Bioactive Cembranoids from the South China Sea Soft Coral Sarcophyton elegans
Abstract
:1. Introduction

2. Results and Discussion
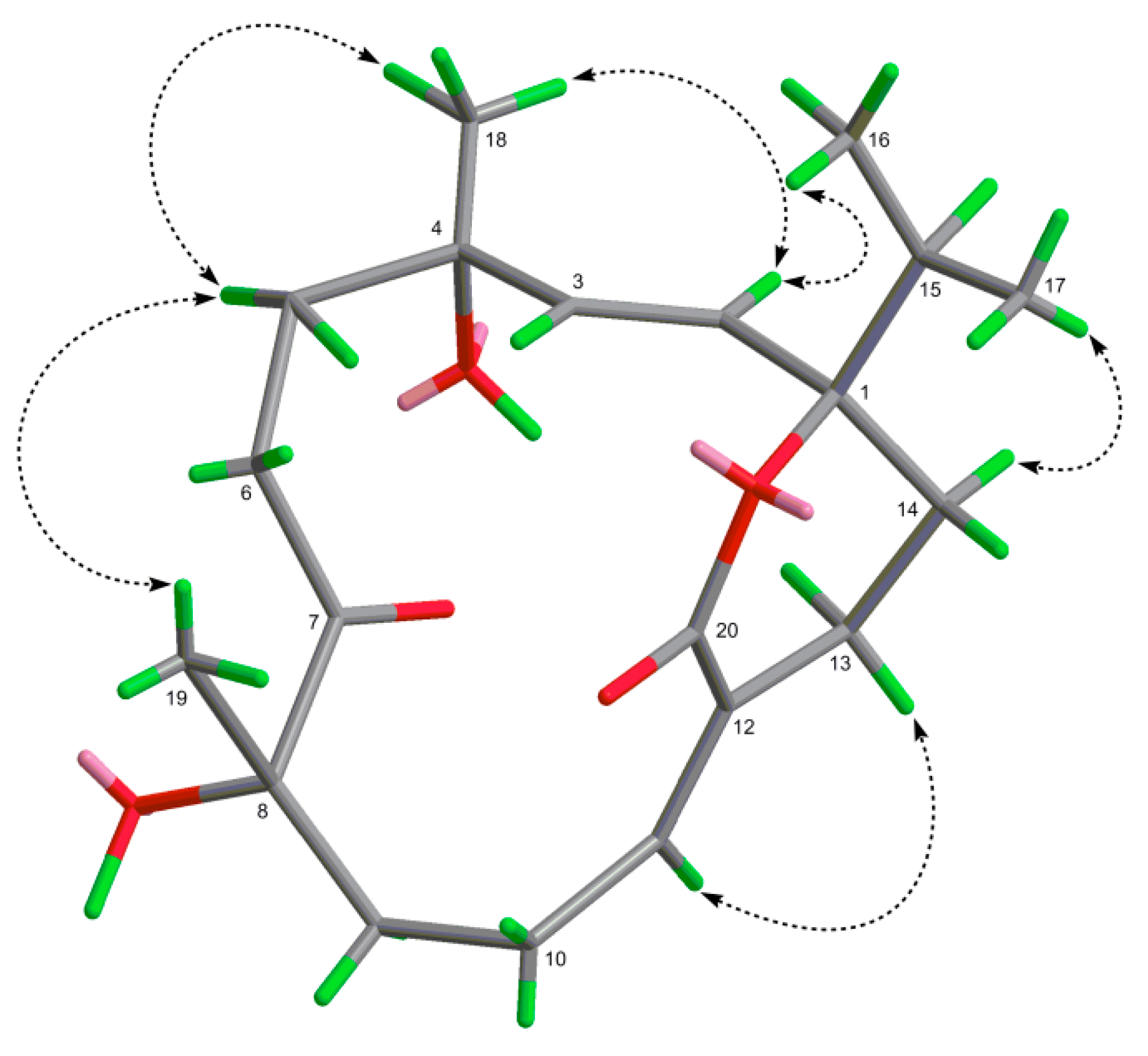
2.1. Structural Elucidation of New Compounds
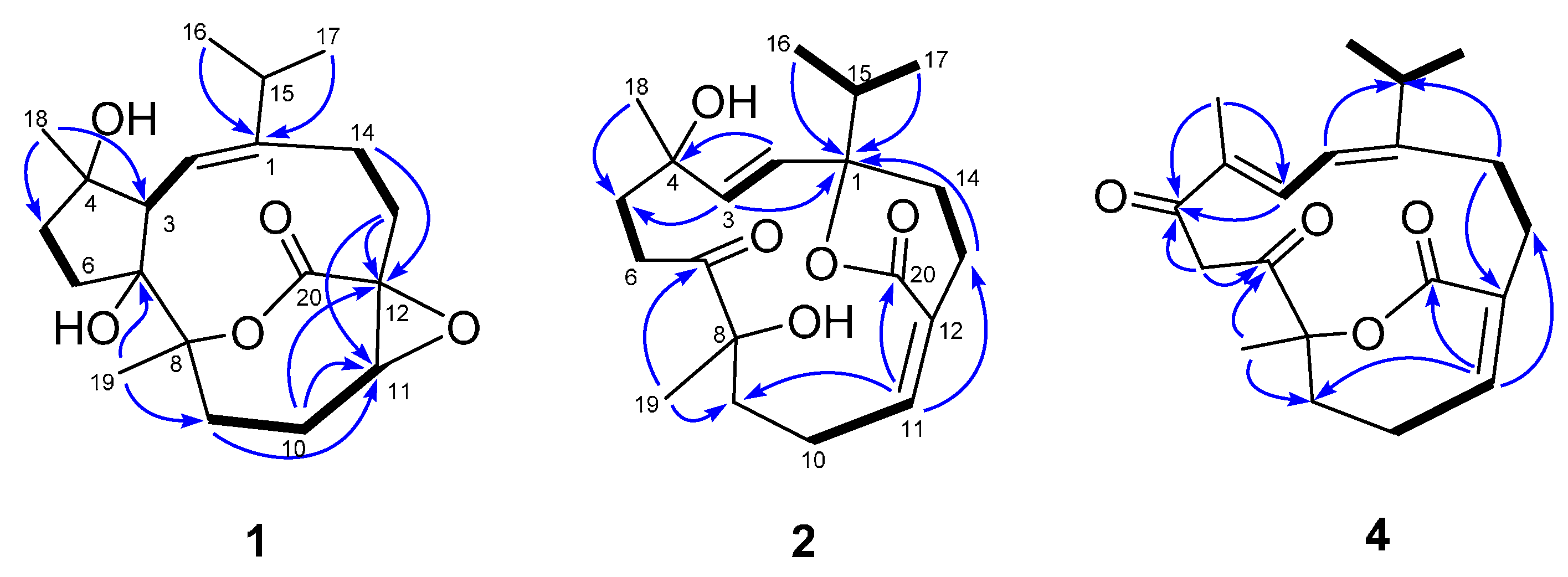
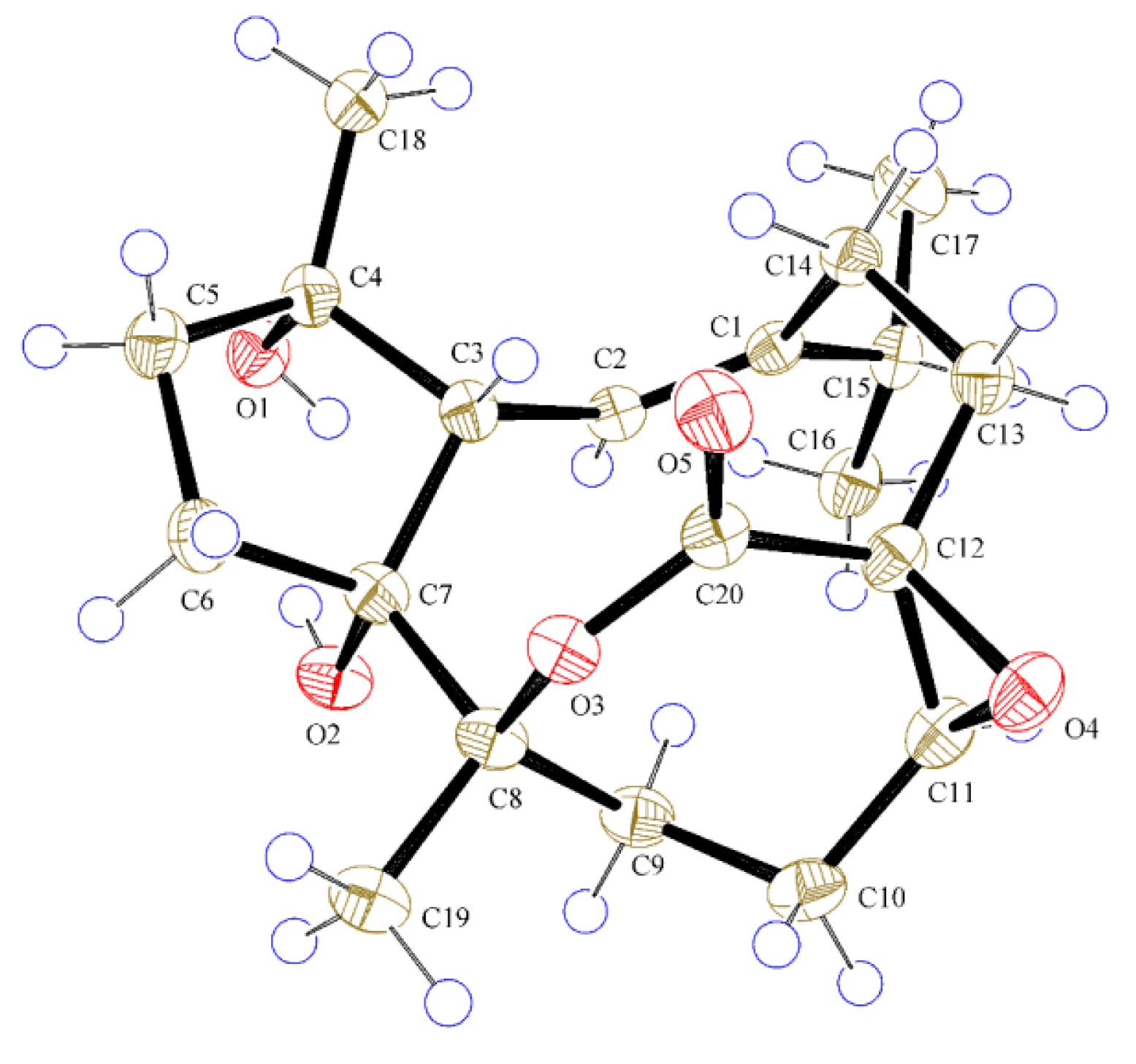
| Position | 1 a | 2 a | 3 a | 4 b |
|---|---|---|---|---|
| 2 | 5.69, d (11.3) | 5.55, d (16.7) | 5.54, d (16.3) | 6.23 d (11.4) |
| 3 | 2.35, m (overlapped) | 5.69, d (16.7) | 5.74, d (16.3) | 6.72, brd (11.4) |
| 5a | 1.98, m | 1.77, m (overlapped) | 1.83, m (overlapped) | |
| 5b | 1.87, m | 1.55, ddd (14.2, 10.5, 7.4) | 1.46, m | |
| 6a | 2.21, m | 3.04, ddd (20.9, 10.5, 7.4) | 1.70, m | 4.90, d (16.8) |
| 6b | 1.90, m | 2.44, m (overlapped) | 1.38, m | 3.29, d (16.8) |
| 7 | 3.25, brd (11.0, 1.4) | |||
| 9a | 2.82, dd (14.4, 14.4) | 1.96, m | 1.88, m | 2.40, m (overlapped) |
| 9b | 1.73, dd (14.4, 7.6) | 1.77, m (overlapped) | 1.79, m | 2.01, m |
| 10a | 2.31, m | 3.40, m | 3.44, m | 2.73, m |
| 10b | 1.38, m | 2.17, m | 2.05, m | 2.31, m |
| 11 | 3.13, dd (7.0, 7.0) | 5.67, m | 6.19, dd (10.1, 5.1) | 6.10, dd (4.1, 4.1) |
| 13a | 2.62, m | 2.45, m (overlapped) | 2.60, m | 3.19, m |
| 13b | 1.30, m | 2.55, m | 1.92, m | |
| 14a | 2.51, m | 2.12, m | 2.16, m | 2.56, dd (13.8, 13.8) |
| 14b | 2.12, m | 1.76 m | 1.84, m (overlapped) | 2.23, dd (13.8, 7.8) |
| 15 | 2.34, m (overlapped) | 1.86, m | 1.87, m | 2.41, m (overlapped) |
| 16 | 1.19, d (6.9) | 0.98, d (6.8) | 0.98, d (7.1) | 1.07, d (6.8) |
| 17 | 1.09, d (6.9) | 0.95, d (6.8) | 0.96, d (7.1) | 1.09, d (6.8) |
| 18 | 1.10, s | 1.39, s | 1.33, s | 1.83, s |
| 19 | 1.34, s | 1.21, s | 1.15, s | 1.54, s |
| Position | 1 a | 2 a | 3 a | 4 b |
|---|---|---|---|---|
| 1 | 147.1, C | 87.8, C | 88.0, C | 158.8, C |
| 2 | 120.1, CH | 128.2, CH | 128.3, CH | 119.4, CH |
| 3 | 52.0, CH | 140.4, CH | 140.8, CH | 137.8, CH |
| 4 | 83.4, C | 72.8, C | 73.4, C | 133.3, C |
| 5 | 38.5, CH2 | 36.5, CH2 | 41.0, CH2 | 195.1, C |
| 6 | 34.7, CH2 | 35.2, CH2 | 25.4, CH2 | 45.7, CH2 |
| 7 | 88.9, C | 219.3, C | 76.0, CH | 204.3, C |
| 8 | 91.9, C | 79.7, C | 75.8, C | 86.1, C |
| 9 | 29.9, CH2 | 42.0, CH2 | 39.0, CH2 | 33.3, CH2 |
| 10 | 24,1, CH2 | 25.9, CH2 | 25.5, CH2 | 27.2, CH2 |
| 11 | 65.6, CH | 150.3, CH | 149.5, CH | 143.6, CH |
| 12 | 60.4, C | 125.1, C | 125.5, C | 130.9, C |
| 13 | 33.0, CH2 | 25.0, CH2 | 25.8, CH2 | 37.1, CH2 |
| 14 | 25.8, CH2 | 27.5, CH2 | 27.5, CH2 | 27.6, CH2 |
| 15 | 34.0, CH | 38.4, CH | 38.9, CH | 36.3, CH |
| 16 | 21.3, CH3 | 17.4, CH3 | 17.4, CH3 | 22.4, CH3 |
| 17 | 23.9, CH3 | 17.1, CH3 | 17.1, CH3 | 21.7, CH3 |
| 18 | 26.2, CH3 | 29.3, CH3 | 30.2, CH3 | 10.9, CH3 |
| 19 | 25.1, CH3 | 28.5, CH3 | 24.4, CH3 | 28.9, CH3 |
| 20 | 173.9, C | 168.8, C | 169.5, C | 165.8, C |
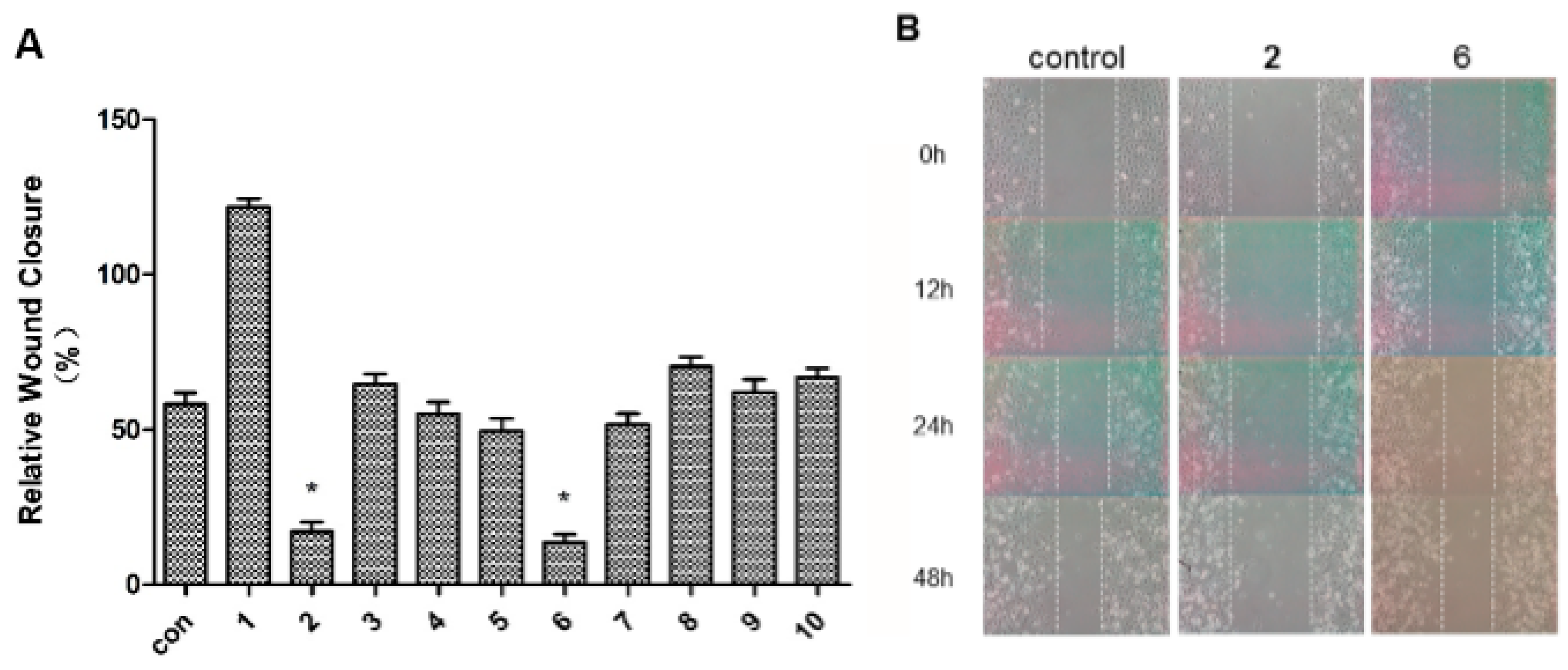
2.2. Antimigratory Activity
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Wound-Healing Assays
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.; Peng, L.; Zhang, T. Progress of studies on the natural cembranoids from the soft coral species of Sarcophyton genus. Med. Chem. Bioact. Nat. Prod. 2006, 19, 257–300. [Google Scholar]
- Gross, H.; Konig, G.M. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochem. Rev. 2006, 5, 115–141. [Google Scholar] [CrossRef]
- Jia, R.; Guo, Y.W.; Chen, P.; Yang, Y.M.; Mollo, E.; Gavagnin, M.; Cimino, G. Biscembranoids and their probable biogenetic precursor from the Hainan soft coral Sarcophyton tortuosum. J. Nat. Prod. 2007, 70, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Li, J.; Su, J.Y.; Liang, Y.J.; Yang, X.P.; Zheng, K.C.; Zeng, L.M. Cytotoxic diterpenoids from the soft coral Sarcophyton crassocaule. J. Nat. Prod. 2006, 69, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Youssef, D.T.A.; Reiland, J.; Ferniz, M.; Marchetti, D.; El Sayed, K.A. Biocatalytic and antimetastatic studies of the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine. J. Nat. Prod. 2006, 69, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Wright, A.D.; Beil, W.; Konig, G.M. Two new bicyclic cembranolides from a new Sarcophyton species and determination of the absolute configuration of sarcoglaucol-16-one. Org. Biomol. Chem. 2004, 2, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.; Kim, J.; Kim, S.; Kim, D. A general strategy for synthesis of both (6Z)- and (6E)-cladiellin diterpenes: Total syntheses of (−)-cladiella-6,11-dien-3-ol, (+)-polyanthellin A, (−)-cladiell-11-ene-3,6,7-triol, and (−)-deacetoxyalcyonin acetate. J. Am. Chem. Soc. 2006, 128, 15851–15855. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, M.T.; Stauffer, C.S.; Mans, M.C. Total syntheses of (+)-vigulariol and (−)-sclerophytin A. Org. Lett. 2011, 13, 4890–4893. [Google Scholar] [CrossRef] [PubMed]
- Bie, W.; Deng, Z.W.; Xu, M.J.; Lin, W.H. Structural elucidation of a new cembranoid diterpene from the Chinese soft coral Sarcophyton sp. J. Chin. Pharm. Sci. 2008, 17, 221–224. [Google Scholar]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; Leen, V.O.; Proksch, P.; Lin, W. Sarcophytolides G–L, new biscembranoids from the soft coral Sarcophyton elegans. Helv. Chim. Acta 2013, 96, 2218–2227. [Google Scholar] [CrossRef]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; Leen, V.O.; Proksch, P.; Lin, W. Sarcophyolides B–E, new cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2013, 11, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Minh, C.V.; Kiem, P.V.; Nguyen, H.N.; Nguyen, X.C.; Nguyen, P.T.; Nguyen, H.D.; Quang, T.H.; Nguyen, T.D.; Thung, D.C.; Thuy, D.T.T. Carotenoids from the soft coral Sarcophyton elegans. Tap Chi Hoa Hoc. 2010, 48, 627–631. [Google Scholar]
- Bishara, A.; Rudi, A.; Benayahu, Y.; Kashman, Y. Three biscembranoids and their monomeric counterpart cembranoid, a biogenetic Diels-Alder precursor, from the soft coral Sarcophyton elegans. J. Nat. Prod. 2007, 70, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, A.S.R.; Gowri, P.M.; Venugopal, M.J.R.V.; Sarada, P.; Murthy, M.V.R.K.; Rao, G.V.; Murthy, P.S.N.; Rao, C.V.; Kumar, G. Novel diterpenoids from the Indian Ocean soft coral Sarcophyton elegans. J. Ind. Chem. Soc. 1999, 76, 656–659. [Google Scholar]
- Moldowan, J.M.; Tursch, B.M.; Djerassi, C. 24ξ-Methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate, a novel polyhydroxylated steroid from an alcyonarian. Steroids 1974, 24, 387–398. [Google Scholar] [CrossRef]
- Moldowan, J.M.; Tan, W.L.; Djerassi, C. 24ξ-Methylcholestane-3β,5α,6β,12β,25-pentol 25-monoacetate, a novel polyoxygenated marine sterol. Steroids 1975, 26, 107–128. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Deng, Y.L.; Fan, C.Q.; Han, Q.H.; Lin, S.L.; Tang, G.H.; Luo, H.B.; Yin, S. Prostaglandin derivatives: nonaromatic phosphodiesterase-4 inhibitors from the soft coral Sarcophyton ehrenbergi. J. Nat. Prod. 2014, 77, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Cai, Y.H.; Fan, C.Q.; Tang, G.H.; Luo, H.B.; Yin, S. Six new tetraprenylated alkaloids from the South China Sea Gorgonian Echinogorgia pseudossapo. Mar. Drugs 2014, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.B.; Xiao, H.; Fan, C.Q.; Lu, Y.N.; Zhang, G.; Yin, S. Bioactive polyhydroxylated sterols from the marine sponge Haliclona crassiloba. Steroids 2013, 78, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.F.; Kurtan, T.; Mandi, A.; Gao, L.X.; Li, J.; Zhang, W.; Guo, Y.W. Sarsolenane and capnosane diterpenes from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller as PTP1B Iinhibitors. Eur. J. Org. Chem. 2014, 2014, 1841–1847. [Google Scholar] [CrossRef]
- Liang, L.F.; Lan, L.F.; Orazio, T.S.; Guo, Y.W. Sartrolides A–G and bissartrolide, new cembranolides from the South China Sea soft coral Sarcophyton trocheliophorum Marenzeller. Tetrahedron 2013, 69, 7381–7386. [Google Scholar] [CrossRef]
- Zou, G.A.; Ding, G.; Su, Z.H.; Yang, J.S.; Zhang, H.W.; Peng, C.Z.; Aisa, H.A.; Zou, Z.M. Lactonecembranoids from Croton laevigatus. J. Nat. Prod. 2010, 73, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Willis, R.H. Studies of Australian soft corals. XXVII. Two novel diterpenes from Sarcophyton glaucum. Aust. J. Chem. 1982, 35, 621–627. [Google Scholar] [CrossRef]
- Uchio, Y.; Nitta, M.; Nakayama, M.; Iwagawa, T.; Hase, T. Ketoemblide and sarcophytolide, two new cembranolides with ε-lactone function from the soft coral Sarcophyta elagans. Chem. Lett. 1983, 4, 613–616. [Google Scholar] [CrossRef]
- Huang, G.J.; Yang, C.M.; Chang, Y.S.; Amagaya, S.; Wang, H.C.; Hou, W.C.; Huang, S.S.; Hu, M.L. Hispolon suppresses SK-Hep1 human hepatoma cell metastasis by inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen activator through the PI3K/Akt and ERK signaling pathways. J. Agric. Food Chem. 2010, 58, 9468–9475. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Q.; Liu, X.F.; Cao, X.W.; Xue, M.Z.; Liu, K.D.; Zhao, Z.J.; Shen, X.; Jiang, H.L.; Xu, Y.F.; Huang, J.; et al. Shafts: A hybrid approach for 3D molecular similarity calculation. 2. Prospective case study in the discovery of diverse p90 ribosomal S6 protein kinase 2 inhibitors to suppress cell migration. J. Med. Chem. 2011, 54, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–10 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, J.; Liu, Q.; Tang, G.; Wang, H.; Fan, C.; Yin, S. Bioactive Cembranoids from the South China Sea Soft Coral Sarcophyton elegans. Molecules 2015, 20, 13324-13335. https://doi.org/10.3390/molecules200713324
Liu X, Zhang J, Liu Q, Tang G, Wang H, Fan C, Yin S. Bioactive Cembranoids from the South China Sea Soft Coral Sarcophyton elegans. Molecules. 2015; 20(7):13324-13335. https://doi.org/10.3390/molecules200713324
Chicago/Turabian StyleLiu, Xin, Junsheng Zhang, Qiao Liu, Guihua Tang, Hongsheng Wang, Chengqi Fan, and Sheng Yin. 2015. "Bioactive Cembranoids from the South China Sea Soft Coral Sarcophyton elegans" Molecules 20, no. 7: 13324-13335. https://doi.org/10.3390/molecules200713324





