Chloro({2-[mesityl(quinolin-8-yl-κN)boryl]-3,5-dimethyl-phenyl}methyl-κC)palladium(II) as a Catalyst for Heck Reactions
Abstract
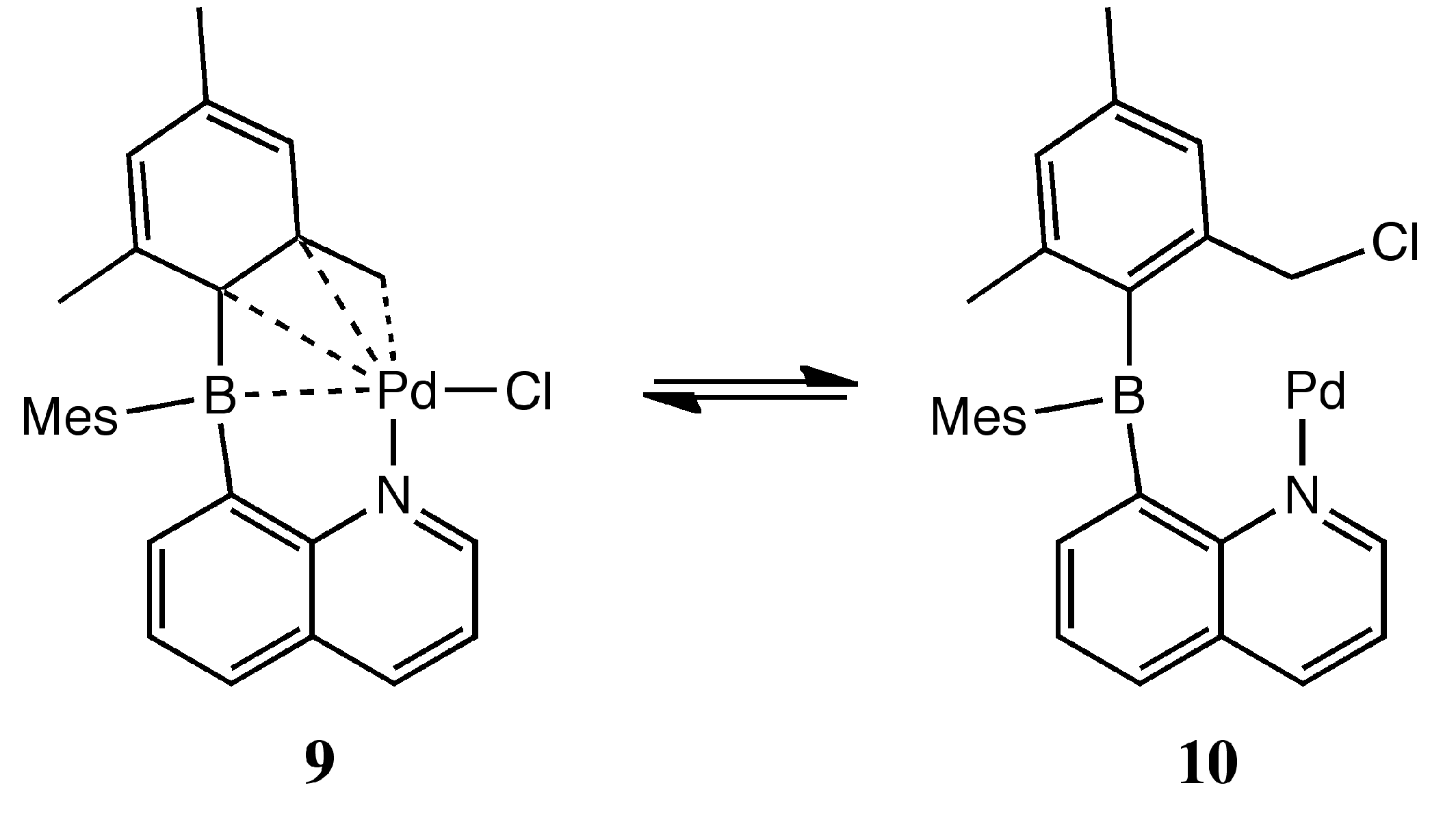
:1. Introduction


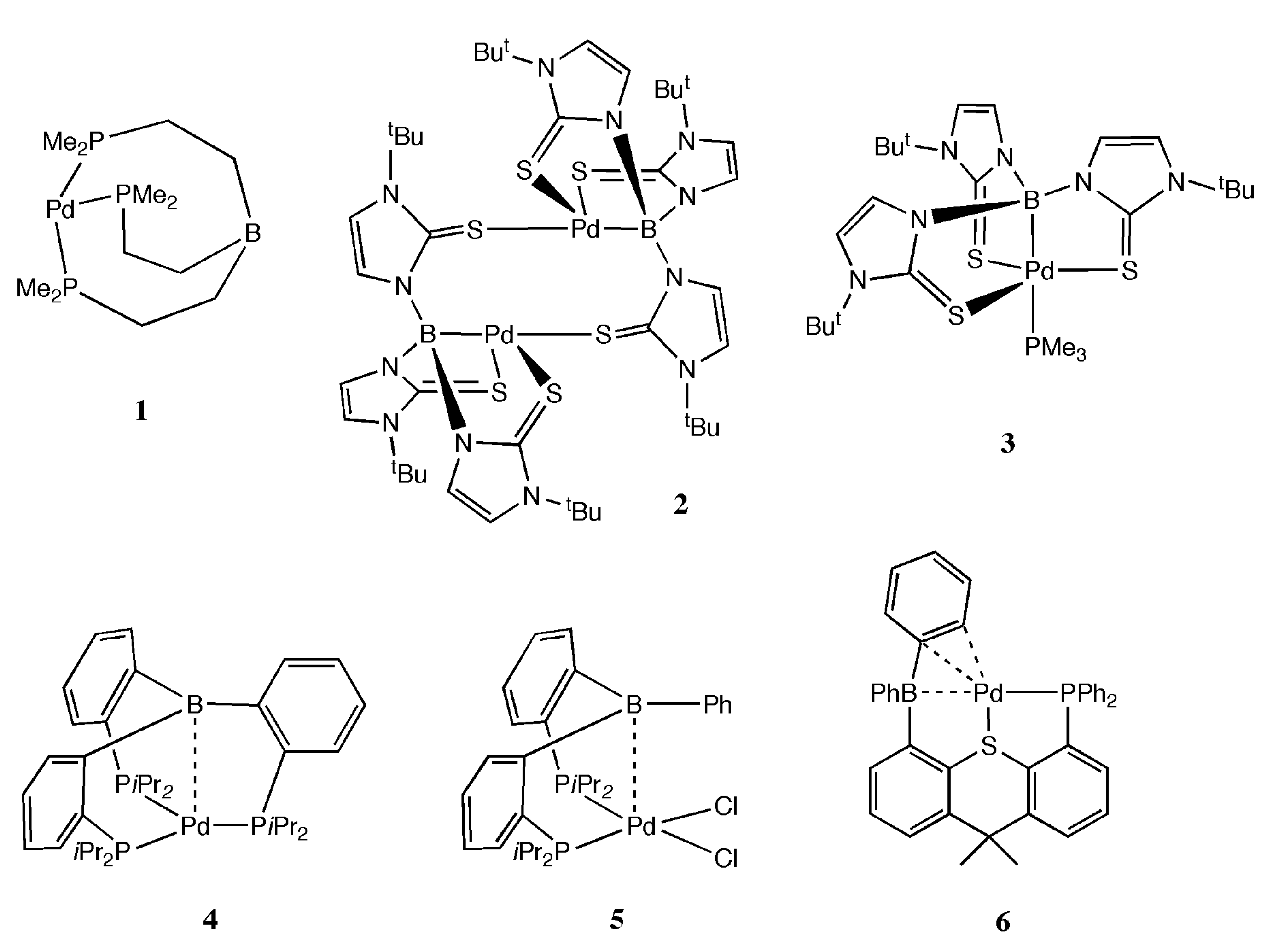
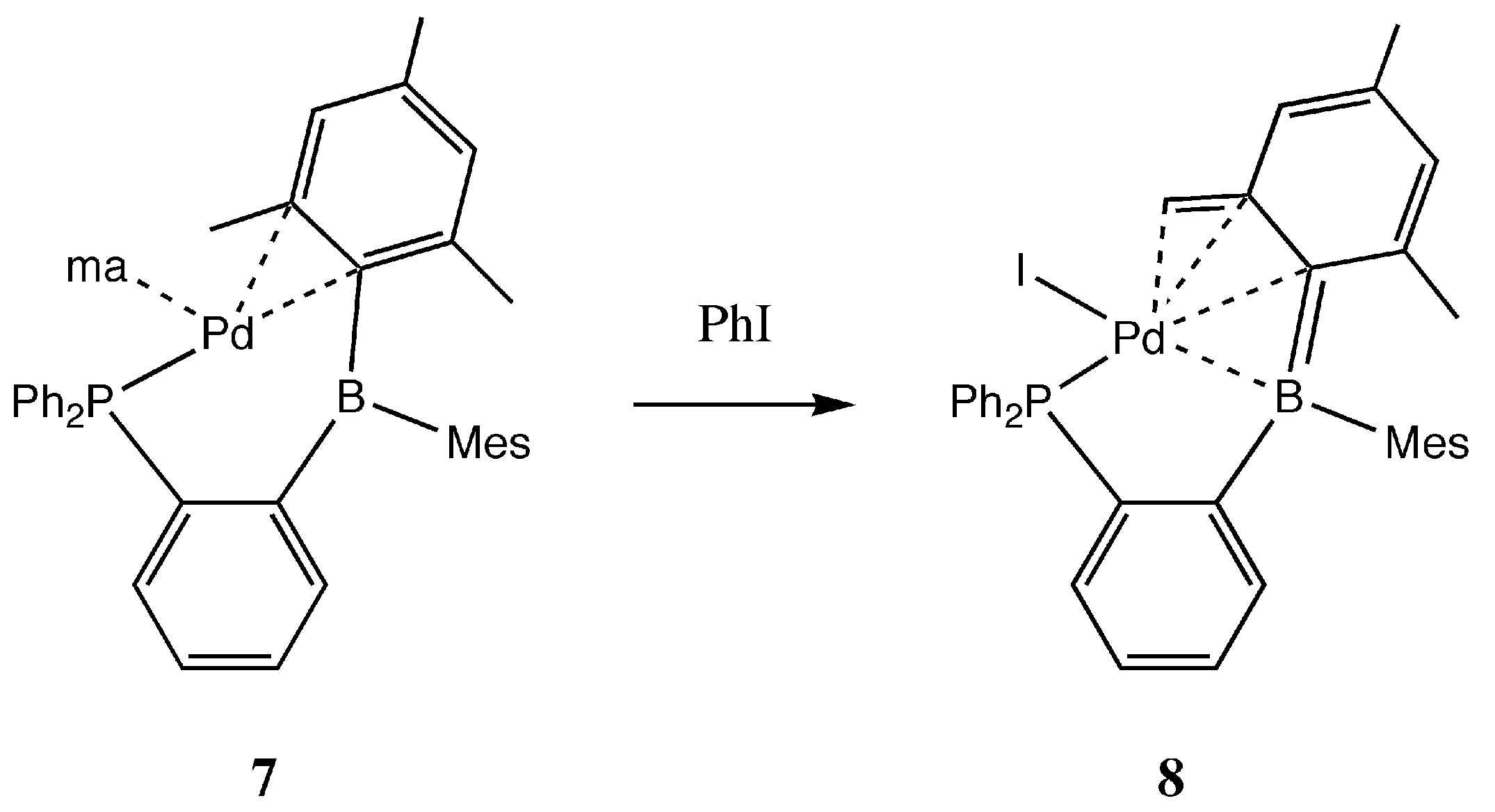
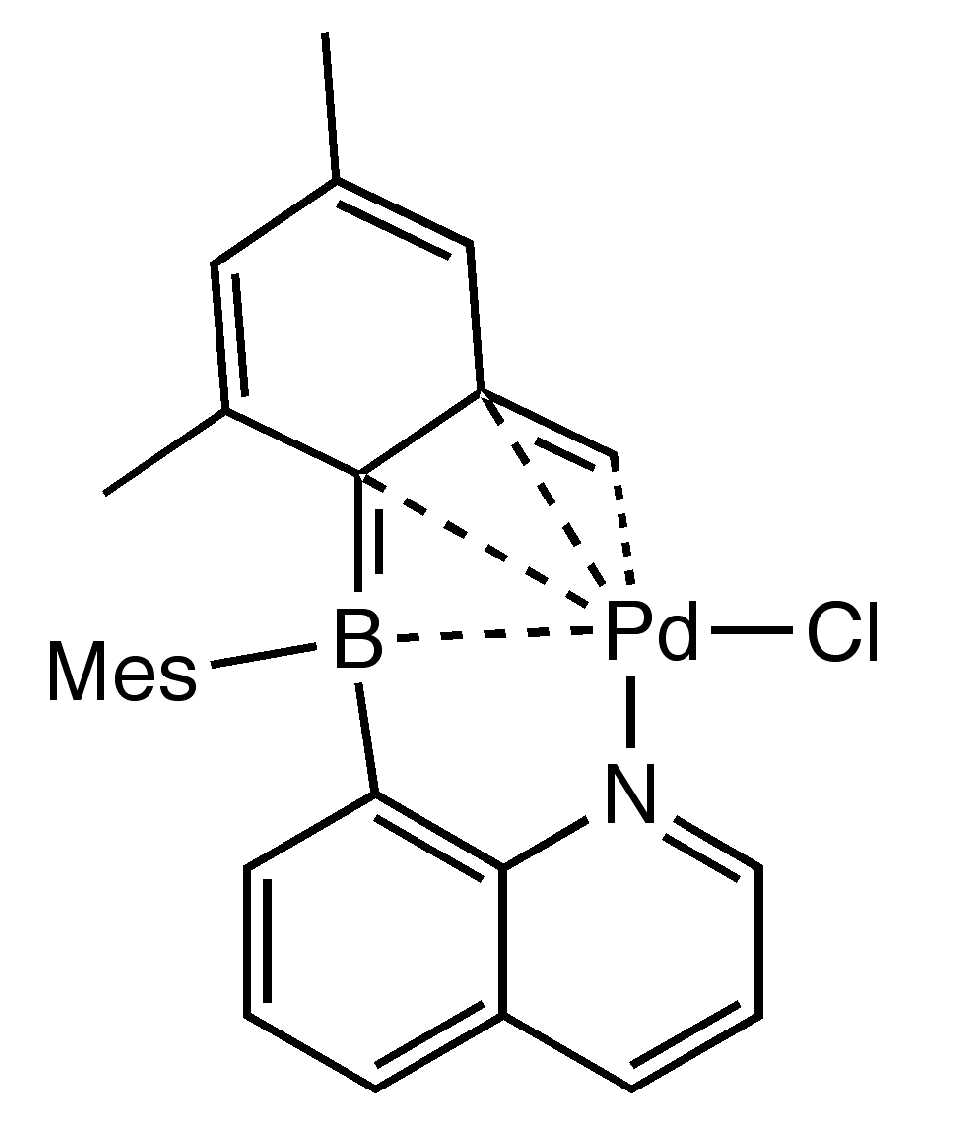
2. Results and Discussion

| Catalyst | X | R | mol % Catalyst | T (°C) | Time/h | Yield (%) a | TON |
|---|---|---|---|---|---|---|---|
| 9 | I | H | 0.05 | 140 | 5 | 88 | 1800 |
| 9 | I | H | 0.01 | 140 | 5 | 94 | 9400 |
| 9 | I | H | 0.005 | 140 | 10 | 89 | 18,000 |
| 9 | I | H | 0.001 | 140 | 20 | 90 | 90,000 |
| 9 | I | p-NO2 | 0.01 | 140 | 10 | 83 | 8300 |
| 9 | I | p-OCH3 | 0.01 | 140 | 10 | 79 | 7900 |
| 9 | Br | p-CN | 0.01 | 140 | 10 | 58 | 5800 |
| 9 | Br | H | 0.01 | 140 | 5 | 0 | 0 |
| Quinoline:PdCl2 = 2:1 | I | H | 0.01 | 140 | 5 | 93 | 9300 |
| Pd(OAc)2 [54] | I | H | 0.5 | 80 | 2 | 96 | 192 |
| PR3:Pd(OAc)2 = 2:1 [49] | Br | p-C(O)CH3 | 0.0001 | 130 | 24 | 100 | 106 |
| Ref. [47] | I | H | 0.01 | 130 | 30 | 87 | 8700 |
| Ref. [46] | I | H | 0.01 | 145 | 20 | 95 | 9500 |
| Ref. [46] | Br | p-OCH3 | 0.01 | 145 | 20 | 93 | 9300 |
| Ref. [44] | I | H | 0.0007 | 160 | 14 | 100 | 1.4 × 105 |
| Pd@PDEB [45] | I | H | 0.001 | 100 | 48 | 100 | 105 |
| Ref. [50] | I | H | 2.73 × 10−5 | 100 | 145 | 99 | 3.6 × 106 |
| Ref. [52] | I | H | 10−8 | 160 | 72 | 98 | 1010 |
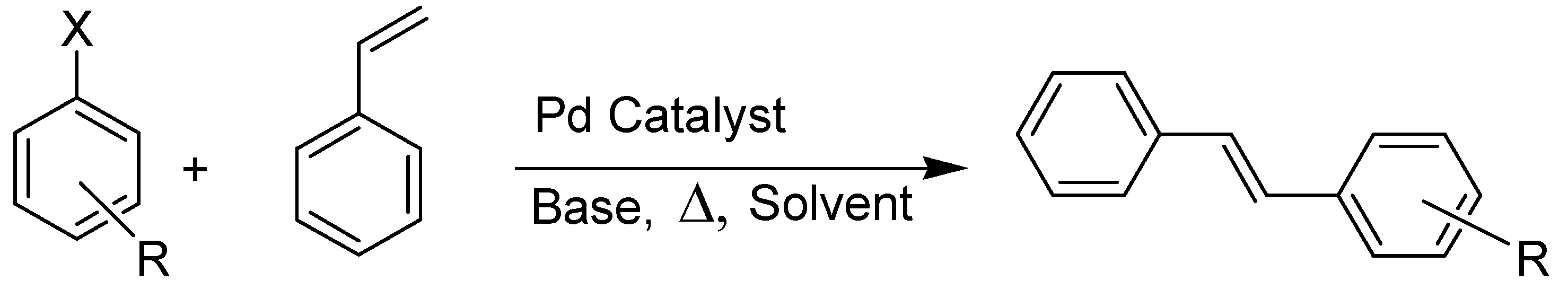
| Catalyst | X | R | mol % Catalyst | T (°C) | Time/h | Yield (%) a | TON |
|---|---|---|---|---|---|---|---|
| 9 | I | H | 0.01 | 140 | 5 | 20 | 2000 |
| 9 | I | H | 0.01 | 140 | 10 | 37 | 3700 |
| 9 | I | p-OCH3 | 0.01 | 140 | 10 | 42 | 4200 |
| 9 | Br | p-CN | 0.01 | 140 | 10 | 30 | 3000 |
| 9 | Br | H | 0.01 | 140 | 5 | 0 | 0 |
| PPh3:Pd(OAc)2 = 2:1 [49] | Br | p-C(O)CH3 | 0.0001 | 130 | 72 | 94 | 940,000 |
| Ref. [46] | I | H | 0.01 | 145 | 20 | 96 | 9600 |
| Ref. [46] | Br | p-OCH3 | 0.01 | 145 | 20 | 95 | 9500 |
| Ref. [44] | I | H | 0.0007 | 140 | 60 | 93 | 133,000 |
| Ref. [52] | Br | p-OCH3 | 0.001 | 160 | 16 | 97 | 97,000 |
| Ref. [51] | I | H | 0.0007 | 140 | 80 | 74 | 106,000 |
| Ref. [55] | Br | p-CHO | 1 | 80 | 15 | 95 | 95 |
| Ref. [56] | I | p-OCH3 | 2 | 120 | 8 | 94 | 47 |
| Ref. [57] | Br | p-C(O)CH3 | 0.00001 | 180 | 69 | 57.5 | 5,750,000 |
| Ref. [58] | Br | H | 0.005 | 140 | 1 | 83 | 16,600 |
3. Experimental Section
3.1. General Information
3.2. Heck Cross Coupling Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Li, H.; Johansson Seechurn, C.C.C.; Colacot, T.J. Development of Preformed Pd Catalysts for Cross-Coupling Reactions, Beyond the 2010 Nobel Prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Cabri, W.; Candiani, I. Recent Developments and New Perspectives in the Heck Reaction. Acc. Chem. Res. 1995, 28, 2–7. [Google Scholar] [CrossRef]
- Ozawa, F.; Kubo, A.; Hayashi, T. Generation of tertiary phosphine-coordinated Pd(0) species from Pd(OAc)2 in the catalytic Heck reaction. Chem. Lett. 1992, 11, 2177–2180. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Heck Reactions in the Presence of P(t-Bu)3: Expanded Scope and Milder Reaction Conditions for the Coupling of Aryl Chlorides. J. Org. Chem. 1999, 64, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.C. The Development of Versatile Methods for Palladium-Catalyzed Coupling Reactions of Aryl Electrophiles through the Use of P(t-Bu)3 and PCy3 as Ligands. Acc. Chem. Res. 2008, 41, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- We note that numerous supported Pd(0) catalysts, such as Pd/C or zeolite supported Pd, exist and that these are phosphine-free.
- Selvakumar, K.; Zapf, A.; Beller, M. New Palladium Carbene Catalysts for the Heck Reaction of Aryl Chlorides in Ionic Liquids. Org. Lett. 2002, 4, 3031–3033. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Alper, H. Highly Efficient, Recyclable Pd(II) Catalysts with Bisimidazole Ligands for the Heck Reaction in Ionic Liquids. Org. Lett. 2003, 5, 3209–3212. [Google Scholar] [CrossRef] [PubMed]
- Consorti, C.S.; Zanini, M.L.; Leal, S.; Ebeling, G.; Dupont, J. Chloropalladated Propargyl Amine: A Highly Efficient Phosphine-Free Catalyst Precursor for the Heck Reaction. Org. Lett. 2003, 5, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Kashin, A.N.; Karlstedt, N.B.; Mitin, A.V.; Cheprakov, A.V.; Kazankov, G.M. NC-palladacycles as highly effective cheap precursors for the phosphine-free Heck reactions. J. Organomet. Chem. 2001, 622, 89–96. [Google Scholar] [CrossRef]
- Mino, T.; Shirae, Y.; Sasai, Y.; Sakamoto, M.; Fujita, T. Phosphine-free palladium catalyzed Mizoroki-Heck reaction using hydrazone as a ligand. J. Org. Chem. 2006, 71, 6834–6839. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.S.; Pozebon, D.; Monteiro, A.L.; Dupont, J. On the use of phosphine-free PdCl2(SEt2)2 complex as catalyst precursor for the Heck reaction. Tetrahedron Lett. 2001, 42, 7345–7348. [Google Scholar] [CrossRef]
- Herrman, W.A.; Reisinger, C.P.; Spiegler, M. Chelating N-heterocyclic carbene ligands in palladium-catalyzed heck-type reactions. J. Organomet. Chem. 1998, 557, 93–96. [Google Scholar] [CrossRef]
- Joost, M.; Zeineddine, A.; Estevez, L.; Mallet-Ladeira, S.; Miqueu, K.; Amgoune, A.; Bourissou, D. Facile Oxidative Addition of Aryl Iodides to Gold(I) by Ligand Design: Bending Turns on Reactivity. J. Am. Chem. Soc. 2014, 136, 14654–14657. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W.; Erker, G. Frustrated Lewis Pairs: Metal-free Hydrogen Activation and More. Angew. Chem. Int. Ed. 2010, 49, 46–76. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W. Frustrated Lewis pairs: A new strategy to small molecule activation and hydrogenation catalysis. Dalton Trans. 2009, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Rokob, T.A.; Hamza, A.; Papai, I. Rationalizing the Reactivity of Frustrated Lewis Pairs: Thermodynamics of H2 Activation and the Role of Acid-Base Properties. J. Am. Chem. Soc. 2009, 131, 10701–10710. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.C.; San Juan, R.R.; Masuda, J.D.; Stephan, D.W. Reversible, Metal-Free Hydrogen Activation. Science 2006, 314, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.C.; Stephan, D.W. Facile Heterolytic Cleavage of Dihydrogen by Phosphines and Boranes. J. Am. Chem. Soc. 2007, 129, 1880–1881. [Google Scholar] [CrossRef] [PubMed]
- Momming, C.M.; Otten, E.; Kehr, G.; Frohlich, R.; Grimme, S.; Stephan, D.W.; Erker, G. Reversible Metal-Free Carbon Dioxide Binding by Frustrated Lewis Pairs. Angew. Chem. Int. Ed. 2009, 48, 6643–6646. [Google Scholar] [CrossRef] [PubMed]
- Berkefeld, A.; Piers, W.E.; Parvez, M. Tandem Frustrated Lewis Pair/Tris(pentafluorophenyl)borane-Catalyzed Deoxygenative Hydrosilylation of Carbon Dioxide. J. Am. Chem. Soc. 2010, 132, 10660–10661. [Google Scholar] [CrossRef] [PubMed]
- Ashley, A.E.; Thompson, A.L.; O’Hare, D. Non-Metal-Mediated Homogeneous Hydrogenation of CO2 to CH3OH. Angew. Chem. Int. Ed. 2009, 48, 9839–9843. [Google Scholar] [CrossRef] [PubMed]
- Appelt, C.; Westenberg, H.; Bertini, F.; Ehlers, A.W.; Slootweg, J.C.; Lammertsma, K.; Uhl, W. Geminal Phosphorus/Aluminum-Based Frustrated Lewis Pairs: C-H versus C≡C Activation and CO2 Fixation. Angew. Chem. Int. Ed. 2011, 50, 3925–3928. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, Y.; Liu, J.; Lin, Y.J.; Li, Z.H.; Wang, H. Synthesis and Reactivity of the CO2 Adducts of Amine/Bis(2,4,6-tris(trifluoromethyl)phenyl)borane Pairs. Organometallics 2013, 32, 6753–6758. [Google Scholar] [CrossRef]
- Rochette, E.; Courtemanche, M.A.; Pulis, A.P.; Bi, W.; Fontaine, F.G. Ambiphilic Frustrated Lewis Pair Exhibiting High Robustness and Reversible Water Activation: Towards the Metal-Free Hydrogenation of Carbon Dioxide. Molecules 2015, 20, 11902–11914. [Google Scholar]
- Roesler, R.; Piers, W.E.; Parvez, M. Synthesis, structural characterization and reactivity of the aminoborane 1-(NPh2)-2-[B(C6F5)2]C6H4. J. Organomet. Chem. 2003, 680, 218–222. [Google Scholar] [CrossRef]
- Fromel, S.; Frohlich, R.; Daniliuc, C.G.; Kehr, G.; Erker, G. Halogen Addition to a Frustrated Lewis Pair. Eur. J. Inorg. Chem. 2012, 23, 3774–3779. [Google Scholar] [CrossRef]
- Son, J.H.; Tamang, S.R.; Hoefelmeyer, J.D. Bis(3-bromomesityl)8-quinolyliniumboron(III) tribromide. Acta Cryst. 2015, in press. [Google Scholar]
- Sircoglou, M.; Bouhadir, G.; Saffon, N.; Miqueu, K.; Bourissou, D. A Zwitterionic Gold(I) Complex from an Ambiphilic Diphosphino–Alane Ligand. Organometallics 2008, 27, 1675–1678. [Google Scholar] [CrossRef]
- Dureen, M.A.; Welch, G.C.; Gilbert, T.M.; Stephan, D.W. Heterolytic Cleavage of Disulfides by Frustrated Lewis Pairs. Inorg. Chem. 2009, 48, 9910–9917. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.G.; Boudreau, J.; Thibault, M.H. Coordination Chemistry of Neutral (Ln)-Z Amphoteric and Ambiphilic Ligands. Eur. J. Inorg. Chem. 2008, 35, 5439–5454. [Google Scholar] [CrossRef]
- Amgoune, A.; Bourissou, D. σ-Acceptor, Z-type ligands for transition metals. Chem. Commun. 2011, 47, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Grobe, J.; Lutke-Brochtrup, K.; Krebs, B.; Lage, M.; Niemeyer, H.H.; Wurthwein, E.U. Alternative Ligands, XXXVII. Phosphane Ligands with Boron as Lewis-acidic Centre: Synthesis and Coordinating Properties. Z. Naturforsch. B 2006, 61, 882–895. [Google Scholar] [CrossRef]
- Pang, K.; Quan, S.M.; Parkin, G. Palladium complexes with Pd→B dative bonds: Analysis of the bonding in the palladaboratrane compound [κ4B(mimBut)3]Pd(PMe3). Chem. Commun. 2006, 42, 5015–5017. [Google Scholar] [CrossRef] [PubMed]
- Sircoglou, M.; Bontemps, S.; Bouhadir, G.; Saffon, N.; Miqueu, K.; Gu, W.; Mercy, M.; Chen, C.H.; Foxman, B.M.; Maron, L.; et al. Group 10 and 11 Metal Boratranes (Ni, Pd, Pt, CuCl, AgCl, AuCl, and Au+) Derived from a Triphosphine–Borane. J. Am. Chem. Soc. 2008, 130, 16729–16738. [Google Scholar]
- Bontemps, S.; Sircoglou, M.; Bouhadir, G.; Puschmann, H.; Howard, J.A.K.; Dyer, P.W.; Miqueu, K.; Bourissou, D. Ambiphilic Diphosphine–Borane Ligands: Metal→Borane Interactions within Isoelectronic Complexes of Rhodium, Platinum and Palladium. Chem. Eur. J. 2008, 14, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F. An Unambiguous Electron-Counting Notation for Metallaboratranes. Organometallics 2006, 25, 4741–4743. [Google Scholar] [CrossRef]
- Emslie, D.J.H.; Harrington, L.E.; Jenkins, H.A.; Robertson, C.M.; Britten, J.F. Group 10 Transition-Metal Complexes of an Ambiphilic PSB-Ligand: Investigations into η3(BCC)-Triarylborane Coordination. Organometallics 2008, 27, 5317–5325. [Google Scholar] [CrossRef]
- Sircoglou, M.; Bontemps, S.; Mercy, M.; Saffon, N.; Takahashi, M.; Bouhadir, G.; Maron, L.; Bourissou, D. Transition-Metal Complexes Featuring Z-Type Ligands: Agreement or Discrepancy between Geometry and dn Configuration? Angew. Chem. Int. Ed. 2007, 46, 8583–8586. [Google Scholar]
- Malacea, R.; Saffon, N.; Gomez, M.; Bourissou, D. A new insight into ortho-(dimesitylboryl)diphenylphosphines: Applications in Pd-catalyzed Suzuki–Miyaura couplings and evidence for secondary π-interaction. Chem. Commun. 2011, 47, 8163–8165. [Google Scholar] [CrossRef] [PubMed]
- Malacea, R.; Chahdoura, F.; Devillard, M.; Saffon, N.; Gomez, M.; Bourissou, D. ortho-(Dimesitylboryl)phenylphosphines: Positive Boryl Effect in the Palladium-Catalyzed Suzuki–Miyaura Coupling of 2-Chloropyridines. Adv. Synth. Catal. 2013, 355, 2274–2284. [Google Scholar] [CrossRef]
- Son, J.H.; Pudenz, M.A.; Hoefelmeyer, J.D. Reactivity of the Bifunctional Ambiphilic Molecule 8-(dimesitylboryl)quinoline: Hydrolysis and Coordination to Cu(I), Ag(I) and Pd(II). Dalton Trans. 2010, 39, 11081–11090. [Google Scholar] [CrossRef] [PubMed]
- Ohff, M.; Ohff, A.; van der Boom, M.E.; Milstein, D. Highly Active Pd(II) PCP-Type Catalysts for the Heck Reaction. J. Am. Chem. Soc. 1997, 119, 11687–11688. [Google Scholar] [CrossRef]
- Dong, Z.; Ye, Z. Reusable, Highly Active Heterogeneous Palladium Catalyst by Convenient Self-Encapsulation Cross-Linking Polymerization for Multiple Carbon—Carbon Cross-Coupling Reactions at ppm to ppb Palladium Loadings. Adv. Synth. Catal. 2014, 356, 3401–3414. [Google Scholar] [CrossRef]
- Kantam, M.L.; Srinivas, P.; Yadav, J.; Likhar, P.R.; Bhargava, S. Trifunctional N,N,O-Terdentate Amido/Pyridyl Carboxylate Ligated Pd(II) Complexes for Heck and Suzuki Reactions. J. Org. Chem. 2009, 74, 4882–4885. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, J.; Zhang, Z.P.; Fu, Y.; Liu, L.; Guo, Q.X. Pd(quinoline-8-carboxylate)2 as a Low-Priced, Phosphine-Free Catalyst for Heck and Suzuki Reactions. J. Org. Chem. 2007, 72, 9342–9345. [Google Scholar] [CrossRef] [PubMed]
- Farina, V. High-Turnover Palladium Catalysts in Cross-Coupling and Heck Chemistry: A Critical Overview. Adv. Synth. Catal. 2004, 346, 1553–1582. [Google Scholar] [CrossRef]
- Herrman, W.A.; Brossmer, C.; Reisinger, C.P.; Riermeier, T.H.; Ofele, K.; Beller, M. Palladacycles: Efficient New Catalysts for the Heck Vinylation of Aryl Halides. Chem. Eur. J. 1997, 3, 1357–1364. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, J.; Yun, H.; Cui, X.; Yuan, R. Cyclopalladated ferrocenylimines: Highly active catalysts for Heck reactions. J. Organomet. Chem. 2001, 637, 793–795. [Google Scholar] [CrossRef]
- Ohff, M.; Ohff, A.; Milstein, D. Highly active PdII cyclometallated imine catalysts for the Heck reaction. Chem. Commun. 1999, 357–358. [Google Scholar] [CrossRef]
- Alonso, D.A.; Najera, C.; Pacheco, M.C. Oxime-Derived Palladium Complexes as Very Efficient Catalysts for the Heck-Mizoroki Reaction. Adv. Synth. Catal. 2002, 344, 172–183. [Google Scholar] [CrossRef]
- Alonso, D.A.; Najera, C.; Pacheco, M.C. Oxime Palladacycles: Stable and Efficient Catalysts for Carbon–Carbon Coupling Reactions. Org. Lett. 2000, 2, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Bagherzadeh, M.; Moradi-Shoeili, Z.; Boghaei, D.M. Pd(OAc)2 without added ligand as an active catalyst for Mizoroki–Heck reaction in aqueous media. RSC Adv. 2012, 2, 12091–12095. [Google Scholar] [CrossRef]
- Özdemir, I.; Yiğit, M.; Çetinkaya, E.; Çetinkaya, B. Synthesis of novel palladium N-heterocyclic-carbene complexes as catalysts for Heck and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2006, 20, 187–192. [Google Scholar] [CrossRef]
- Wang, R.; Twamley, B.; Shreeve, J.M. A Highly Efficient, Recyclable Catalyst for C–C Coupling Reactions in Ionic Liquids: Pyrazolyl-Functionalized N-Heterocyclic Carbene Complex of Palladium(II). J. Org. Chem. 2005, 71, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Albisson, D.A.; Bedford, R.B.; Scully, P.N. Orthopalladated triarylphosphite complexes as highly efficient catalysts in the Heck reaction. Tetrahedron Lett. 1998, 39, 9793–9796. [Google Scholar] [CrossRef]
- Heidenreich, R.G.; Köhler, K.; Krauter, J.G.E.; Pietsch, J. Pd/C as a Highly Active Catalyst for Heck, Suzuki and Sonogashira Reactions. Synlett 2002, 1118–1122. [Google Scholar] [CrossRef]
- Herrman, W.A.; Bohm, V.P.W.; Reisinger, C.P. Application of palladacycles in Heck type reactions. J. Organomet. Chem. 1999, 576, 23–41. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Spencer, J. The Potential of Palladacycles: More than Just Precatalysts. Chem. Rev. 2005, 105, 2527–2571. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.; Hartwig, J.F. A Route to Pd0 from PdII Metallacycles in Amination and Cross-Coupling Chemistry. Angew. Chem. Int. Ed. 1996, 35, 2359–2360. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, J.R.; Tsai, F.Y. Palladium(II)/Cationic 2,2′-Bipyridyl System as Highly Efficient and Reusable Catalyst for the Mizoroki-Heck Reaction in Water. Molecules 2010, 15, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamang, S.R.; Hoefelmeyer, J.D. Chloro({2-[mesityl(quinolin-8-yl-κN)boryl]-3,5-dimethyl-phenyl}methyl-κC)palladium(II) as a Catalyst for Heck Reactions. Molecules 2015, 20, 12979-12991. https://doi.org/10.3390/molecules200712979
Tamang SR, Hoefelmeyer JD. Chloro({2-[mesityl(quinolin-8-yl-κN)boryl]-3,5-dimethyl-phenyl}methyl-κC)palladium(II) as a Catalyst for Heck Reactions. Molecules. 2015; 20(7):12979-12991. https://doi.org/10.3390/molecules200712979
Chicago/Turabian StyleTamang, Sem Raj, and James D. Hoefelmeyer. 2015. "Chloro({2-[mesityl(quinolin-8-yl-κN)boryl]-3,5-dimethyl-phenyl}methyl-κC)palladium(II) as a Catalyst for Heck Reactions" Molecules 20, no. 7: 12979-12991. https://doi.org/10.3390/molecules200712979






