Chemical Composition and Antioxidant Activity of Essential Oil of Six Pinus Taxa Native to China
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Composition
| No. | Name of Components | RI | Relative Peak Area (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt | Ptf | Pvm | Pvu | Ph | Pm | |||||||
| 1 | Tricyclene | 921 | 1.81 | 1.11 | 0.39 | - | 0.21 | - | ||||
| 2 | α-Thujene | 925 | 0.13 | 1.48 | 0.24 | - | 1.72 | 2.02 | ||||
| 3 | α-Pinene | 932 | 11.08 | 6.78 | 16.55 | 20.55 | 9.68 | 8.16 | ||||
| 4 | Camphene | 945 | 0.38 | 0.45 | 0.58 | - | 0.34 | 0.63 | ||||
| 5 | Sabinene | 970 | 0.15 | - | - | - | 0.18 | - | ||||
| 6 | β-Pinene | 978 | 2.29 | 0.95 | 1.45 | - | 0.35 | 2.99 | ||||
| 7 | Myrcene | 982 | - | - | 0.72 | - | 1.04 | - | ||||
| 8 | α-Phellandrene | 1003 | 0.26 | - | - | - | - | - | ||||
| 9 | δ-3-Carene | 1008 | 0.31 | 0.19 | 0.38 | - | 0.13 | 0.13 | ||||
| 10 | α-Terpinene | 1014 | - | - | 0.11 | - | 0.12 | 0.29 | ||||
| 11 | Limonene | 1024 | 0.41 | - | - | - | 0.09 | - | ||||
| 12 | β-Phellandrene | 1027 | 0.12 | 0.30 | 0.57 | - | 0.37 | 0.66 | ||||
| 13 | β-Ocimene | 1041 | - | - | 0.22 | - | 0.15 | - | ||||
| 14 | γ-Terpinene | 4056 | 0.48 | 0.22 | 0.33 | - | 0.13 | 0.52 | ||||
| 15 | α-Terpinolene | 4086 | 0.24 | 0.37 | 0.95 | - | 0.59 | 0.17 | ||||
| 16 | Terpinen-4-ol | 1161 | - | - | - | - | 0.15 | - | ||||
| 17 | α-Terpineol | 1175 | 3.43 | 2.62 | 3.32 | - | 1.32 | 3.34 | ||||
| 18 | Bornyl acetale | 1267 | 4.13 | 3.32 | 3.93 | 12.71 | 2.96 | 3.83 | ||||
| 19 | α-Cubebene | 1346 | 0.21 | 0.32 | 0.28 | - | 0.26 | 0.24 | ||||
| 20 | α-Rlangene | 1371 | 0.24 | 0.26 | 0.30 | - | 0.31 | 0.24 | ||||
| 21 | α-Copaene | 1379 | - | - | 0.20 | - | - | 0.14 | ||||
| 22 | β-Bourbonene | 1388 | - | - | - | 1.13 | 0.12 | - | ||||
| 23 | β-Elemene | 1391 | 1.26 | 2.13 | 1.82 | - | 1.41 | 2.93 | ||||
| 24 | Longifolene | 1411 | 0.54 | 0.29 | 0.12 | 1.52 | 0.24 | - | ||||
| 25 | β-Caryophellene | 1421 | 22.36 | 20.83 | 24.08 | 26.31 | 18.26 | 18.48 | ||||
| 26 | α-Caryophellene | 1423 | 3.72 | - | - | - | 2.64 | 3.36 | ||||
| 27 | α-Guaiene | 1437 | 2.80 | 2.07 | 1.23 | 8.19 | 2.48 | 3.67 | ||||
| 28 | Aromadendrene | 1440 | 2.15 | 3.18 | 3.54 | - | 2.22 | 2.31 | ||||
| 29 | (E)-β-Farnesene | 1456 | 1.45 | 0.77 | - | 1.25 | 2.59 | - | ||||
| 30 | β-Santalene | 1458 | 0.47 | - | 0.83 | 1.27 | - | - | ||||
| 31 | α-Humulene | 1461 | - | - | 2.65 | - | 0.85 | - | ||||
| 32 | γ-Muurolene | 1472 | 1.06 | - | - | 2.30 | - | - | ||||
| 33 | Germacrene D | 1482 | 7.43 | 8.97 | 9.93 | 1.26 | 2.71 | 9.78 | ||||
| 34 | α-Amorphene | 1484 | 0.42 | 0.86 | 0.60 | 0.27 | 0.72 | 0.80 | ||||
| 35 | Aristolochene | 1486 | 2.10 | 3.56 | 2.95 | - | 3.03 | 3.71 | ||||
| 36 | β-Selinene | 1488 | 1.60 | - | 0.22 | 1.01 | 1.23 | - | ||||
| 37 | Phenylethyl-3 methyl butanoate | 1490 | 0.41 | 0.47 | - | 1.18 | 0.27 | - | ||||
| 38 | Phenylethyl isovalerate | 1491 | 0.74 | 1.69 | 1.49 | - | 0.14 | 1.23 | ||||
| 39 | epi-Cubebol | 1495 | 0.44 | 0.24 | - | 1.37 | 1.17 | - | ||||
| 40 | α-Selinene | 1496 | 1.72 | 2.42 | 2.34 | - | 3.53 | 2.91 | ||||
| 41 | Bicyclogermacrene | 1498 | - | 0.35 | - | 0.83 | 2.97 | - | ||||
| 42 | α-Muurolene | 1500 | 0.62 | - | - | 1.79 | - | - | ||||
| 43 | β-Cadinene | 1508 | 2.00 | - | 1.07 | - | - | 0.92 | ||||
| 44 | γ-Cadinene | 1517 | 3.72 | 9.74 | 2.19 | 3.45 | 2.23 | 1.62 | ||||
| 45 | δ-Cadinene | 1521 | 2.33 | 1.31 | - | - | 2.57 | - | ||||
| 46 | Cadina-1,4-diene | 1525 | - | - | - | - | 0.12 | 1.55 | ||||
| 47 | α-Cadinene | 1533 | - | - | - | - | 0.19 | 1.36 | ||||
| 48 | α-Calacorene | 1542 | 0.61 | 0.16 | 0.35 | - | 0.11 | 0.51 | ||||
| 49 | (E)-Nerolidol | 1546 | 0.24 | 1.31 | 0.35 | - | 1.17 | 2.50 | ||||
| 50 | Occidentalol | 1553 | - | - | - | - | 2.33 | - | ||||
| 51 | Germacrene B | 1557 | 0.20 | 1.50 | 0.81 | - | 0.22 | 0.12 | ||||
| 52 | (Z)-3-Hexenyl benzoate | 1567 | 0.16 | 0.18 | 0.58 | - | 0.71 | 1.22 | ||||
| 53 | Longipinaol | 1573 | - | 0.15 | - | - | 1.77 | 0.64 | ||||
| 54 | Spathulenol | 1579 | 0.23 | - | 0.60 | 1.25 | 0.65 | - | ||||
| 55 | Caryophyllene oxide | 1585 | 0.30 | 3.59 | 1.16 | - | - | 1.74 | ||||
| 56 | Globulol | 1593 | 0.15 | 0.45 | 1.40 | - | 2.62 | 1.18 | ||||
| 57 | β-Calacorene | 1596 | 2.25 | 1.40 | 2.44 | 3.64 | 1.28 | - | ||||
| 58 | β-Oplopenone | 1608 | 0.49 | 2.87 | 0.81 | - | 0.18 | 1.91 | ||||
| 59 | δ-Cadinol | 1646 | - | 0.44 | 0.61 | - | 2.98 | 0.91 | ||||
| 60 | α-Cadinol | 1652 | - | - | - | 1.55 | - | - | ||||
| 61 | α-Eudesmol | 1654 | 0.17 | 0.74 | - | - | 1.74 | 1.76 | ||||
| 62 | β-Bisabolal | 1672 | 0.15 | - | - | - | 0.76 | 1.98 | ||||
| 63 | Pentadecanal | 1718 | 0.17 | 0.18 | - | - | 0.48 | 1.21 | ||||
| 64 | Benzyl benzoate | 1733 | 1.10 | 0.90 | 0.69 | - | - | - | ||||
| 65 | Octadecane | 1798 | 0.23 | 2.07 | - | - | 2.54 | - | ||||
| 66 | Cubitene | 1877 | 0.56 | - | 0.29 | 0.48 | - | - | ||||
| 67 | Laurenene | 1881 | 0.52 | - | - | - | 0.36 | - | ||||
| 68 | Pimaradiene | 1943 | 0.23 | 0.62 | - | - | 0.47 | - | ||||
| 69 | Neocembrene | 1960 | 0.14 | 0.11 | - | 1.69 | 1.87 | 0.18 | ||||
| 70 | Sclareol | 1973 | 0.28 | 0.89 | 0.26 | - | 1.38 | - | ||||
| 71 | Manoyl oxide | 1993 | 0.57 | - | - | - | - | - | ||||
| 72 | Palustradiene | 2005 | - | 0.22 | - | - | 0.36 | 2.18 | ||||
| Total percentage a | 0.51 | 0.50 | 0.47 | 0.42 | 0.53 | 0.48 | ||||||
| Essential oil (%) content | 93.76 | 95.03 | 95.93 | 95.00 | 95.77 | 97.03 | ||||||
2.2. Total Phenolic Content
2.3. Antioxidant Activity
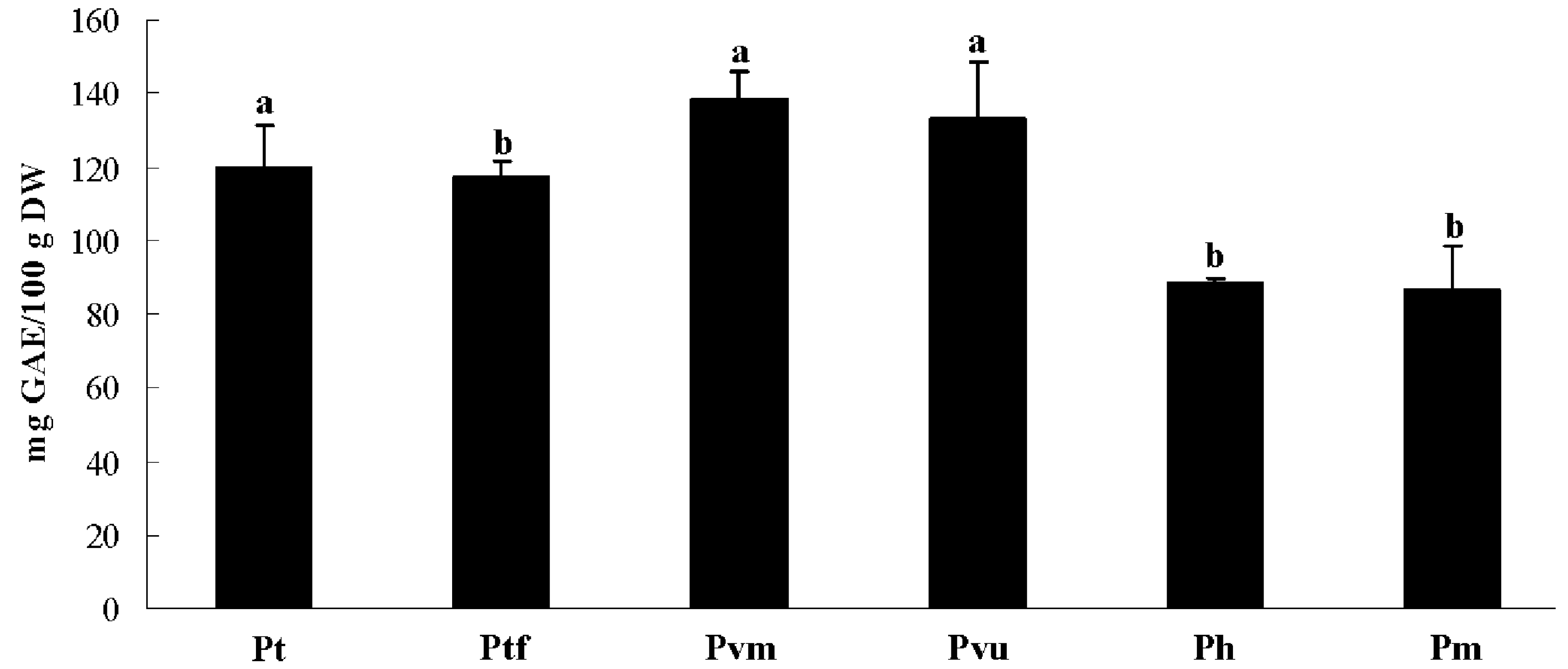
| Taxa | DPPH | FRAP | ABTS |
|---|---|---|---|
| Pt | 1775.22 ± 138.17 NF | 1036.68 ± 51.14 a | 3078.52 ± 278.59 a |
| Ptf | 1844.19 ± 180.55 | 904.72 ± 90.73 b | 2467.85 ± 141.63 b |
| Pvm | 1851.65 ± 151.19 | 1134.45 ± 36.14 ab | 3584.41 ± 315.63 a |
| Pvu | 1817.25 ± 131.19 | 814.72 ± 112.41 b | 3486.33 ± 140.75 c |
| Ph | 918.28 ± 25.37 | 584.78 ± 68.67 c | 2151.43 ± 215.03 b |
| Pm | 892.45 ± 78.31 | 477.78 ± 48.67 c | 1461.01 ± 131.03 c |
2.4. Correlation Analysis
| DPPH | FRAP | ABTS | |
|---|---|---|---|
| Panel A | |||
| TPC | 0.94 ** | 0.93 ** | 0.86 * |
| Panel B | |||
| DPPH | 1.00 | ||
| FRAP | 0.90 * | 1.00 | |
| ABTS | 0.84 * | 0.63 | 1.00 |
3. Experimental Section
3.1. Chemicals and Plant Materials
| Taxa | Origin | Latitude(°N)/Longitude(°E) | Elevation (m) |
|---|---|---|---|
| P. tabulaeformis | Huanglong, Shaanxi | 35.632/109.772 | 1127 |
| P. tabulaeformis f. shekanensis | Fuxian, Shaanxi | 35.998/108.690 | 1316 |
| P. tabulaeformis var. mukdensis | Anshan, Liaoning | 40.960/123.147 | 294 |
| P. tabulaeformis var. umbraculifera | Anshan, Liaoning | 41.009/123.124 | 250 |
| P. massoniana | Yangxian, Shaanxi | 33.326/107.624 | 722 |
| P. henryi | Nanzheng, Shaanxi | 32.857/106.586 | 1254 |
3.2. Identification of the Chemical Components of the Essential Oils
3.3. Determination of Total Phenolic
3.4. Antioxidant Capacity Determined by DPPH
3.5. Antioxidant Capacity Determined by Ferric Reducing Antioxidant Power (FRAP)
3.6. Antioxidant Capacity Determined by Radical Cation (ABTS)
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aqqarwal, B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Bonjean, K.; Cayeux, K.; Defraigne, J.O. Mecanismes physiologiques de la defense antioxidant. Clin. Nutr. Metab. 2002, 16, 233–239. [Google Scholar] [CrossRef]
- Glizczynska-Swiglo, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Weisburger, J.H. Lycopene and tomato products in health promotion. Exp. Biol. Med. 2002, 227, 924–927. [Google Scholar]
- Cheng, H.Y.; Li, R.X.; Chuang, L.Y. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules 2012, 17, 7294–7304. [Google Scholar]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Raseta, M. Antioxidant activities of Melittis melissophyllum L. (Lamiaceae). Molecules 2011, 16, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.P.; Fang, B.; Zhao, Q.Y.; Jiao, B.N.; Zhou, Z.Q. Falvonoid composition and antioxidant activities of Chinese local pummelo (Citrus grandis Osbeck.) varieties. Food Chem. 2014, 161, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lagha-Benamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crop. Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Graikou, K.; Gortzi, O.; Mantanis, G.; Chinou, I. Chemical composition and biological activity of the essential oil from the wood of Pinus heldreichii Christ. var. leucodermis. Eur. J. Wood Prod. 2012, 70, 615–620. [Google Scholar] [CrossRef]
- Song, Z.Q. Oleoresin Characteristics and Chemical Classification of Pinus, 2rd ed.; University of Science and Technology of China Press: Hefei, China, 2009; p. 7. [Google Scholar]
- Watanabe, K.; Momose, F.; Handa, H. Interaction between influenza virus pine cone antitumor substances that inhibit the virus multiplication. Biochem. Biophys. Res. Commun. 1995, 214, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Chung, H.J. Flavor compounds of pine sprout tea and pine needle tea. J. Agric. Food Chem. 2000, 48, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Lee, E. Effects of powdered pine needle (Pinus densiflora seib et Zucc.) on serum and liber lipid composition and antioxidative capacity in rats fed high oxidized fat. J. Korean Soc. Food Sci. Nutr. 2003, 32, 926–930. [Google Scholar]
- Kim, H.; Lee, B.; Yun, K.W. Comparison of chemical composition and antimicrobial activity of essential oils from three Pinus species. Ind. Crop. Prod. 2013, 44, 323–329. [Google Scholar] [CrossRef]
- Su, X.Y.; Wang, Z.Y.; Liu, J.R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]
- Bo, C.Y.; Zheng, G.Y.; Song, Q. Comparative study on chemical components of essential oils from Pinus massoniana, P. sylvestris var. mongolica and Abies nephrolepis needles. Chem. Ind. For. Prod. 2010, 30, 45–50. [Google Scholar]
- Zafar, I.; Mohammd, Z.U.R.; Shaista, J.K.; Aneela, F.; Shahid, M. GC-MS studies of needles essential oils of Pinus roxburghaii and their antimicrobial activity. Pak. J. Biochem. Mol. Biol. 2011, 44, 36–38. [Google Scholar]
- Yang, J.K.; Kang, B.K.; Kim, T.H.; Hong, S.C.; Seo, W.T.; Choi, M.S. Efficient extraction methods and analysis of essential oil from softwood leaves. Korean J. Biotechnol. Bioeng. 2002, 17, 357–364. [Google Scholar]
- Amri, I.; Hamrouni, L.; Hanana, M.; Gargouri, S.; Fezzani, T.; Jamoussi, B. Chemical composition, physico-chemical properties, antifungal and herbicidal activities of Pinus halepensis Miller essential oils. Biol. Agric. Hortic. 2013, 29, 91–106. [Google Scholar] [CrossRef]
- Dob, T.; Berramdane, T.; Chelgoum, C. Chemical composition of essential oil of Pinus halepensis Miller growing in Algeria. C. R. Chim. 2005, 8, 1939–1945. [Google Scholar]
- Chang, C.W.; Chang, W.L.; Chang, S.T.; Cheng, S.S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008, 42, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Chua, M.T.; Chang, E.H.; Huang, C.G.; Chen, W.J.; Chang, S.T. Variations in insecticidal activity and chemical compositions of leaf essential oils from Cryptomeria japonica at different ages. Bioresour. Technol. 2008, 100, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, Ö.; Koçak, A. Essential oil composition of six Pinus L. taxa (Pinaceae) from Canada and their chemotaxonomy. J. Agric. Sci. Technol. 2014, 4, 67–73. [Google Scholar]
- Jin, Y.J.; Wu, J.K.; Sun, F.; Deng, W.H.; Wang, H.J.; Sun, W.C. Terpene composition of needle oil from Pinus tabulaeformis and the comparison with other two-needle pines. J. Beijing For. Coll. 1994, 16, 38–47. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Giorgi, A.; Madeo, M.; Speranza, G.; Cocucci, M. Influence of environmental factors on composition of phenolic antioxidants of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2010, 24, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Rieger, G.; Muller, M.; Guttenberger, H.; Bucar, F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Wildi, B.; Lutz, C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996, 19, 138–146. [Google Scholar] [CrossRef]
- Aruoma, O. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 523–524, 9–20. [Google Scholar] [CrossRef]
- Lissi, E.A.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinous exudates from Heliotropium sp. A comparison of ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem. Toxicol. 2006, 44, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.N.; Cao, X.F.; Xu, F.; Sun, R.C.; Jones, G.L. Structural features and antioxidant activities of lignins from steam-exploded bamboo (Phyllostachys pubescens). J. Agric. Food Chem. 2014, 62, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Woo, K.S.; Ko, J.Y.; Jeong, H.S. Effect of milling time on antioxidant compounds and activities of methanol extracts of sorghum [Sorghum bicolor (L.) Moench]. Food Sci. Biotechnol. 2014, 23, 1741–1746. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Barcia, M.T.; Rodrigues, D.; Cruz, P.N.; Hermosín-Gutiérrez, I.; Smith, R.; Godoy, H.T. Antioxidant activity of hydrophilic and lipophilic extracts of Brazilian blueberries. Food Chem. 2014, 164, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Tavano, O.L. Use of different spices as potential natural antioxidant additives on cooked beans (Phaseolus vulgaris). Increase of DPPH radical scavenging activity and total phenolic content. Plant Foods Hum. Nutr. 2014, 69, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Fernanda, D.B.; Abadio, F.; Dietmar, R.K.; Reinhold, C.; Tseng, W.H.; Böser, S.; Graeve, L. Antioxidant activity and characterization of phenolic compounds from bacaba (Oenocarpus bacaba Mart.) fruit by HPLC-DAD-MS. J. Agric. Food Chem. 2012, 22, 7665–7673. [Google Scholar]
- Du, G.M.; Li, M.J.; Ma, F.W.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Wang, L.J.; Su, S.; Wu, J.; Du, H.; Li, S.S.; Huo, J.W.; Zhang, Y.; Wang, L.S. Variation of anthocyanins and flavonols in Vaccinium uliginosum berry in Lesser Khingan Mountains and its antioxidant activity. Food Chem. 2014, 160, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H.; Talebi, M. Total phenolic content and antioxidant activity of three Iranian endemic Achillea species. Ind. Crop. Prod. 2013, 50, 154–158. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Co.: Carol Stream, IL, USA, 1995. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the essential oils are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Liu, Z.; Li, Z. Chemical Composition and Antioxidant Activity of Essential Oil of Six Pinus Taxa Native to China. Molecules 2015, 20, 9380-9392. https://doi.org/10.3390/molecules20059380
Xie Q, Liu Z, Li Z. Chemical Composition and Antioxidant Activity of Essential Oil of Six Pinus Taxa Native to China. Molecules. 2015; 20(5):9380-9392. https://doi.org/10.3390/molecules20059380
Chicago/Turabian StyleXie, Qing, Zhihong Liu, and Zhouqi Li. 2015. "Chemical Composition and Antioxidant Activity of Essential Oil of Six Pinus Taxa Native to China" Molecules 20, no. 5: 9380-9392. https://doi.org/10.3390/molecules20059380





