian J. Exp. Biol. 2009, 47, 584–890. [Google Scholar] [PubMed]
- Marzouk, A.M. Hepatoprotective Triterpenes from Hairy Root Cultures of Ocimum basilicum L. Z. Naturforsch. C 2009, 64, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Moreno-Cuevas, J.E.; González-Garza, M.T.; Rodríguez-Montalvo, C.; Cruz-Vega, D.E. Liver Fibrosis and Protection Mechanisms Action of Medicinal Plants Targeting Apoptosis of Hepatocytes and Hepatic Stellate Cells. Adv. Pharmacol. Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bilal, A.; Jahan, N.; Ahmed, A.; Bilal, S.N.; Habib, S.; Hajra, S. Phytochemical and pharmacological studies on Ocimum basilicum linn—A review. Int. J. Curr. Res. Rev. 2012, 4, 73–83. [Google Scholar]
- Nahak, G.; Mishra, R.C.; Sahu, R.K. Phytochemical investigation and in vitro antioxidant evaluation of some Ocimum species. J. Pharm. Res. 2011, 4, 2340–2343. [Google Scholar]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.S.; Daoud, Z.; Nassar, M. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac. J. Trop. Med. 2012, 5, 294–298. [Google Scholar] [CrossRef]
- Yacout, G.A.; Elguindy, N.M.; El Azab, E.F. Hepatoprotective effect of basil (Ocimum basilicum L.) on CCl4-induced liver fibrosis in rats. Afr. J. Biotechnol. 2012, 11, 15702–15711. [Google Scholar] [CrossRef]
- Hassanpouraghdam, B.M.; Hassani, A.; Shalamzari, S.M. Menthone and Estragole-rich Essential Oil of Cultivated Ocimum basilicum L. from Northwest Iran. Chemija 2010, 21, 59–62. [Google Scholar]
- Ismail, M. Central properties and chemical composition of Ocimum basilicum essential oil. Pharm. Biol. 2006, 44, 619–626. [Google Scholar] [CrossRef]
- Stefan, M.; Zamfirache, M.M.; Padurariu, C.; Truta, E.; Gostin, I. The composition and antibacterial activity of essential oils in three Ocimum species growing in Romania. Cent. Eur. J. Biol. 2013, 8, 600–608. [Google Scholar] [CrossRef]
- Abduelrahman, A.H.N.; Elhussein, S.A.; Osman, N.A.; Nour, A.H. Morphological variability and chemical composition of essential oils from nineteen varieties of basil (Ocimum basilicum L.) growing in Sudan. Int. J. Chem. Technol. 2009, 1, 1–10. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Byun, S.; Kim, S.; Kim, M.; Park, D.; Lee, J. Isomenthone protects human dermal fibroblasts from TNF-α-induced death possibly by preventing activation of JNK and p38 MAPK. Food Chem. Toxicol. 2012, 50, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; p. 339. [Google Scholar]
- Tamayo, P.R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology 1983, 3, 112–120. [Google Scholar] [CrossRef]
- Lamireau, T.; Desmouliere, A.; Bioulac-Sage, P. Mechanisms of hepatic fibrogenesis. Arch. Pediatr. 2002, 9, 392–405. [Google Scholar] [CrossRef]
- Hsu, C.L.; Hsu, C.C.; Yen, G.C. Hepatoprotection by freshwater clam extract against CCl4-induced hepatic damage in rats. Am. J. Chin. Med. 2010, 38, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Marra, F.; Pinzani, M. Myofibroblast—Like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol. Asp. Med. 2008, 29, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Ahmed, D. Protective effects of Digera muricata (L.) Mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem. Toxicol. 2009, 47, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Iwai, S.; Karim, R.; Kitano, M.; Sukata, T.; Min, W.; Morimura, K.; Wanibuchi, H.; Seki, S.; Fukushima, S. Role of oxidative DNA damage caused by carbon tetrachloride-induced liver injury—Enhancement of MeIQ-induced glutathione S-transferase placental form-positive foci in rats. Cancer Lett. 2002, 179, 15–24. [Google Scholar] [CrossRef]
- Sagor, A.T.; Chowdhury, M.R.H.; Tabassum, N.; Hossain, H.; Rahman, M.; Alam, A. Supplementation of fresh ucche (Momordica charantia L. var. muricata Willd) prevented oxidative stress, fibrosis and hepatic damage in CCl4 treated rats. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdel Salam, O.M.; Sleem, A.A.; Shafee, N. Effect of trazodone and nefazodone on hepatic injury induced by carbon tetrachloride. Drug Discov. Ther. 2010, 4, 285–297. [Google Scholar] [PubMed]
- Leung, T.M.; Fung, M.L.; Liong, E.C.; Lau, T.Y.; Nanji, A.A.; Tipoe, G.L. Role of nitric oxide in the regulation of fibrogenic factors in experimental liver fibrosis in mice. Histol. Histopathol. 2011, 26, 201–211. [Google Scholar] [PubMed]
- Carpino, G.; Morini, S.; Ginanni Corradini, S.; Franchitto, A.; Merli, M.; Siciliano, M.; Gentili, F.; Onetti Muda, A.; Berloco, P.; Rossi, M.; et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig. Liver Dis. 2005, 37, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Jakovac, H.; Tomac, J.; Šain, I. Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol. Appl. Pharmacol. 2009, 241, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Parikh, J.G.; Kulkarni, A.; Johns, C. α-Smooth muscle actin-positive fibroblasts correlate with poor survival in hepatocellular carcinoma. Oncol. Lett. 2014, 7, 573–575. [Google Scholar] [PubMed]
- Rockey, D.C.; Weymouth, N.; Shi, Z. Smooth Muscle α Actin (Acta2) and Myofibroblast Function during Hepatic Wound Healing. PLoS ONE 2013, 8, e77166. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Robino, G. Oxidative stress-related molecules and liver fibrosis. J. Hepatol. 2001, 35, 297–306. [Google Scholar] [CrossRef]
- Teraoka, R.; Shimada, T.; Aburada, M. The molecular mechanisms of the hepatoprotective effect of gomisin A against oxidative stress and inflammatory response in rats with carbon tetrachloride-induced acute liver injury. Biol. Pharm. Bull. 2012, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.I.; Mars, W.M.; Michalopoulos, G.K. Signals and Cells Involved in Regulating Liver Regeneration. Cells 2012, 1, 1261–1292. [Google Scholar] [CrossRef] [PubMed]
- Tekkesin, N.; Taga, Y.; Sav, A.; Almaata, I.; İbrisim, D. Induction of HGF and VEGF in Hepatic Regeneration after Hepatotoxin-Induced Cirrhosis in Mice. Hepato-Gastroenterol. 2011, 58, 971–979. [Google Scholar]
- Zhang, J.; Zhou, S.; Zhou, Y.; Feng, F.; Wang, Q.; Zhu, X.; Ai, H.; Huang, X.; Zhang, X. Hepatocyte Growth Factor Gene-Modified Adipose-Derived Mesenchymal Stem Cells Ameliorate Radiation Induced Liver Damage in a Rat Model. PLoS ONE 2014, 9, e114670. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Uchiyama, K.; Nakamori, M.; Ueda, K.; Iwahashi, M.; Ueno, H.; Muragaki, Y.; Ooshima, A.; Yamaue, H. Combination gene therapy of HGF and truncated type II TGF-beta receptor for rat liver cirrhosis after partial hepatectomy. Surgery 2006, 139, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Gibran, A.; Masoud, M.S. Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl4-induced fibrotic liver. Saudi J. Gastroenterol. 2012, 18, 263–267. [Google Scholar]
- Xia, J.; Dai, C.; Michalopoulos, G.K.; Liu, Y. Hepatocyte Growth Factor Attenuates Liver Fibrosis Induced by Bile Duct Ligation. Am. J. Pathol. 2006, 168, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Xie, X.; Qiu, H.; Deng, Y.; Zhu, D.; Fan, J. Astragalus mongholicus ameliorates renal fibrosis by modulating HGF and TGF-β in rats with unilateral ureteral obstruction. J. Zhejiang Univ. Sci. B 2009, 10, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Riddick, D.S.; Lee, C.; Bhathena, A.; Timsit, Y.E.; Cheng, P.Y.; Morgan, E.T.; Prough, R.A.; Ripp, S.L.; Miller, K.K.; Jahan, A.; et al. Transcriptional suppression of cytochrome P450 genes by endogenous and exogenous chemicals. Drug Metab. Dispos. 2004, 32, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wong, C.C.; Cheng, K.W.; Huang, L.; Constantinides, P.P.; Rigas, B. Regioselective oxidation of phospho-NSAIDs by human cytochrome P450 and flavin monooxygenase isoforms: Implications for their pharmacokinetic properties and safety. Br. J. Pharmacol. 2012, 167, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [PubMed]
- Placer, Z.A.; Crushman, L.; Johnson, B.C. Estimation of product of lipid peroxidation (malondialdhyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kellin, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Nandi, A.; Chatterjee, I.B. Assay of superoxide dismutase activity in animal tissues. J. Biosci. 1988, 13, 305–315. [Google Scholar] [CrossRef]
- Sinha, K.A. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: London, UK; New York, NY, USA; Tokyo, Japan, 1996; Chapter 6; pp. 99–112. [Google Scholar]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; de Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Li, S.J.; Cao, Y.F.; Man, X.B.; Yu, W.F.; Wang, H.Y.; Wu, M.C. Different alterations of cytochrome P450 3A4 isoform and its gene expression in livers of patients with chronic liver diseases. World J. Gastroenterol. 2003, 9, 359–363. [Google Scholar] [PubMed]
- Galal, M.K.; Khalaf, A.A.; Ogaly, H.A.; Ibrahim, M.A. Vitamin E attenuates neurotoxicity induced by deltamethrin in rats. BMC Complement. Altern. Med. 2014, 14, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ogaly, H.A.; Khalaf, A.A.; Ibrahim, M.A.; Galal, M.K.; Abd-Elsalam, R.M. Influence of green tea extract on oxidative damage and apoptosis induced by deltamethrin in rat brain. Neurotoxicol. Teratol. 2015, 50, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogaly, H.A.; Eltablawy, N.A.; El-Behairy, A.M.; El-Hindi, H.; Abd-Elsalam, R.M. Hepatocyte Growth Factor Mediates the Antifibrogenic Action of Ocimum bacilicum Essential Oil against CCl4-Induced Liver Fibrosis in Rats. Molecules 2015, 20, 13518-13535. https://doi.org/10.3390/molecules200813518
Ogaly HA, Eltablawy NA, El-Behairy AM, El-Hindi H, Abd-Elsalam RM. Hepatocyte Growth Factor Mediates the Antifibrogenic Action of Ocimum bacilicum Essential Oil against CCl4-Induced Liver Fibrosis in Rats. Molecules. 2015; 20(8):13518-13535. https://doi.org/10.3390/molecules200813518
Chicago/Turabian StyleOgaly, Hanan A., Nadia A. Eltablawy, Adel M. El-Behairy, Hatim El-Hindi, and Reham M. Abd-Elsalam. 2015. "Hepatocyte Growth Factor Mediates the Antifibrogenic Action of Ocimum bacilicum Essential Oil against CCl4-Induced Liver Fibrosis in Rats" Molecules 20, no. 8: 13518-13535. https://doi.org/10.3390/molecules200813518





