Covalently Cross-Linked Arabinoxylans Films for Debaryomyces hansenii Entrapment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and Characterization of WEAX
| Component | Content |
|---|---|
| Arabinose a | 25.00 ± 1.50 |
| Xylose a | 41.00 ± 1.10 |
| Glucose a | 3.90 ± 0.30 |
| Protein a | 3.70 ± 0.05 |
| Ferulic acid b | 2.100 ± 0.001 |
| Diferulic acids b | 0.040 ± 0.001 |
| Triferulic acid b | traces |
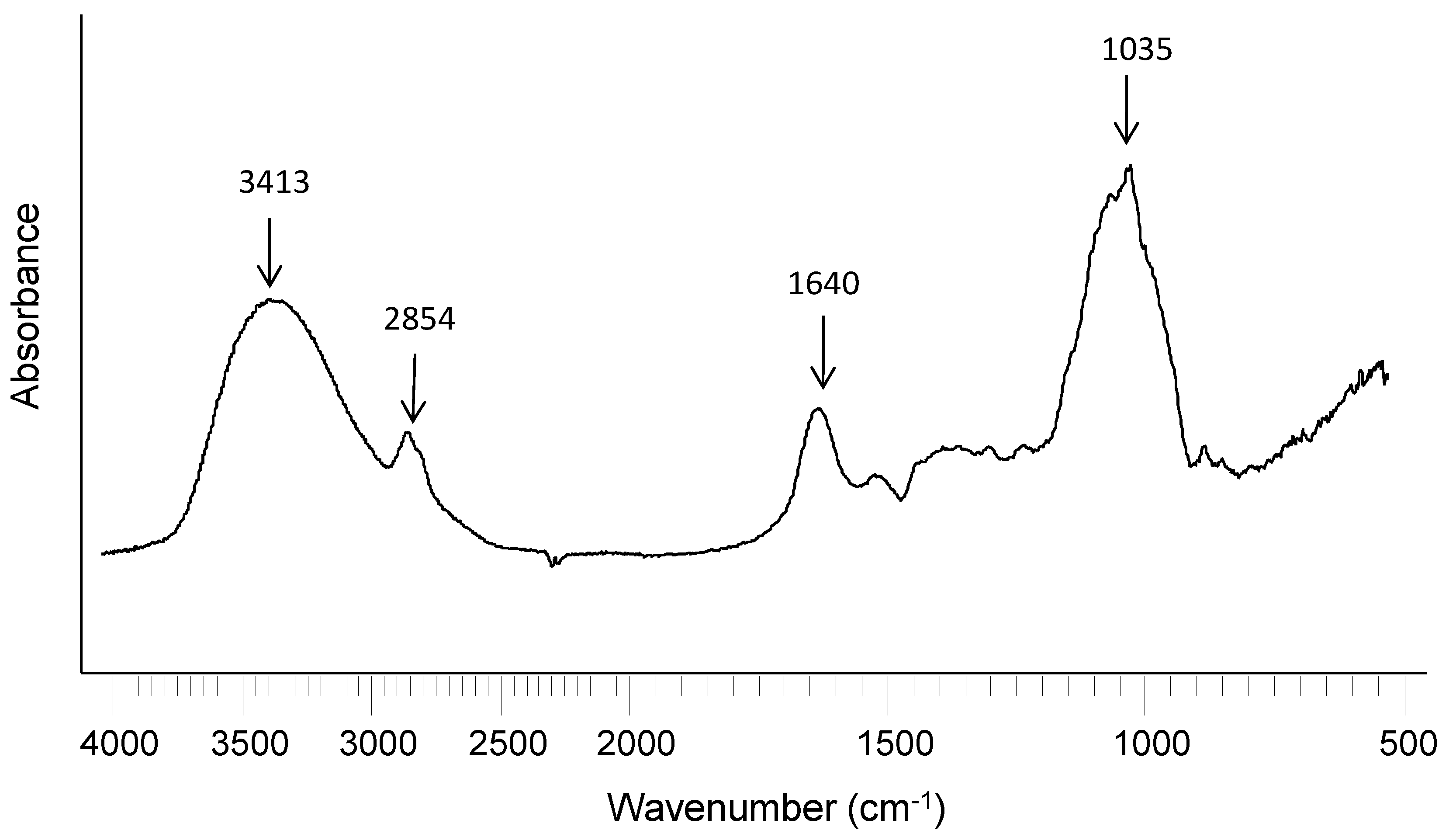
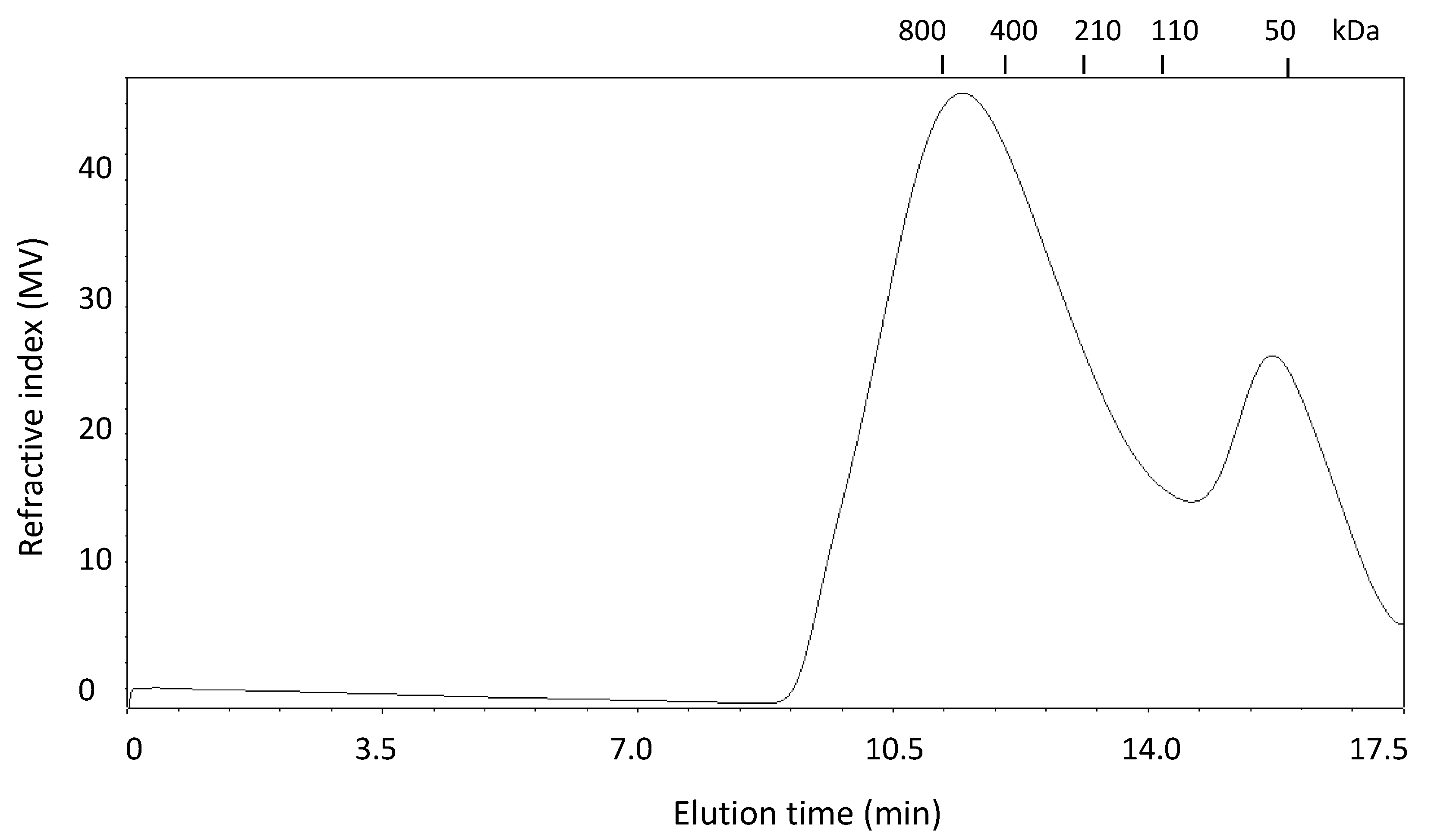
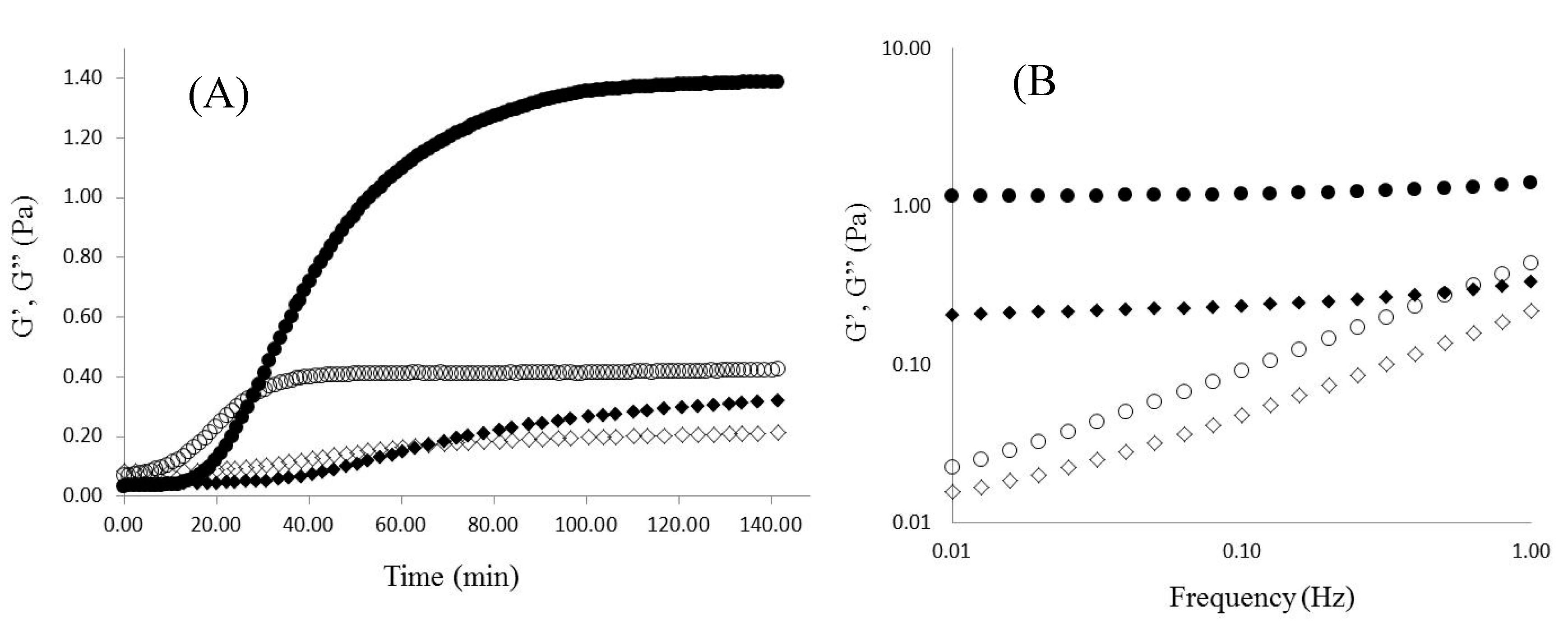
2.2. WEAX Gelation
 ), G” (
), G” (  ).
).
 ), G” (
), G” (  ).
).
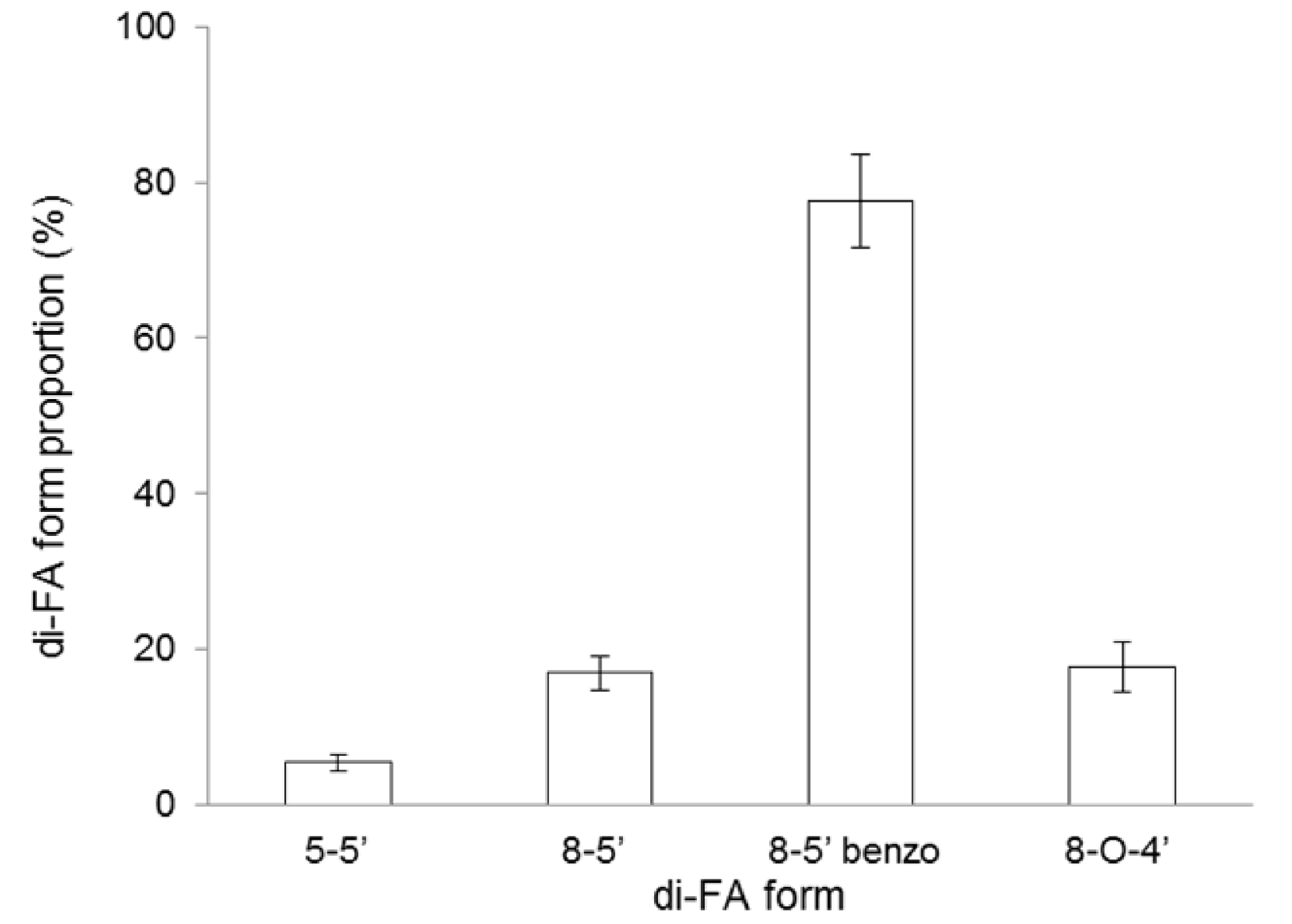
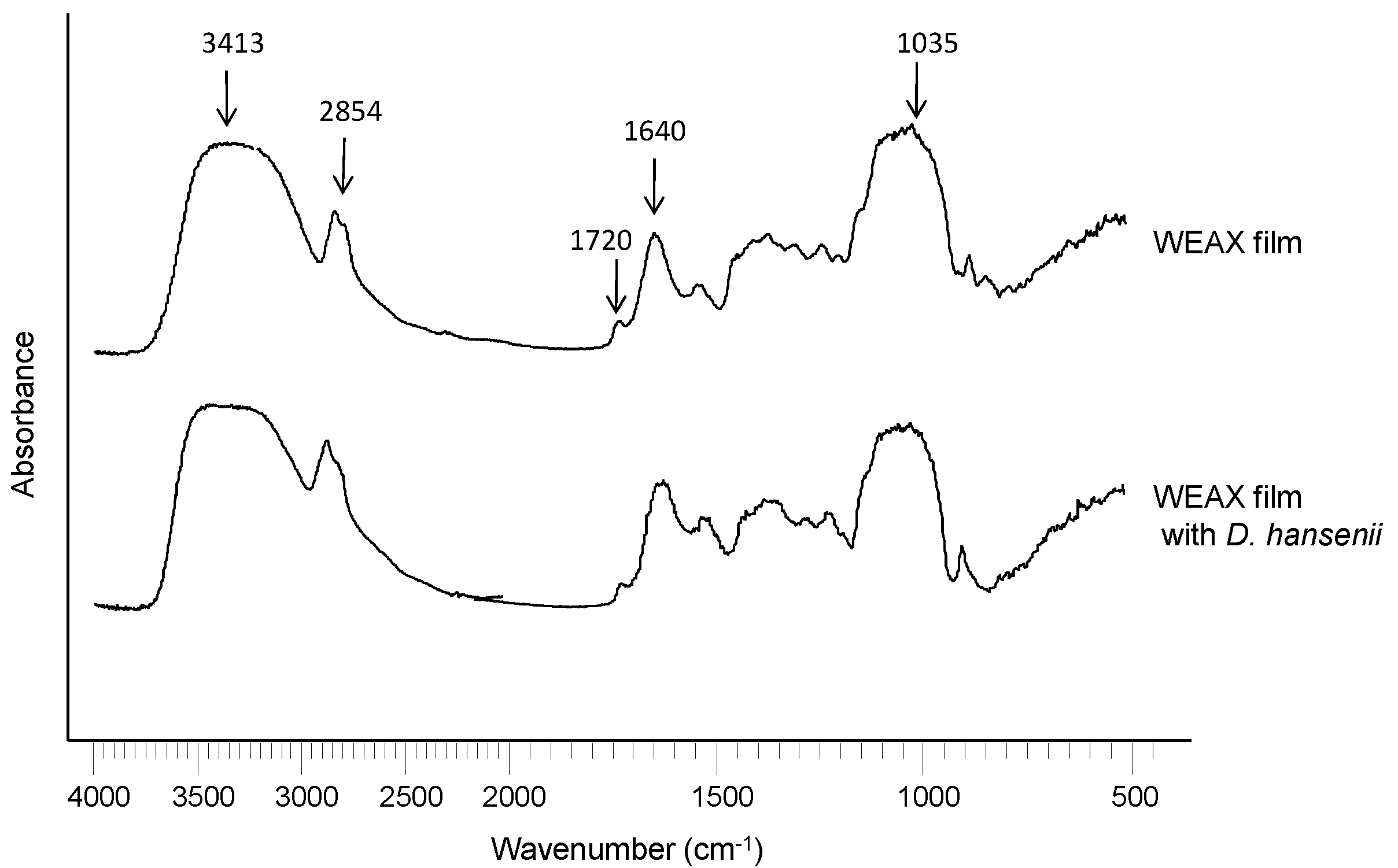
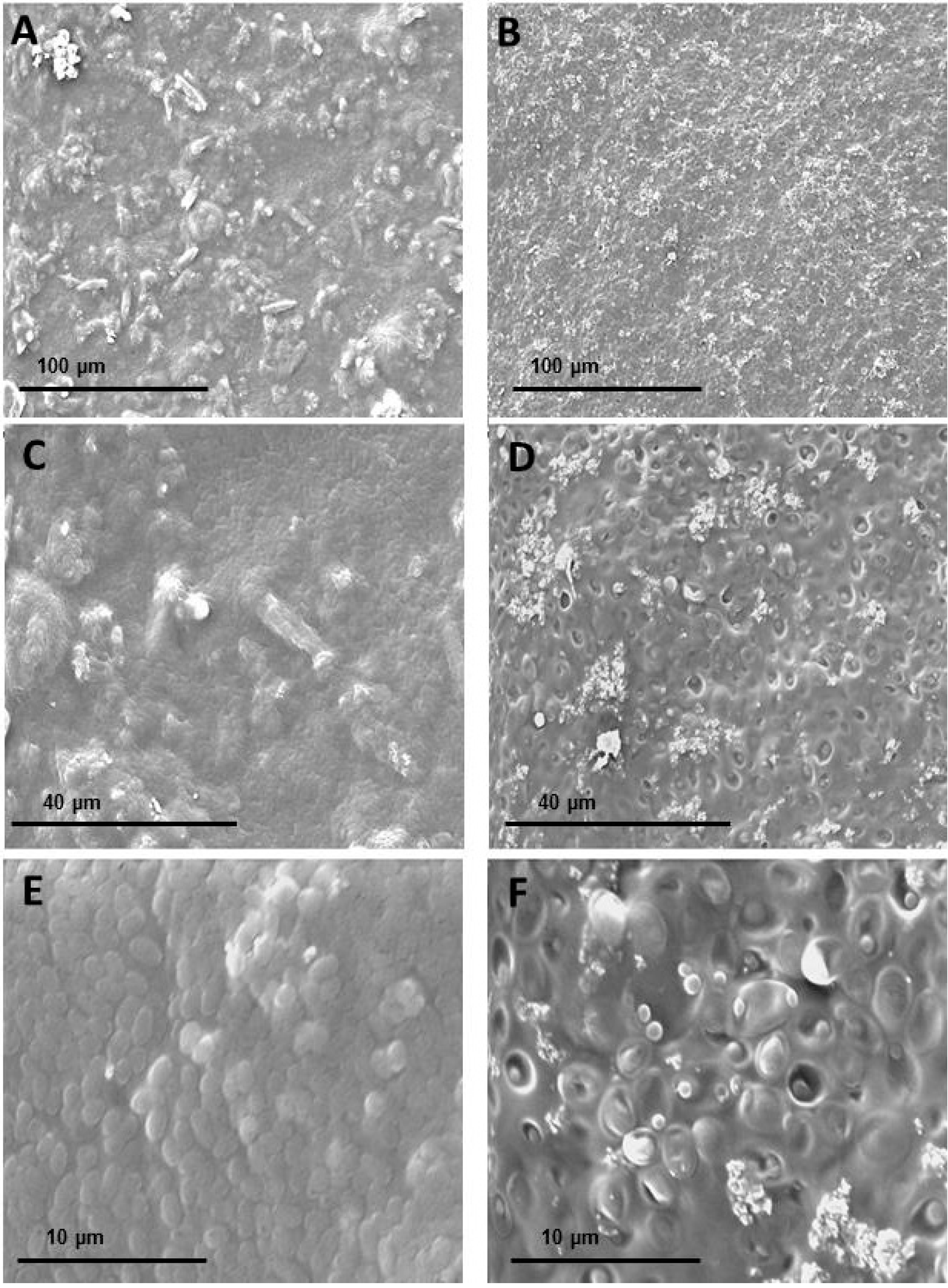
2.3. WEAX Films
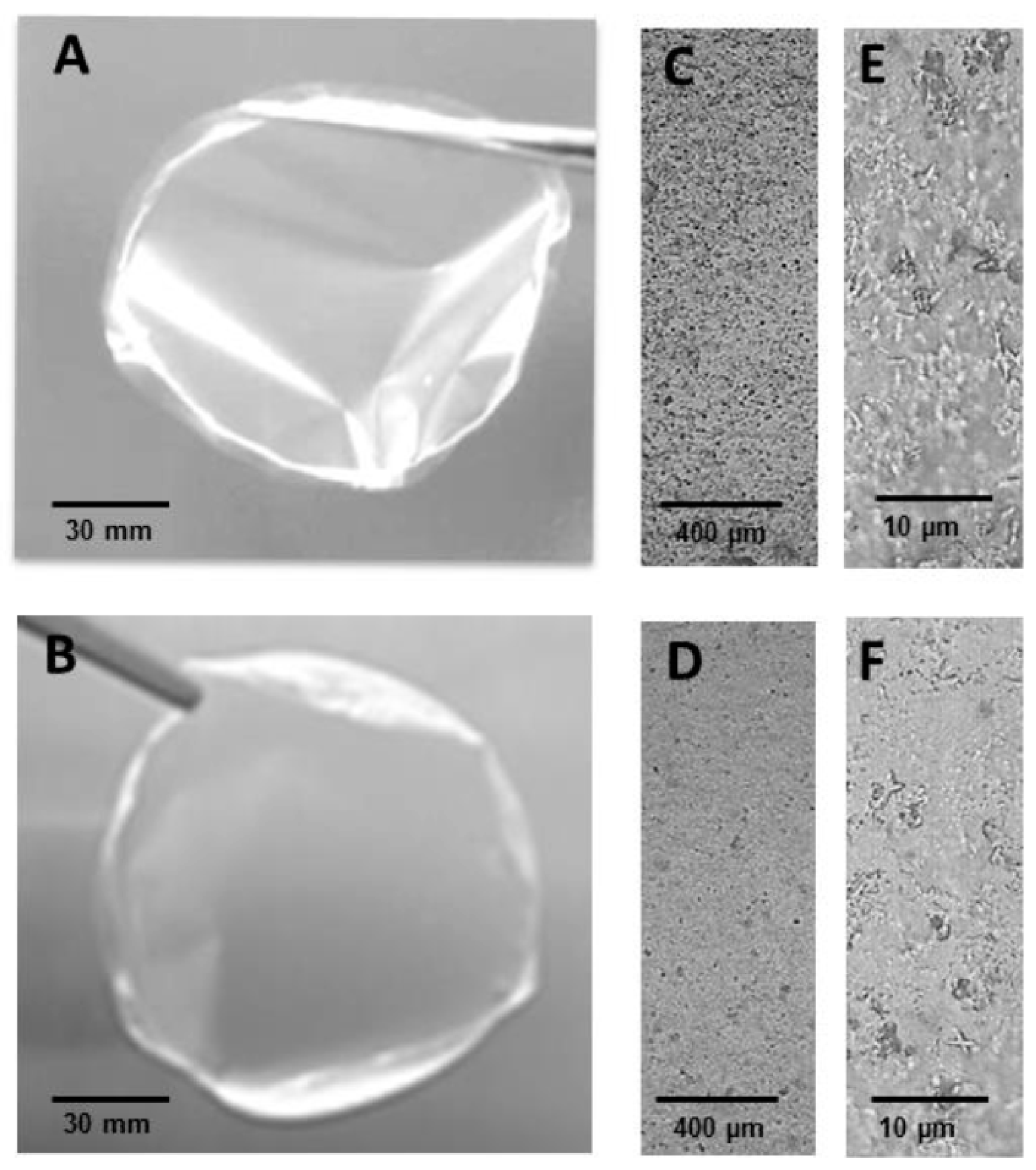
| WEAX Film | WEAX Film Containing D. hansenii | |
|---|---|---|
| L | 95.86 ± 0.89a | 94.63 ± 0.06b |
| a | –0.15 ± 0.03a | –0.27 ± 0.04b |
| b | 4.62 ± 0.27a | 9.77 ± 0.16b |
| YI | 6.88 ± 0.44a | 14.75 ± 0.25b |
| Film | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| WEAX film | 7.00 ± 1.00 | 12.80 ± 2.0 | 410 ± 51 |
| WEAX film with D. hansenii | 6.00 ± 1.70 | 10.30 ± 1.0 | 375 ± 10 |
3. Experimental Section
3.1. Materials
3.2. WEAX Extraction and Characterization
3.3. WEAX Gelation
3.4. WEAX Films Preparation and Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dervilly-Pinel, G.; Thibault, J.F.; Saulnier, L. Experimental evidence for a semi-flexible conformation for arabinoxylans. Carbohydr. Res. 2001, 330, 365–372. [Google Scholar] [CrossRef]
- Smith, M.M.; Hartley, R.D. Occurrence and nature of ferulic acid substitution of cell-wall polysaccharides in graminaceous plants. Carbohydr. Res. 1983, 118, 65–80. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G.; Bushuk, W. Physical properties of water-soluble pentosans from different wheat varieties. Cereal Chem. 1991, 68, 145–150. [Google Scholar]
- Figueroa-Espinoza, M.C.; Rouau, X. Oxidative cross-linking of pentosans by a fungal laccase and a horseradish peroxidase: Mechanism of linkage between feruloylated arabinoxylans. Cereal Chem. 1998, 75, 259–265. [Google Scholar] [CrossRef]
- Bollag, J.M.; Leonowicz, A. Comparative studies of extracellular fungal laccase. Appl. Environ. Microb. 1984, 48, 849–856. [Google Scholar]
- Ralph, J.; Quideau, S.; Grabber, J.H.; Hatfield, R.D. Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Soc. Perkin Trans. 1 1994, 3485–3498. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Morel, M.H.; Rouau, X. Effect of lysine, tyrosine, cysteine and glutathione on the oxidative cross-linking of feruloylated arabinoxylans by a fungal laccase. J. Agric. Food Chem. 1998, 46, 2583–2589. [Google Scholar] [CrossRef]
- Schooneveld-Bergmans, M.E.F.; Dignum, M.J.W.; Grabber, J.H.; Beldman, G.; Voragen, A.G.J. Studies on oxidative cross-linking of feruloylated arabinoxylans from wheat flour and wheat bran. Carbohydr. Polym. 1999, 38, 309–317. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Guigliarelli, B.; Belle, V.; Rouau, X.; Micard, V. Storage stability of arabinoxylan gels. Carbohydr. Polym. 2005, 59, 181–188. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Vansteenkiste, E.; Babot, C.; Rouau, X.; Micard, V. Oxidative gelation of feruloylated arabinoxylan as affected by protein. Influence on protein enzymatic hydrolysis. Food Hydrocoll. 2004, 18, 557–564. [Google Scholar] [CrossRef]
- Chanliaud, E.; Saulnier, L.; Thibault, J.F. Alkaline extraction and characterization of heteroxylans from maize bran. J. Cereal Sci. 1995, 21, 195–203. [Google Scholar] [CrossRef]
- Pérez Espitia, P.J.; Du, W.X.; Avena-Bustillos, R.J.; Ferreira Soares, N.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Gerasopoulos, D.; Sfakiotakis, E. Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar]
- Fan, Y.; Xu, Y.; Wang, D.; Zhang, L.; Sun, J.; Sun, L.; Zhang, B. Effect of alginate coating combined with yeast antagonist on strawberry (Fragaria × ananassa) preservation quality. Postharvest Biol. Technol. 2009, 53, 84–90. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.; Larralde-Corona, C.; Vero, S.; López-Aburto, M.; Ochoa, J.; Ascencio-Valle, F. Characterization of yeast Debaryomyces hansenii for the biological control of blue mold decay of Mexican lemon. CyTA-J. Food 2010, 8, 49–56. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.H.; Rouau, X.; Doublier, J.L.; Micard, V. Arabinoxylan gels: impact of the feruloylation degree on their structure and properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, M.; Biliaderis, C.G.; Bushuk, W. Comparison of the structure and composition of water-soluble pentosans from different wheat varieties. Cereal Chem. 1991, 68, 139–144. [Google Scholar]
- Saulnier, L.; Sado, P.E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281. [Google Scholar] [CrossRef]
- Dervilly, G.; Saulnier, L.; Roger, P.; Thibault, J.F. Isolation of homogeneous fractions of wheat water-soluble arabinoxylan. Influence of structure on their macromolecular characteristics. J. Agric. Food Chem. 2000, 48, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Fincher, G.B.; Stone, B.A. A water-soluble arabinogalactan–peptide from wheat endosperm. Aust. J. Biol. Sci. 1974, 27, 117–132. [Google Scholar]
- Barron, C.; Rouau, X. FTIR and Raman signatures of wheat grain peripheral tissues. Cereal Chem. 2008, 85, 619–625. [Google Scholar] [CrossRef]
- Cyran, M.R.; Saulnier, L. Cell wall fractions isolated from outer layers of rye grain by sequential treatment with α-amylase and proteinase: Structural investigation of polymers in two ryes with contrasting breadmaking quality. J. Agric. Food Chem. 2005, 53, 9213–9224. [Google Scholar] [CrossRef] [PubMed]
- Séné, C.F.B.; McCann, M.C.; Wilson, R.H.; Grinter, R. Fourier-Transform Raman and Fourier-Transform Infrared Spectroscopy. An investigation of five higher plant cell walls and their components. Plant Physiol. 1994, 106, 1623–1631. [Google Scholar] [PubMed]
- Morales-Ortega, A.; Carvajal-Millan, E.; Brown-Bojorquez, F.; Rascón-Chu, A.; Torres-Chavez, P.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Martínez-López, A.L.; Campa-Mada, A.C. Entrapment of probiotics in water extractable arabinoxylan gels: Rheological and microstructural characterization. Molecules 2014, 19, 3628–3637. [Google Scholar] [CrossRef] [PubMed]
- Doublier, J.L.; Cuvelier, G. Gums and hydrocolloids: Functional aspects. In Carbohydrates in Food; Eliasson, A.C., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 283–318. [Google Scholar]
- Ross-Murphy, S.B. Rheological methods. In Biophysical Methods in Food Research; Chan, H.W.S., Ed.; Blackwell Scientific Publications: Oxford, UK, 1984; pp. 138–199. [Google Scholar]
- Bolin, H.R.; Huxsoll, C.C. Control of minimally processed carrot (Daucus carota) surface discoloration caused by abrasion peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar]
- Martínez-López, A.L.; Carvajal-Millan, E.; López-Franco, Y.L.; Lizardi-Mendoza, J.; Rascón-Chu, A. Antioxidant activity of maize bran arabinoxylan microspheres. In Food Composition and Analysis. Methods and Strategies; Haghi, A.K., Carvajal-Millan, E., Eds.; Apple Academic Press, Inc.: Waretown, NJ, USA, 2014; ISBN 978-1-92-689-585-7. pp. 19–28. [Google Scholar]
- Boeriu, C.G.; Oudgenoeg, G.; Spekking, W.T.; Berendsen, L.B.; Vancon, L.; Boumans, H.; Gruppen, H.; van Berkel, W.J.; Laane, C.; Voragen, A.G. Horseradish peroxidase-catalyzed cross-linking of feruloylated arabinoxylans with beta-casein. J Agric. Food Chem. 2004, 52, 6633–6639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Whistler, R.L. Mechanical properties and water vapor permeability of thin film from corn hull arabinoxylan. J. Appl. Polym. Sci. 2004, 93, 2896–2902. [Google Scholar] [CrossRef]
- Goksu, E.I.; Karamanlioglu, M.; Bakir, U.; Yilmaz, L.; Yilmazer, U. Production and characterization of films from cotton stalk xylan. J. Agric. Food Chem. 2007, 55, 10685–10691. [Google Scholar] [CrossRef] [PubMed]
- Novák, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Procházka, V.; Synytsya, A.; Blahovec, J.; Hejlová, A.; Čopíková, J. Yeast β(1,3),(1,6)-d-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Rouilly, A.; Geneau-Sbartaï, C.; Rigal, L. Thermo-mechanical processing of sugar beet pulp. III. Study of extruded films improvement with various plasticizers and cross-linkers. Bioresour. Technol. 2009, 100, 3076–3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höije, A.; Sternemalm, E.; Heikkinen, S.; Tenkanen, M.; Gatenholm, P. Material properties of films from enzymatically tailored arabinoxylans. Biomacromolecules 2008, 9, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC Intl. Method 991.43, 16th ed.; Association of Official Analytical Communities: Arlington, VA, USA, 1995. [Google Scholar]
- Alves, V.; Ferreira, A.; Costa, N.; Freitas, F.; Reis, M.; Coelhoso, I. Characterization of biodegradable films from the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr. Polym. 2011, 83, 1582–1590. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Estrada, R.; Calderón-Santoyo, M.; Carvajal-Millan, E.; Valle, F.D.J.A.; Ragazzo-Sánchez, J.A.; Brown-Bojorquez, F.; Rascón-Chu, A. Covalently Cross-Linked Arabinoxylans Films for Debaryomyces hansenii Entrapment. Molecules 2015, 20, 11373-11386. https://doi.org/10.3390/molecules200611373
González-Estrada R, Calderón-Santoyo M, Carvajal-Millan E, Valle FDJA, Ragazzo-Sánchez JA, Brown-Bojorquez F, Rascón-Chu A. Covalently Cross-Linked Arabinoxylans Films for Debaryomyces hansenii Entrapment. Molecules. 2015; 20(6):11373-11386. https://doi.org/10.3390/molecules200611373
Chicago/Turabian StyleGonzález-Estrada, Ramsés, Montserrat Calderón-Santoyo, Elizabeth Carvajal-Millan, Felipe De Jesús Ascencio Valle, Juan Arturo Ragazzo-Sánchez, Francisco Brown-Bojorquez, and Agustín Rascón-Chu. 2015. "Covalently Cross-Linked Arabinoxylans Films for Debaryomyces hansenii Entrapment" Molecules 20, no. 6: 11373-11386. https://doi.org/10.3390/molecules200611373






