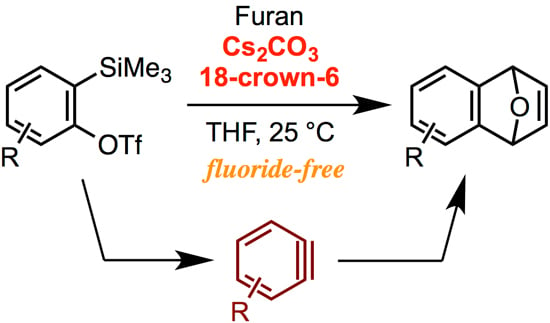
An Alternative Method for Generating Arynes from ortho-Silylaryl Triflates: Activation by Cesium Carbonate in the Presence of a Crown Ether
Abstract
:1. Introduction
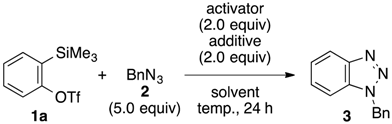
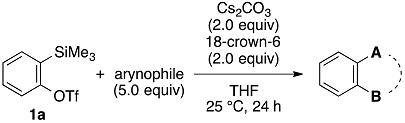
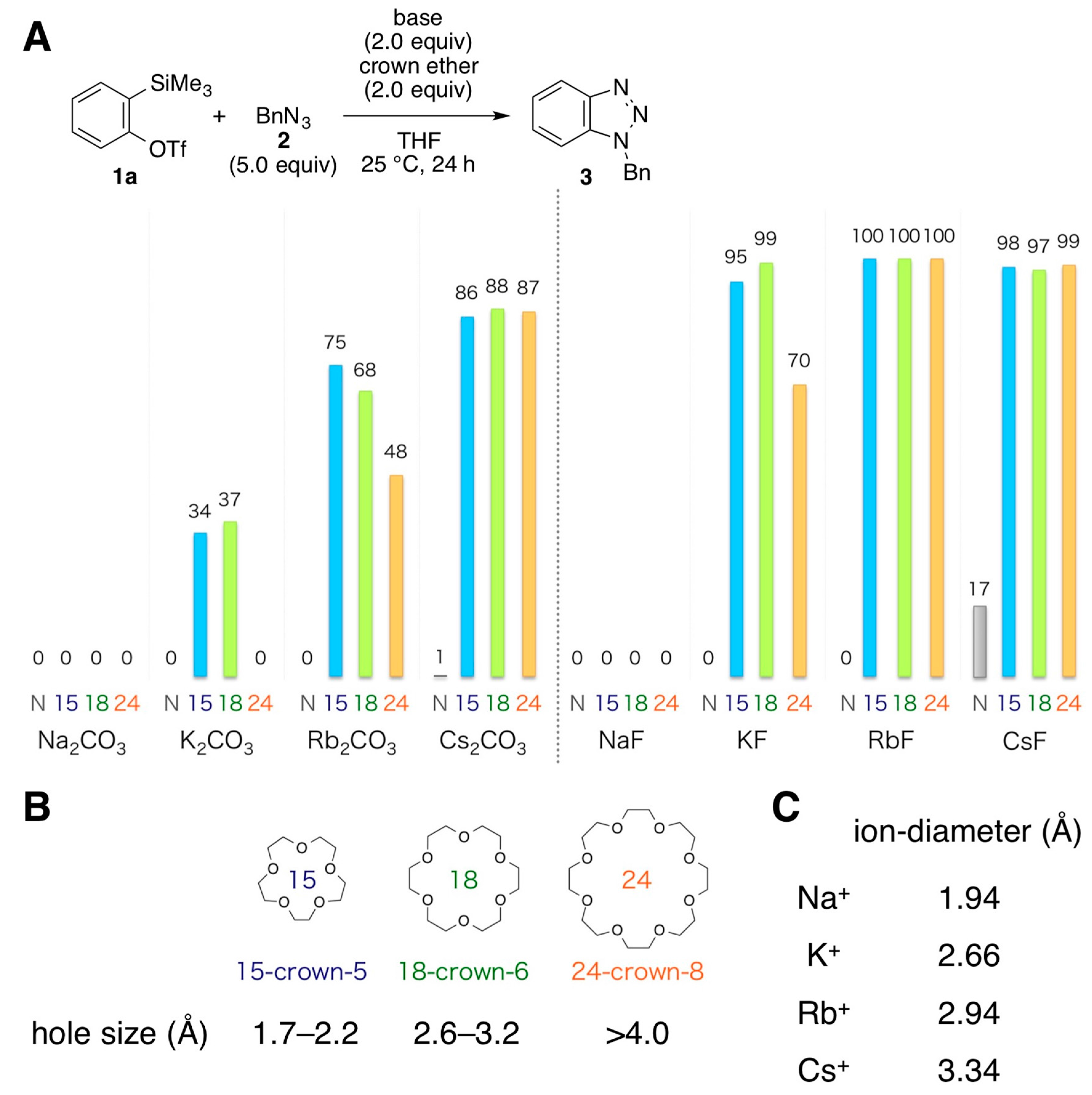
2. Results and Discussion
| Entry | Activator | Additive | Solvent | Temp. (°C) | Yield (%) a |
|---|---|---|---|---|---|
| 1 | Cs2CO3 | ––– | THF | 25 | 1 |
| 2 | Cs2CO3 | ––– | THF | 60 | 32 |
| 3 | Cs2CO3 | ––– | MeCN | 25 | 38 |
| 4 | Cs2CO3 | ––– | MeCN | 60 | 79 |
| 5 | Cs2CO3 | 18-crown-6 | THF | 25 | 88 (86) b |
| 6 | Cs2CO3 | 18-crown-6 | MeCN | 25 | 72 |
| 7 | Cs2CO3 | 18-crown-6 | CH2Cl2 | 25 | 13 |
| 8 | Cs2CO3 | 18-crown-6 | toluene | 25 | 59 |
| 9 | Cs2CO3 | 18-crown-6 | DME | 25 | 88 |
| 10 | CsHCO3 | 18-crown-6 | THF | 25 | 0 |
| 11 | K2CO3 | 18-crown-6 | THF | 25 | 37 |
| 12 c | K2CO3 | 18-crown-6 | THF | 25 | 76 |
| 13 | K3PO4 | 18-crown-6 | THF | 25 | 37 |
| 14 | KF | 18-crown-6 | THF | 25 | 99 |
| 15 | CsF | ––– | MeCN | 25 | quant. |
| Entry | Arynophile | Product | Yield (%) a | ||
|---|---|---|---|---|---|
| 1 |  | 4 |  | 5a | 73 |
| 2 | 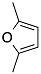 | 6 |  | 7 | 87 |
| 3 | 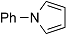 | 8 |  | 9 | 74 |
| 4 | 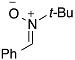 | 10 |  | 11 | 84 |
| 5 | 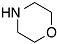 | 12 |  | 13 | 75 |
| Entry | Arynophile | Product | Yield (%) a | ||
|---|---|---|---|---|---|
| 1 | 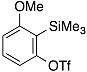 | 1b | 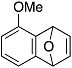 | 5b | 89 |
| 2 | 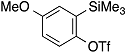 | 1c |  | 5c | 86 |
| 3 | 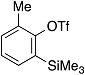 | 1d |  | 5d | 88 |
| 4 | 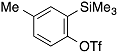 | 1e | 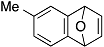 | 5e | 89 |
| 5 | 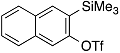 | 1f | 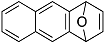 | 5f | 76 |
| 6 |  | 1g |  | 5g | 85 |

3. Experimental Section
3.1. General Remarks
3.2. Typical Procedure for Aryne Generation from ortho-Silylaryl Triflate with Cesium Carbonate in the Presence of 18-Crown-6
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Hoffmann, R.W. Dehydrobenzene and Cycloalkynes; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Pellissier, H.; Santelli, M. The use of arynes in organic synthesis. Tetrahedron 2003, 59, 701–730. [Google Scholar] [CrossRef]
- Yoshida, H.; Takaki, K. Multicomponent Coupling Reaction of Arynes for Construction of Heterocyclic Skeletons. Heterocycles 2012, 85, 1333–1349. [Google Scholar] [CrossRef]
- Yoshida, H.; Takaki, K. Aryne Insertion Reactions into Carbon-Carbon σ-Bonds. Synlett 2012, 23, 1725–1732. [Google Scholar] [CrossRef]
- Gampe, C.M.; Carreira, E.M. Arynes and Cyclohexyne in Natural Product Synthesis. Angew. Chem. Int. Ed. 2012, 51, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Tadross, P.M.; Stoltz, B.M. A Comprehensive History of Arynes in Natural Product Total Synthesis. Chem. Rev. 2012, 112, 3550–3577. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskiy, A.V.; Markina, N.A.; Larock, R.C. Use of benzynes for the synthesis of heterocycles. Org. Biomol. Chem. 2013, 11, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.W.; Suzuki, K. A “Hot, Energized” Benzyne. Angew. Chem. Int. Ed. 2013, 52, 2655–2656. [Google Scholar] [CrossRef] [PubMed]
- Himeshima, Y.; Sonoda, T.; Kobayashi, H. Fluoride-Induced 1,2-Elimination of O-Trimethylsilylphenyl Triflate to Benzyne under Mild Conditions. Chem. Lett. 1983, 12, 1211–1214. [Google Scholar] [CrossRef]
- Several ortho-silylaryl triflates are commercially available. For improved synthesis of ortho-silylaryl triflates, see: Bronner, S.M.; Garg, N.K. Efficient Synthesis of 2-(Trimethylsilyl)phenyl Trifluoromethanesulfonate: A Versatile Precursor to o-Benzyne. J. Org. Chem. 2009, 74, 8842–8843. [Google Scholar]
- Peña, D.; Cobas, A.; Pérez, D.; Guitián, E. An Efficient Procedure for the Synthesis of ortho-Trialkylsilylaryl Triflates: Easy Access to Precursors of Functionalized Arynes. Synthesis 2002, 1454–1458. [Google Scholar]
- Candito, D.A.; Lautens, M. Stereoselective Nickel-Catalyzed [2+2+2] Cycloaddition of Enynes and Arynes. Synlett 2011, 1987–1992. [Google Scholar]
- Smith, A.B., III; Kim, W.-S. Diversity-oriented synthesis leads to an effective class of bifunctional linchpins uniting anion relay chemistry (ARC) with benzyne reactivity. Proc. Natl. Acad. Sci. USA 2011, 108, 6787–6792. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.M.; Gilmore, C.D.; Stoltz, B.M. Benzannulated Bicycles by Three-Component Aryne Reactions. Angew. Chem. Int. Ed. 2011, 50, 4488–4491. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, T.; Nishiyama, T.; Shigeta, T.; Mohri, S.; Morita, S.; Takayanagi, S.; Terauchi, Y.; Morikawa, Y.; Takagi, A.; Ishikawa, Y.; et al. ortho-Selective Nucleophilic Addition of Primary Amines to Silylbenzynes: Synthesis of 2-Silylanilines. Angew. Chem. Int. Ed. 2011, 50, 5674–5677. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. A Multicomponent Coupling Reaction Induced by Insertion of Arynes into the C=O Bond of Formamide. Angew. Chem. Int. Ed. 2011, 50, 6638–6642. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yoshida, R.; Takaki, K. Synchronous Ar–F and Ar–Sn Bond Formation through Fluorostannylation of Arynes. Angew. Chem. Int. Ed. 2013, 52, 8629–8632. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Nakamura, K.; Shibano, S.; Ide, S.; Minami, M.; Sato, Y. Addition of Cyclic Ureas and 1-Methyl-2-oxazolidone to Pyridynes: A New Approach to Pyridodiazepines, Pyridodiazocines, and Pyridooxazepines. Org. Lett. 2013, 15, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.E.; Garg, N.K. Regioselective reactions of 3,4-pyridynes enabled by the aryne distortion model. Nat. Chem. 2013, 5, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Henderson, J.L.; Ernouf, G.; Greaney, M.F. Tandem thia-Fries rearrangement—Cyclisation of 2-(trimethylsilyl)phenyl trifluoromethanesulfonate benzyne precursors. Chem. Commun. 2013, 49, 7602–7604. [Google Scholar] [CrossRef] [PubMed]
- Bhojgude, S.S.; Thangaraj, M.; Suresh, E.; Biju, A.T. Tandem [4 + 2]/[2 + 2] Cycloaddition Reactions Involving Indene or Benzofurans and Arynes. Org. Lett. 2014, 16, 3576–3579. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-L.; Chen, J.-R.; Zou, Y.-Q.; Wei, Q.; Xiao, W.-J. Three-Component Coupling Reaction Triggered by Insertion of Arynes into the S=O Bond of DMSO. Org. Lett. 2014, 16, 3768–3771. [Google Scholar] [CrossRef] [PubMed]
- Pandya, V.G.; Mhaske, S.B. Transition-Metal-Free C–S Bond Formation: A Facile Access to Aryl Sulfones from Sodium Sulfinates via Arynes. Org. Lett. 2014, 16, 3836–3839. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hosoya, T. Synthesis of Diverse Aromatic Oxophosphorus Compounds by the Michaelis–Arbuzov-type Reaction of Arynes. Chem. Lett. 2013, 42, 583–585. [Google Scholar] [CrossRef]
- Kii, I.; Shiraishi, A.; Hiramatsu, T.; Matsushita, T.; Uekusa, H.; Yoshida, S.; Yamamoto, M.; Kudo, A.; Hagiwara, M.; Hosoya, T. Strain-promoted double-click reaction for chemical modification of azido-biomolecules. Org. Biomol. Chem. 2010, 8, 4051–4055. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Shiraishi, A.; Kanno, K.; Matsushita, T.; Johmoto, K.; Uekusa, H.; Hosoya, T. Enhanced clickability of doubly sterically-hindered aryl azides. Sci. Rep. 2011, 1, 82. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hatakeyama, Y.; Johmoto, K.; Uekusa, H.; Hosoya, T. Transient Protection of Strained Alkynes from Click Reaction via Complexation with Copper. J. Am. Chem. Soc. 2014, 136, 15059–15062. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Kato, T.; Hosoya, T. Generation of Arynes via Ate Complexes of Arylboronic Esters with an ortho-Leaving Group. Org. Lett. 2013, 15, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Uchida, K.; Hosoya, T. Generation of Arynes Triggered by Sulfoxide–Metal Exchange Reaction of ortho-Sulfinylaryl Triflates. Chem. Lett. 2014, 43, 116–118. [Google Scholar] [CrossRef]
- Yoshida, S.; Nonaka, T.; Morita, T.; Hosoya, T. Modular synthesis of bis- and tris-1,2,3-triazoles by permutable sequential azide-aryne and azide-alkyne cycloadditions. Org. Biomol. Chem. 2014, 12, 7489–7493. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Uchida, K.; Igawa, K.; Tomooka, K.; Hosoya, T. An efficient generation method and remarkable reactivities of 3-triflyloxybenzyne. Chem. Commun. 2014, 50, 15059–15062. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Harada, R.; Kato-Sumida, T.; Johmoto, K.; Uekusa, H.; Hosoya, T. Boron-Selective Biaryl Coupling Approach to Versatile Dibenzoxaborins and Application to Concise Synthesis of Defucogilvocarcin M. Org. Lett. 2014, 16, 6240–6243. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Uchida, K.; Hosoya, T. Generation of Arynes Using Trimethylsilylmethyl Grignard Reagent for Activation of ortho-Iodoaryl or ortho-Sulfinylaryl Triflates. Chem. Lett. 2015, 44, 691–693. [Google Scholar] [CrossRef]
- Yoshida, S.; Karaki, F.; Uchida, K.; Hosoya, T. Generation of cycloheptynes and cyclooctynes via a sulfoxide–magnesium exchange reaction of readily synthesized 2-sulfinylcycloalkenyl triflates. Chem. Commun. 2015, 51, 8745–8748. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Hosoya, T.; Katsuki, M.; Suzuki, K. New Efficient Protocol for Aryne Generation. Selective Synthesis of Differentially Protected 1,4,5-Naphthalenetriols. Tetrahedron Lett. 1991, 32, 6735–6736. [Google Scholar] [CrossRef]
- Ikawa, T.; Takagi, A.; Kurita, Y.; Saito, K.; Azechi, K.; Egi, M.; Kakiguchi, K.; Kita, Y.; Akai, S. Preparation and Regioselective Diels–Alder Reactions of Borylbenzynes: Synthesis of Functionalized Arylboronates. Angew. Chem. Int. Ed. 2010, 49, 5563–5566. [Google Scholar] [CrossRef] [PubMed]
- Cycloadduct 3 was observed as a sole product and starting material 1a was completely consumed. In the case of using 0.1 or 1.0 equiv of 18-crown-6, cycloadduct 3 was obtained in 31% or 74% yield, respectively.
- Ikawa, T.; Nishiyama, T.; Nosaki, T.; Takagi, A.; Akai, S. A Domino Process for Benzyne Preparation: Dual Activation of o-(Trimethylsilyl)phenols by Nonafluorobutanesulfonyl Fluoride. Org. Lett. 2011, 13, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; Wiley: Chichester, UK, 2000. [Google Scholar]
- Gokel, G.W. Advances in Supramolecular Chemistry; JAI Press: Greenwich, CT, USA, 1997; Volume 4. [Google Scholar]
- Pedersen, C.J. Crystalline Salt Complexes of Macrocyclic Polyethers. J. Am. Chem. Soc. 1970, 92, 386–391. [Google Scholar] [CrossRef]
- Frensdorff, H.K. Stability Constants of Cyclic Polyether Complexes with Univalent Cations. J. Am. Chem. Soc. 1971, 93, 600–606. [Google Scholar] [CrossRef]
- Nishimura, T.; Kawamoto, T.; Sasaki, K.; Tsurumaki, E.; Hayashi, T. Rhodium-Catalyzed Asymmetric Cyclodimerization of Oxa- and Azabicyclic Alkenes. J. Am. Chem. Soc. 2007, 129, 1492–1493. [Google Scholar] [CrossRef] [PubMed]
- Lautens, M.; Schmid, G.A.; Chau, A. Remote Electronic Effects in the Rhodium-Catalyzed Nucleophilic Ring Opening of Oxabenzonorbornadienes. J. Org. Chem. 2002, 67, 8043–8053. [Google Scholar] [CrossRef] [PubMed]
- Fillion, E.; Trépanier, V.É.; Heikkinen, J.J.; Remorova, A.A.; Carson, R.J.; Goll, J.M.; Seed, A. Palladium-Catalyzed Intramolecular Reactions of (E)-2,2-Disubstituted 1-Alkenyldimethylalanes with Aryl Triflates. Organometallics 2009, 28, 3518–3531. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Sun, J.-Q.; Wei, X.; Wong, W.-Y.; Lee, A.W.M. Generation of Synthetic Equivalents of Benzdiynes from Benzobisoxadisilole. J. Org. Chem. 2004, 69, 7190–7197. [Google Scholar] [CrossRef] [PubMed]
- Best, W.M.; Collins, P.A.; McCulloch, R.K.; Wege, D. Deoxygenation of 1,4-Epoxy-1,4-dihydroarenes with Enneacarbonyldiiron. Aust. J. Chem. 1982, 35, 843–848. [Google Scholar] [CrossRef]
- Swapna, K.; Kumar, A.V.; Reddy, V.P.; Rao, K.R. Recyclable Heterogeneous Iron Catalyst for C−N Cross-Coupling under Ligand-Free Conditions. J. Org. Chem. 2009, 74, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Hazama, Y.; Sumida, Y.; Yano, T.; Hosoya, T. An Alternative Method for Generating Arynes from ortho-Silylaryl Triflates: Activation by Cesium Carbonate in the Presence of a Crown Ether. Molecules 2015, 20, 10131-10140. https://doi.org/10.3390/molecules200610131
Yoshida S, Hazama Y, Sumida Y, Yano T, Hosoya T. An Alternative Method for Generating Arynes from ortho-Silylaryl Triflates: Activation by Cesium Carbonate in the Presence of a Crown Ether. Molecules. 2015; 20(6):10131-10140. https://doi.org/10.3390/molecules200610131
Chicago/Turabian StyleYoshida, Suguru, Yuki Hazama, Yuto Sumida, Takahisa Yano, and Takamitsu Hosoya. 2015. "An Alternative Method for Generating Arynes from ortho-Silylaryl Triflates: Activation by Cesium Carbonate in the Presence of a Crown Ether" Molecules 20, no. 6: 10131-10140. https://doi.org/10.3390/molecules200610131