Calophyllolide Content in Calophyllum inophyllum at Different Stages of Maturity and Its Osteogenic Activity
Abstract
:1. Introduction
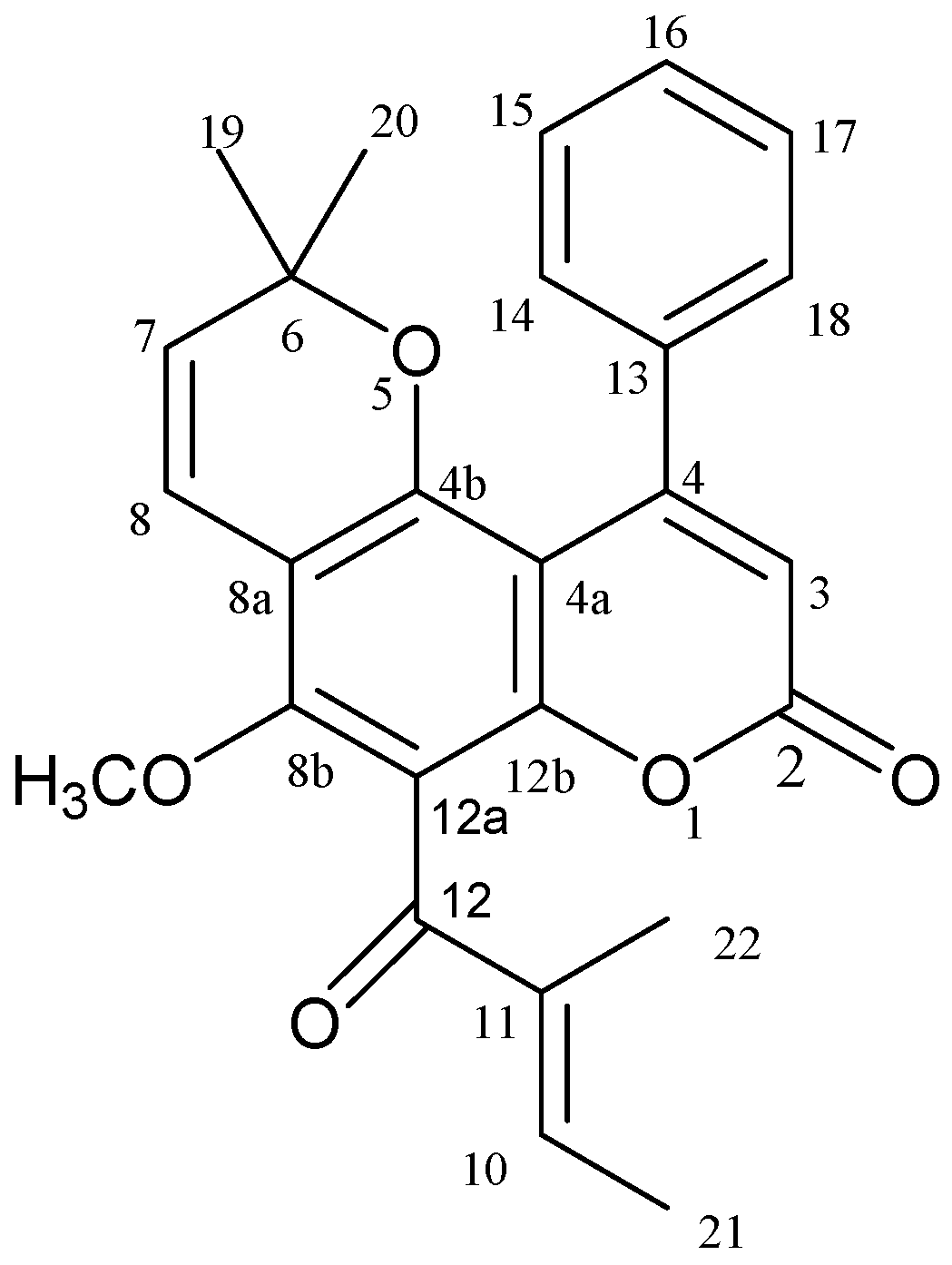
2. Results and Discussion
2.1. Calophyllolide Isolated from C. inophyllum Nuts
2.2. Quantification of the Calophyllolide in Nuts
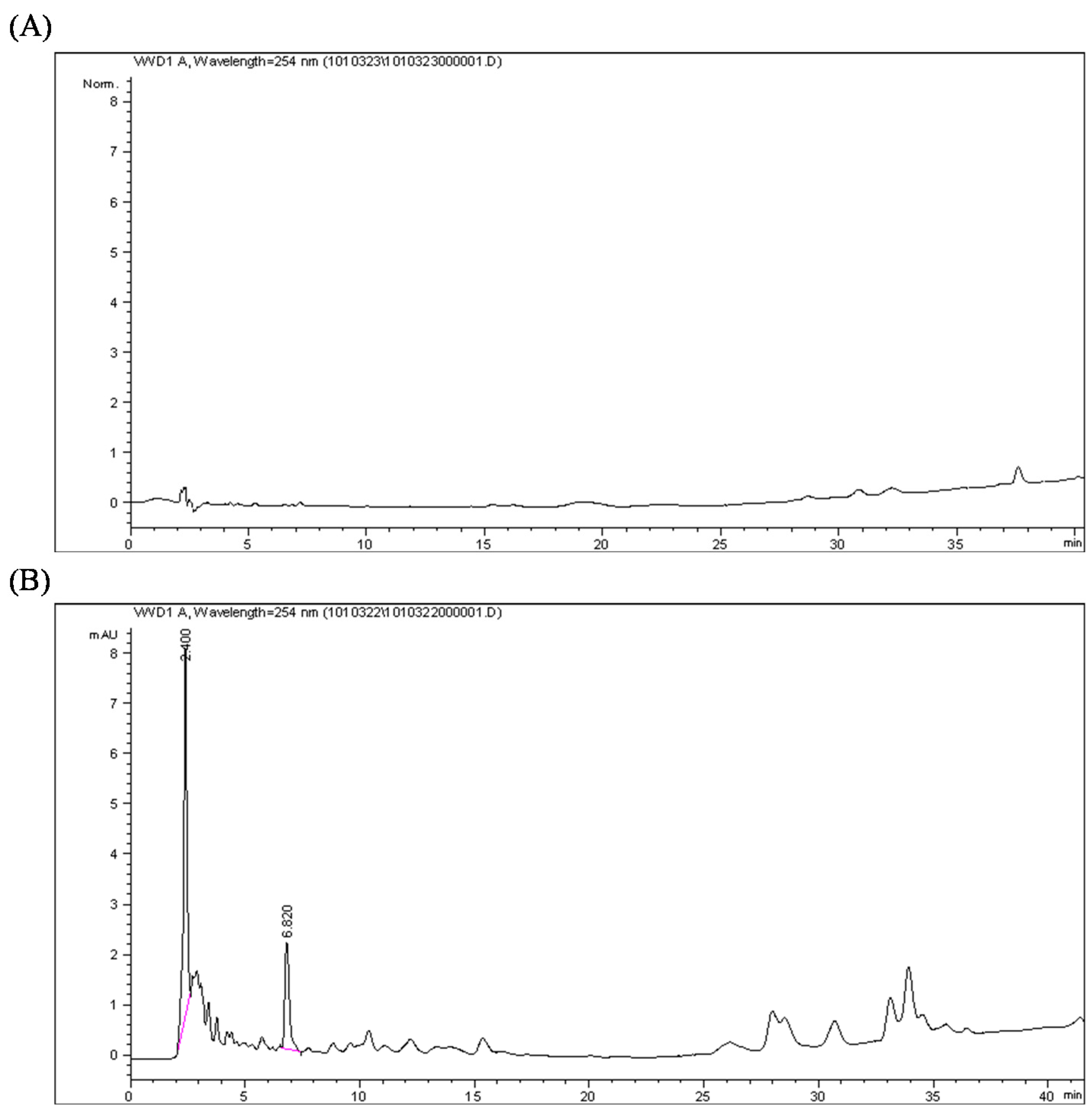

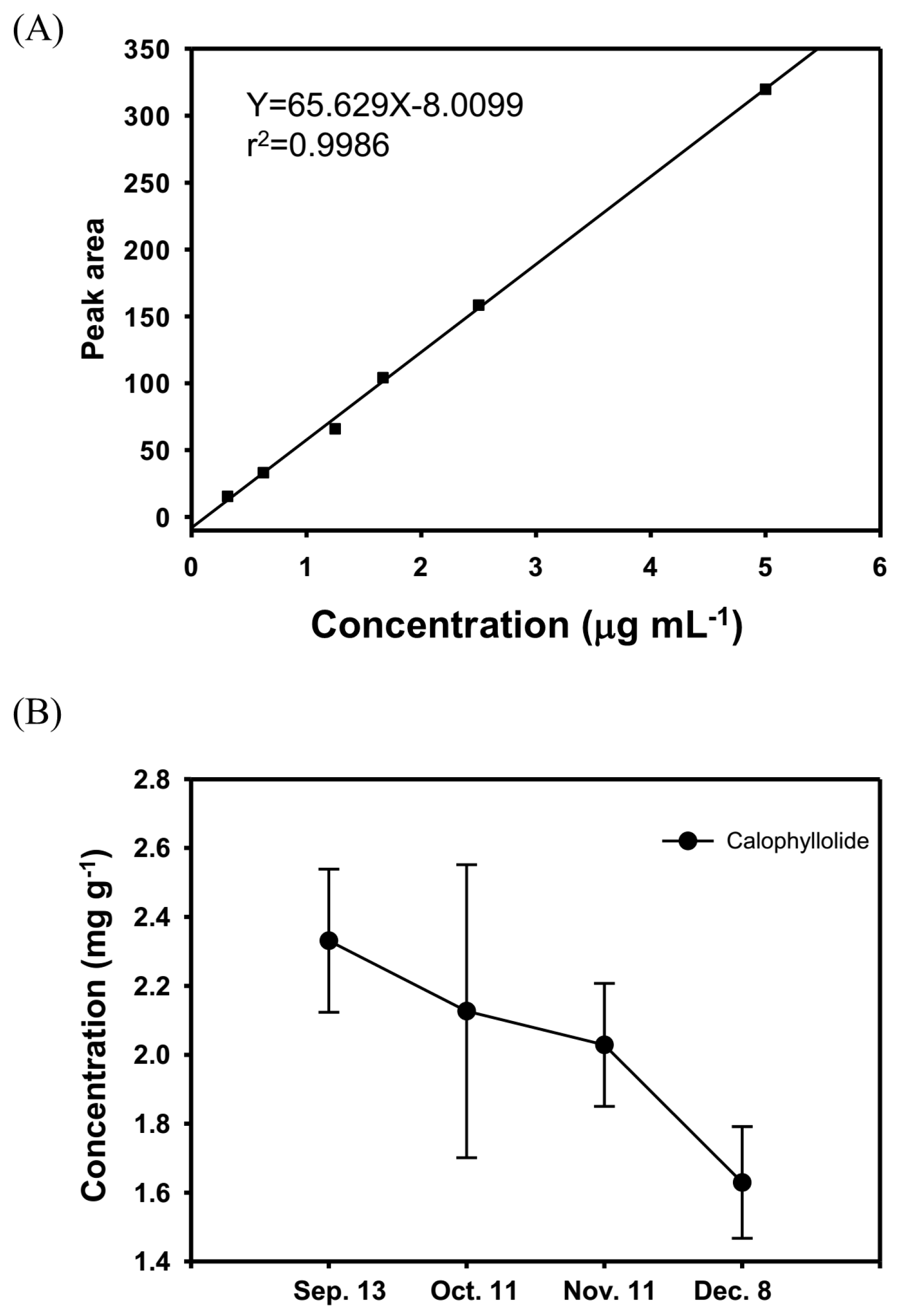
2.3. Calophyllolide-Induced Osteoblast MC3T3-E1 Cell Differentiation

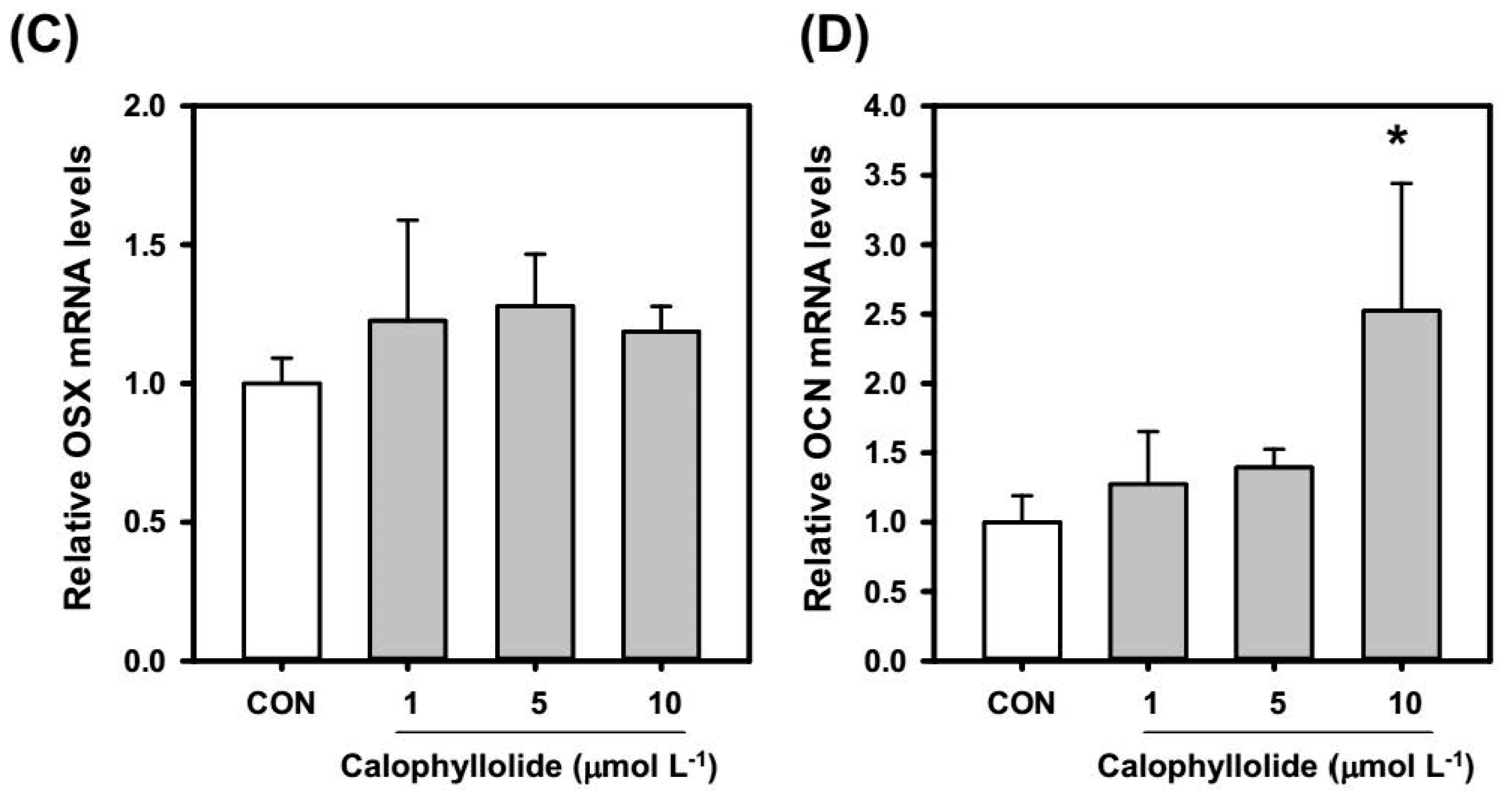
2.4. Calophyllolide Induced Osteoblastic Gene Expression in MC3T3-E1 Cells
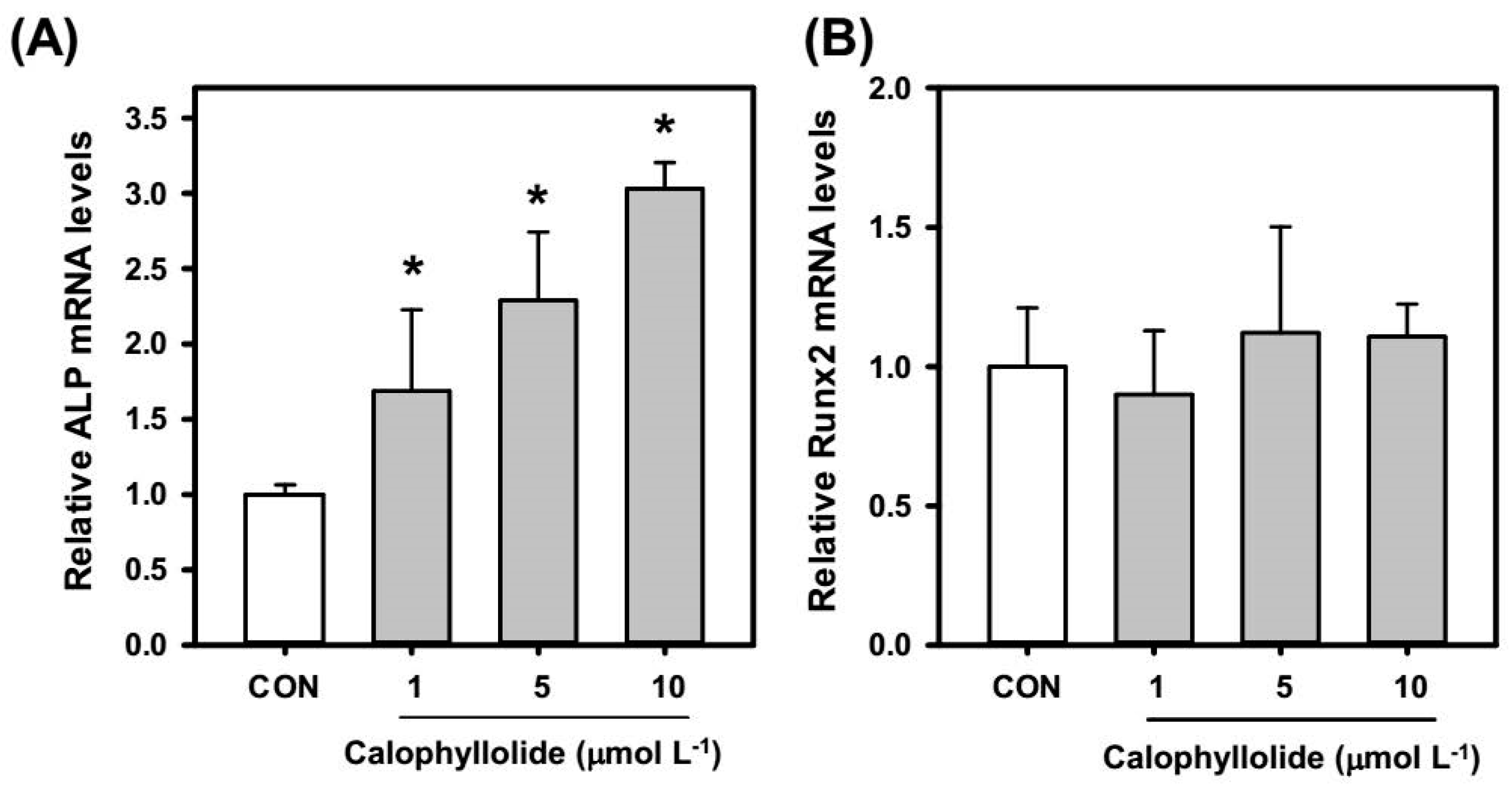
2.5. Calophyllolide Enhanced Mineralization and ALP Expression of MC3T3-E1 Cells

2.6. Discussion
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Chemicals
3.4. Calophyllolide Isolation, Purification and Identification
3.5. Calophyllolide
3.6. Quantitative Analysis of Calophyllolide in the Fruits of C. inophyllum
3.6.1. Preparation of the Calophyllolide Standard Solution
3.6.2. Preparation of the C. inophyllum Nut Sample Solutions
3.6.3. HPLC Analysis
3.7. Cell Culture and Differentiation of MC3T3-E1 Cells
3.8. Alkaline Phosphatase (ALP) Assay
3.9. Quantitative Real-Time RT-PCR
| Gene Name | Primer Sequence | Size (bp) | Annealing Temperature (°C) | Accession No. |
|---|---|---|---|---|
| ALP | F: 5′-TGACCTTCTCTCCTCCATCC-3′ | 126 | 55 | NM_001287176.1 |
| R: 5′-CTTCCTGGGAGTCTCATCCT-3′ | ||||
| RUNX2 | F: 5′-CCCTGAACTCTGCACCAAGT | 147 | 55 | NM_001145920.2 |
| R: 5′-TGGAGTGGATGGATGGGGAT | ||||
| OSX | F: 5′-GTCAAGAGTCTTAGCCAAACTC-3′ | 124 | 55 | NM_130458.3 |
| R: 5′-AAATGATGTGAGGCCAGATGG-3′ | ||||
| OCN | F: 5′-CAATAAGGTAGTGAACAGAC-3′ | 133 | 50 | NM_001305448.1 |
| R: 5′-CTTTAAGCCATACTGGTTT-3′ | ||||
| GAPDH | F: 5′-GTATGACTCCACTCACGGCAAA-3′ | 101 | 60 | NM_008084 |
| R: 5′-GGTCTCGCTCCTGGAAGATG-3′ |
3.10. Mineralized Matrix Formation Assay and ALP Staining
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prasad, J.; Shrivastava, A.; Khanna, A.K.; Bhatia, G.; Awasthi, S.K.; Narender, T. Antidyslipidemic and antioxidant activity of the constituents isolated from the leaves of Calophyllum inophyllum. Phytomedicine 2012, 19, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.J.; Mei, W.L.; Zhong, H.M.; Zeng, Y.B.; Wu, X.D.; Dai, H.F. A new prenylated xanthone from the branches of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011, 13, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ee, G.C.; Mah, S.H.; Rahmani, M.; Taufiq-Yap, Y.H.; Teh, S.S.; Lim, Y.M. A new furanoxanthone from the stem bark of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011, 13, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Hamzah, R.; Rahaman, A.A.; Jong, V.Y.; Khong, H.Y.; Rajab, N.F.; Ee, G.C.; Inayat-Hussain, S.H. The pyranoxanthone inophyllin a induces oxidative stress mediated-apoptosis in jurkat t lymphoblastic leukemia cells. Food Chem. Toxicol. 2012, 50, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Hung, M.C.; Wang, L.T.; Chen, C.Y. Inocalophyllins a, b and their methyl esters from the seeds of Calophyllum inophyllum. Chem. Pharm. Bull. 2003, 51, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Ueda, Y.; Iinuma, M.; Ishiguro, K. Inhibitory effects of xanthones from guttiferae plants on paf-induced hypotension in mice. Planta Med. 2005, 71, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Li, Z.L.; Yin, S.L.; Shi, G.; Liu, M.S.; Jing, Y.K.; Hua, H.M. Triterpenoids from Calophyllum inophyllum and their growth inhibitory effects on human leukemia HL-60 cells. Fitoterapia 2010, 81, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R.; Johnson, R.K.; et al. The inophyllums, novel inhibitors of hiv-1 reverse transcriptase isolated from the malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.B.; Mathur, C.N.; Seth, S.D.S. Calophyllolide, a complex coumarin anticoagulant from Calophyllum inophyllum Linn. J. Pharm. Pharmacol. 1962, 14, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Yimdjo, M.C.; Azebaze, A.G.; Nkengfack, A.E.; Meyer, A.M.; Bodo, B.; Fomum, Z.T. Antimicrobial and cytotoxic agents from Calophyllum inophyllum. Phytochemistry 2004, 65, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.T.; Kim, S.I.; Kwon, H.W.; Ahn, Y.J. Enhanced repellency of binary mixtures of Zanthoxylum piperitum pericarp steam distillate or Zanthoxylum armatum seed oil constituents and Calophyllum inophyllum nut oil and their aerosols to stomoxys calcitrans. Pest Manag. Sci. 2010, 66, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Liang, Y.H.; Chiang, J.H.; Liu, F.C.; Lin, W.H.; Chang, S.J.; Lin, W.Y.; Wu, C.H.; Weng, J.R. Anti-inflammatory effects of Calophyllum inophyllum l. In RAW264.7 cells. Oncol. Rep. 2012, 28, 1096–1102. [Google Scholar] [PubMed]
- Taylor, P.B.; Culp, J.S.; Debouck, C.; Johnson, R.K.; Patil, A.D.; Woolf, D.J.; Brooks, I.; Hertzberg, R.P. Kinetic and mutational analysis of human immunodeficiency virus type 1 reverse transcriptase inhibition by inophyllums, a novel class of non-nucleoside inhibitors. J. Biol. Chem. 1994, 269, 6325–6331. [Google Scholar] [PubMed]
- Saxena, R.C.; Nath, R.; Palit, G.; Nigam, S.K.; Bhargava, K.P. Effect of calophyllolide, a nonsteroidal anti-inflammatory agent, on capillary permeability. Planta Med. 1982, 44, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Murata, T.; Itoigawa, M.; Nakao, K.; Kaneda, N.; Furukawa, H. Apoptosis inducing activity of 4-substituted coumarins from calophyllum brasiliense in human leukaemia hl-60 cells. J. Pharm. Pharmacol. 2006, 58, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Huang, Y.L.; Liu, Y.W. (+)-Vitisin A inhibits osteoclast differentiation by preventing traf6 ubiquitination and traf6-tak1 formation to suppress nfatc1 activation. PLoS ONE 2014, 9, e89159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Liu, Y.W.; Huang, Y.J.; Chiou, W.F. A special ingredient (VtR) containing oligostilbenes isolated from Vitis thunbergii prevents bone loss in ovariectomized mice: In vitro and in vivo study. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Lee, C.H.; Liao, J.F.; Chen, C.C. 8-prenylkaempferol accelerates osteoblast maturation through bone morphogenetic protein-2/p38 pathway to activate runx2 transcription. Life Sci. 2011, 88, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, L.; Mohan Kumar, R.; Vishweshwar, P.; Pichai, R.; Narasimhan, S. 5-Meth-oxy-2,2-dimethyl-6-[(2E)-2-methyl-but-2-eno-yl]-10-phenyl-2H,8H-pyrano[2,3-f]chromen-8-one (calophyllolide). Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o1115. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.J.; Josephs, J.L. Synthesis of the calophyllum coumarins. Part 2. J. Chem. Soc. Perkin Trans. 1995, 1, 3135–3152. [Google Scholar] [CrossRef]
- Laure, F.; Raharivelomanana, P.; Butaud, J.F.; Bianchini, J.P.; Gaydou, E.M. Screening of anti-hiv-1 inophyllums by HPLC-DAD of Calophyllum inophyllum leaf extracts from french polynesia islands. Anal. Chim. Acta 2008, 624, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, T.N.; Saxena, R.C.; Nigam, S.K.; Misra, G.; Bhargava, K.P. Calophyllolide—A new non-steroidal anti-inflammatory agent. Indian J. Med. Res. 1980, 72, 762–765. [Google Scholar] [PubMed]
- Soteriou, G.A.; Kyriacou, M.C.; Siomos, A.S.; Gerasopoulos, D. Evolution of watermelon fruit physicochemical and phytochemical composition during ripening as affected by grafting. Food Chem. 2014, 165, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Gull, J.; Sultana, B.; Anwar, F.; Naseer, R.; Ashraf, M.; Ashrafuzzaman, M. Variation in antioxidant attributes at three ripening stages of guava (Psidium guajava L.) fruit from different geographical regions of pakistan. Molecules 2012, 17, 3165–3180. [Google Scholar] [CrossRef] [PubMed]
- Amira el, A.; Behija, S.E.; Beligh, M.; Lamia, L.; Manel, I.; Mohamed, H.; Lotfi, A. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J. Agric. Food Chem. 2012, 60, 10896–10902. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liang, C.; Yang, S.Y.; Ra, J.C.; Choi, E.M.; Kim, J.A.; Kim, Y.H. Phenolic compounds from Artemisia iwayomogi and their effects on osteoblastic mc3t3-e1 cells. Biol. Pharm. Bull. 2010, 33, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Yang, R.S.; Chien, M.Y.; Chen, C.C.; Fu, W.M. Enhancement of bone morphogenetic protein-2 expression and bone formation by coumarin derivatives via p38 and erk-dependent pathway in osteoblasts. Eur. J. Pharmacol. 2008, 579, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Z.; Yang, F.; Yang, Z.; Huang, J.; Shi, Q.; Chen, D.; Wang, Y.J. Psoralen stimulates osteoblast differentiation through activation of bmp signaling. Biochem. Biophys. Res. Commun. 2011, 405, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Huang, Y.T.; Chang, C.H.; Chang, J.K. Bone morphogenetic protein-2 and -4 (BMP-2 and -4) mediates fraxetin-induced maturation and differentiation in human osteoblast-like cell lines. Biol. Pharm. Bull. 2006, 29, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Z.; Hou, W.; Zhou, Q.; Zhang, M.; Holz, J.; Sheu, T.J.; Li, T.F.; Cheng, S.D.; Shi, Q.; Harris, S.E.; et al. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-bmp signaling. J. Bone Miner. Res. 2010, 25, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2015. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin k promotes osteoblastic differentiation and mineralization by activation of p38 MAPK- and ERK-mediated expression of Runx2 and Osterix. Eur. J. Pharmacol. 2011, 668, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, T.; Tada, K.; Kitajima, I.; Tojo, K.; Sampath, T.K.; Kawabata, M.; Miyazono, K.; Imamura, T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999, 112, 3519–3527. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compound calophyllolide is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-H.; Liu, Y.-W.; Chen, Z.-F.; Chiou, W.-F.; Tsai, Y.-C.; Chen, C.-C. Calophyllolide Content in Calophyllum inophyllum at Different Stages of Maturity and Its Osteogenic Activity. Molecules 2015, 20, 12314-12327. https://doi.org/10.3390/molecules200712314
Liu W-H, Liu Y-W, Chen Z-F, Chiou W-F, Tsai Y-C, Chen C-C. Calophyllolide Content in Calophyllum inophyllum at Different Stages of Maturity and Its Osteogenic Activity. Molecules. 2015; 20(7):12314-12327. https://doi.org/10.3390/molecules200712314
Chicago/Turabian StyleLiu, Wei-Hsien, Yen-Wenn Liu, Zih-Fong Chen, Wen-Fei Chiou, Ying-Chieh Tsai, and Chien-Chih Chen. 2015. "Calophyllolide Content in Calophyllum inophyllum at Different Stages of Maturity and Its Osteogenic Activity" Molecules 20, no. 7: 12314-12327. https://doi.org/10.3390/molecules200712314





