Biofunctional Constituents from Michelia compressa var. lanyuensis with Anti-Melanogenic Properties
Abstract
:1. Introduction
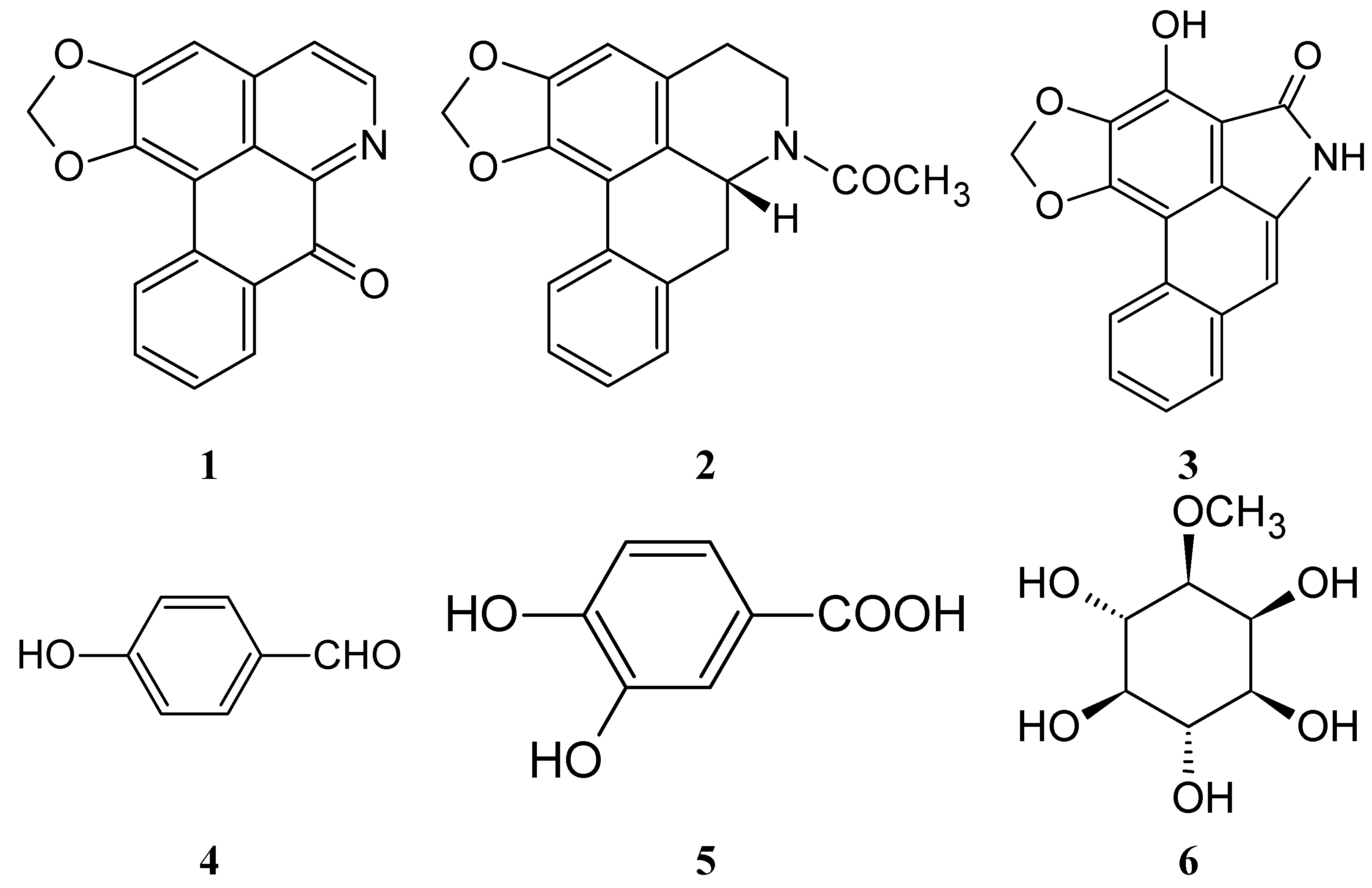
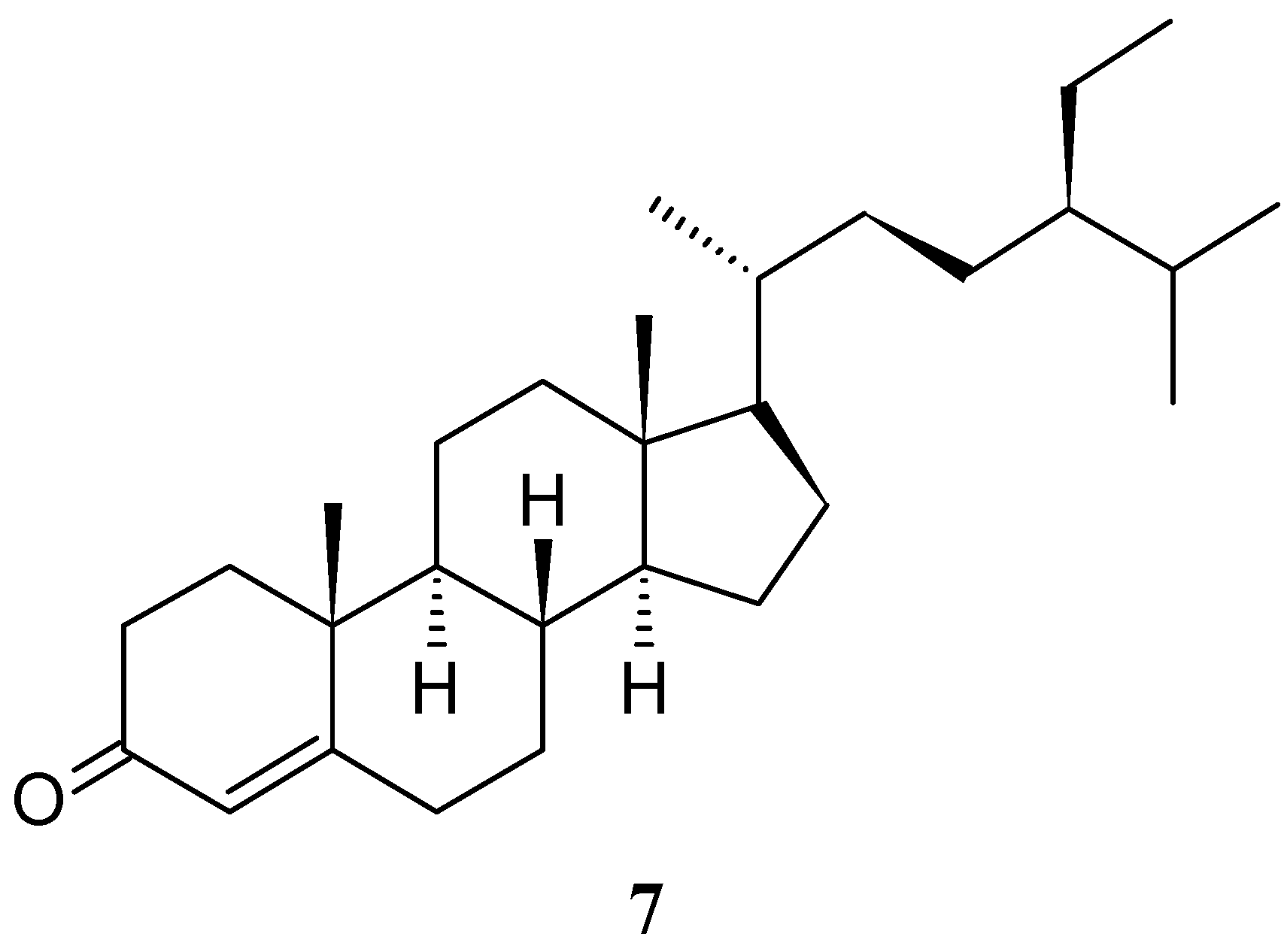
2. Results and Discussion
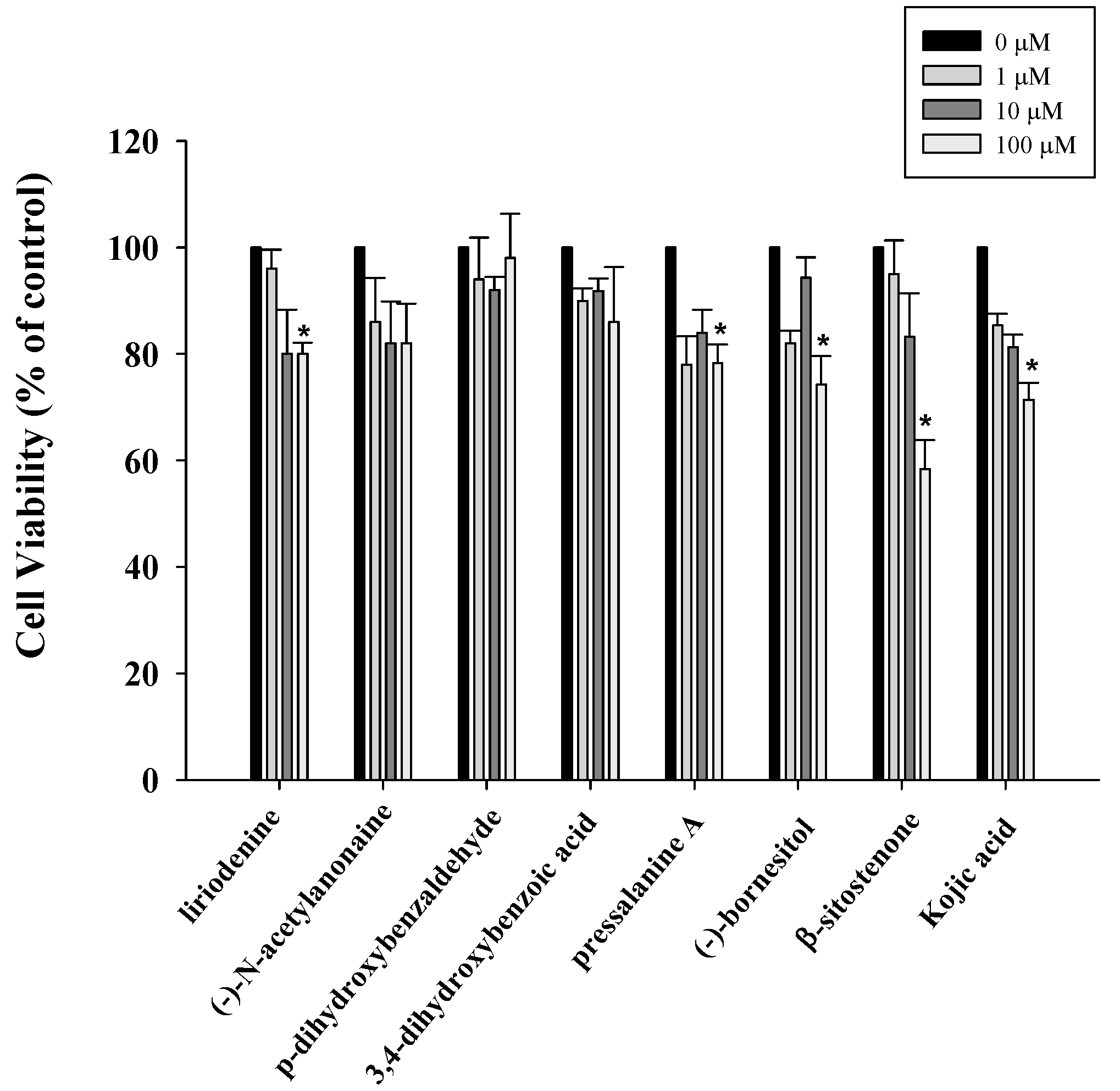
2.1. Anti-Proliferative Properties of Compounds 1–7 from M. compressa var. lanyuensis on B16F10 Cells
2.2. Inhibitory Effects of the Test Compounds on Tyrosinase Activity and Melanin Content
| Compound | Inhibition (%) Tyrosinase Activity | Inhibition (%) Melanin Content |
|---|---|---|
| Liriodenine (1) (5 μM) | 14.52 ± 3.58 | 19.03 ± 4.55 |
| (−)-N-Acetylanonaine (2) (10 μM) | 7.75 ± 1.42 | 17.70 ± 3.18 |
| Pressalanine A (3) (10 μM) | 4.84 ± 0.80 | −12 ± 4.58 |
| p-Dihydroxybenzaldehyde (4) (10 μM) | −7.77 ± 1.80 | −7.90 ± 2.22 |
| 3,4-Dihydroxybenzoic acid (5) (5 μM) | −28.28 ± 7.35 | −18.37 ± 6.43 |
| (−)-Bornesitol (6) (5 μM) | 3.06 ± 0.45 | 0.89 ± 0.08 |
| β-Sitostenone (7) (5 μM) | 25.48 ± 6.30 | 35.86 ± 5.43 |
| Kojic acid (10 μM) | 25.38 ± 1.40 | 20.00 ± 2.12 |
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction, Isolation and Identification
3.4. Cell Culture
3.5. Cell Viability Assay—XTT Assay
3.6. Determination of Melanin Content
3.7. Tyrosinase Activity
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kang, Y.F.; Liu, C.M.; Kao, C.L.; Chen, C.Y. Antioxidant and anticancer constituents from the leaves of Liriodendron tulipifera. Molecules 2014, 19, 4234–4245. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Juang, S.H.; Huang, G.J.; Liao, Y.R.; Chen, Y.F.; Wu, C.C.; Chang, H.T.; Wu, T.S. The constituents of Michelia compressa var. formosana and their bioactivities. Int. J. Mol. Sci. 2014, 15, 10926–10935. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Lee, M.S.; Cha, E.Y.; Lee, J.S.; Sul, J.Y.; Song, I.S.; Kim, J.Y. Magnolol-induced apoptosis in HCT-116 colon cancer cells is associated with the AMP-activated protein kinase signaling pathway. Biol. Pharm. Bull. 2012, 35, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.T.; Huang, J.C.; Kuo, P.L.; Chen, C.Y. Bioactive constituents from Michelia champaca. Nat. Prod. Commun. 2011, 6, 1251–1252. [Google Scholar] [PubMed]
- Chuang, D.Y.; Chan, M.H.; Zong, Y.; Sheng, W.; He, Y.; Jiang, J.H.; Simonyi, A.; Gu, Z.; Fritsche, K.L.; Cui, J.; et al. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. J. Neuroinflamm. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.; Ho, C.L. Chemical composition and anti-mildew activities of essential oils from different parts of Michelia compressa var. formosana. Nat. Prod. Commun. 2015, 10, 665–668. [Google Scholar]
- Cheng, M.J.; Lo, W.L.; Huang, J.C.; Yeh, Y.T.; Hong, Z.L.; Lu, Y.C.; Chang, M.S.; Chen, C.Y. Isolation of a new monoterpenic ester from the leaves of Michelia compressa (Maxim.) Sargent var. formosana Kanehira (Magnoliaceae). Nat. Prod. Res. 2010, 24, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H. Studies on the alkaloids of magnoliaceous plants. 28. Alkaloids of Michelia compressa Maxim. var. formosana Kanehira. Yakugaku Zasshi: J. Pharm. Soc. Jpn. 1962, 82, 794–798. [Google Scholar]
- Marmaras, V.J.; Charalambidis, N.D.; Zervas, C.G. Immune response in insects: The role of phenoloxidase in defense reactions in relation to melanization and sclerotization. Arch. Insect Biochem. Physiol. 1996, 31, 119–133. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res. 1993, 6, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lahtz, C.; Kim, S.I.; Bates, S.E.; Li, A.X.; Wu, X.; Pfeifer, G.P. UVB irradiation does not directly induce detectable changes of DNA methylation in human keratinocytes. F1000Research 2013, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Yelverton, C.B.; Polisetty, S.S.; Housman, T.S.; Williford, P.M.; Teuschler, H.V.; Feldman, S.R. Treatment patterns and cost of nonmelanoma skin cancer management. Dermatol. Surg. 2006, 32, 1266–1271. [Google Scholar]
- Liao, W.T.; Huang, T.S.; Chiu, C.C.; Pan, J.L.; Liang, S.S.; Chen, B.H.; Chen, S.H.; Liu, P.L.; Wang, H.C.; Wen, Z.H.; et al. Biological properties of acidic cosmetic water from seawater. Int. J. Mol. Sci. 2012, 13, 5952–5971. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Chen, C.Y.; Wen, Z.H. Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Exp. Dermatol. 2011, 20, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.-W.; Liu, C.-M.; Chung, M.-I.; Chen, C.-Y. Biofunctional Constituents from Michelia compressa var. lanyuensis with Anti-Melanogenic Properties. Molecules 2015, 20, 12166-12174. https://doi.org/10.3390/molecules200712166
Chu C-W, Liu C-M, Chung M-I, Chen C-Y. Biofunctional Constituents from Michelia compressa var. lanyuensis with Anti-Melanogenic Properties. Molecules. 2015; 20(7):12166-12174. https://doi.org/10.3390/molecules200712166
Chicago/Turabian StyleChu, Chia-Wei, Chi-Ming Liu, Mei-Ing Chung, and Chung-Yi Chen. 2015. "Biofunctional Constituents from Michelia compressa var. lanyuensis with Anti-Melanogenic Properties" Molecules 20, no. 7: 12166-12174. https://doi.org/10.3390/molecules200712166






