Synthesis, In-Vitro Antibacterial, Antifungal, and Molecular Modeling of Potent Anti-Microbial Agents with a Combined Pyrazole and Thiophene Pharmacophore
Abstract
:1. Introduction
2. Results and Discussion
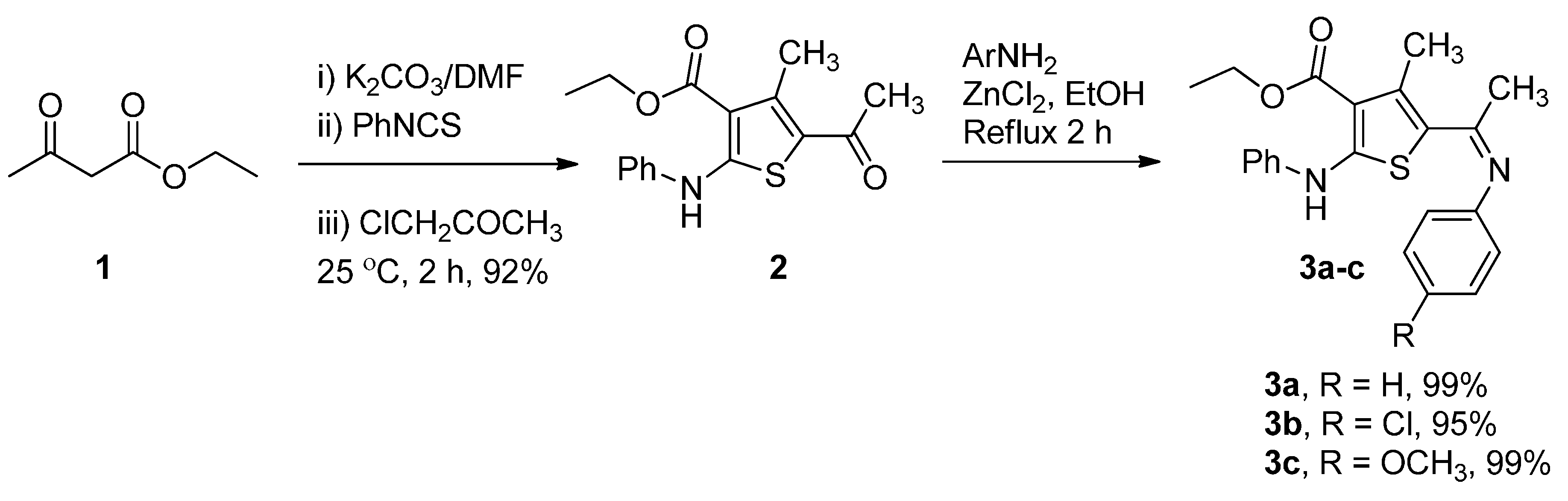
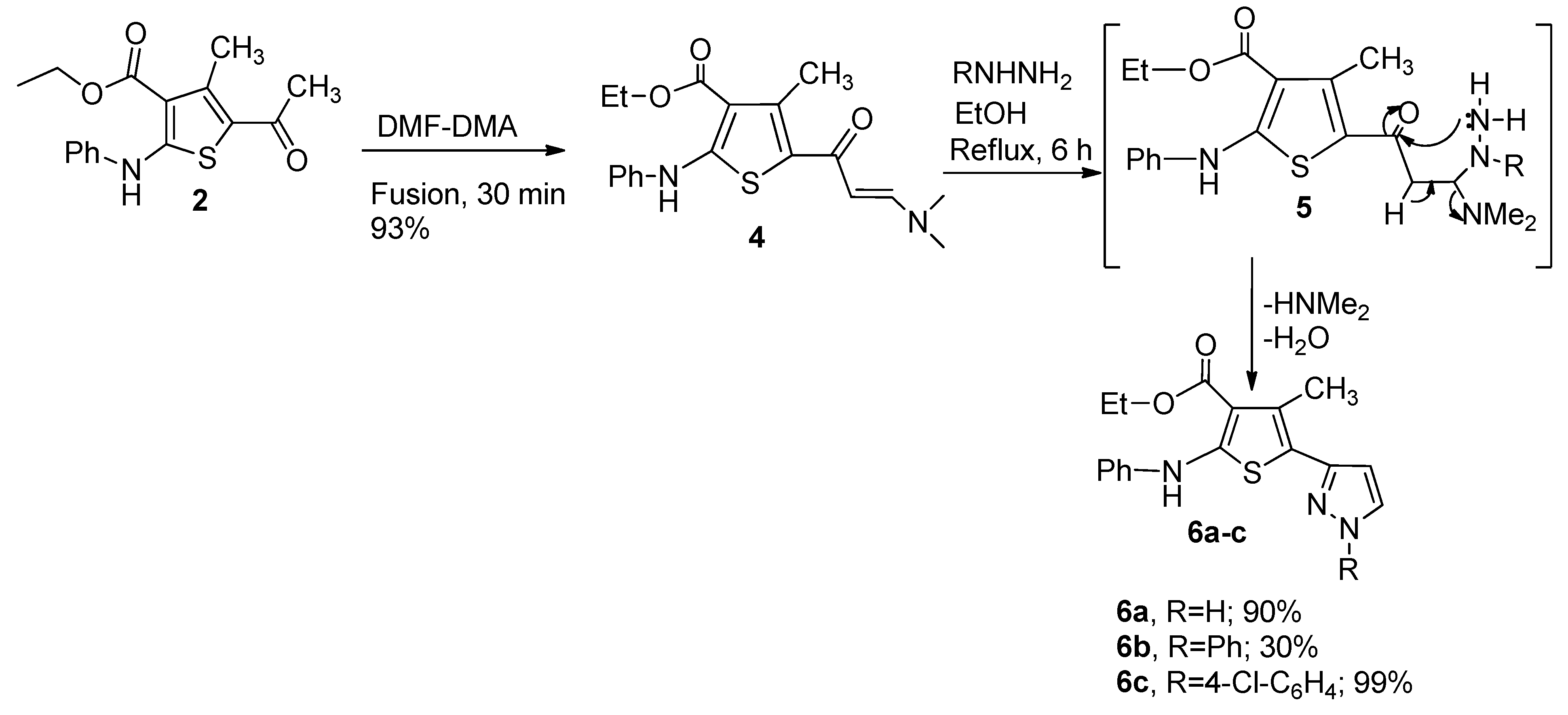
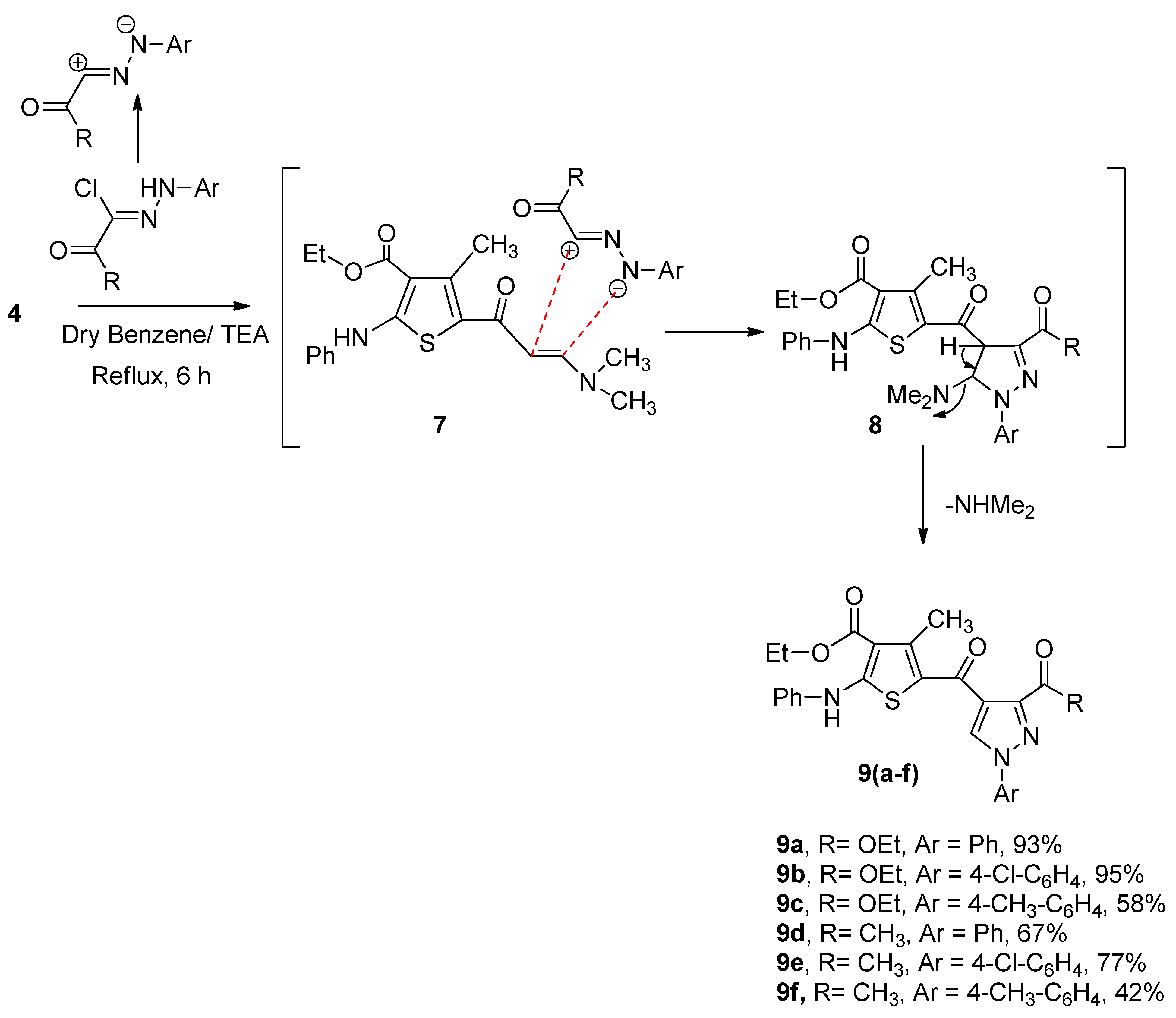
2.1. Synthesis of Compounds 2, 3a–c, 4, 6a–c and 9a–f
2.2. X-ray Crystal Structure of Compound 2
| Crystal Data | |
| C16H17NO3S | V = 1468.7 (4) Å3 |
| Mr = 303.37 | Z = 4 |
| Monoclinic, P21/c | Mo Kα radiation |
| a = 8.5752(16) Å | µ = 0.23 mm−1 |
| b = 21.046(4) Å | T = 100 K |
| c = 8.2941(12) Å | 0.33 × 0.17 × 0.14 mm |
| β = 101.131(6)° | |
| Data Collection | |
| Bruker Kappa APEXII Duo diffractometer | 2854 reflections with I > 2σ(I) |
| Absorption correction: multi-scan Blessing, 1995 | Rint = 0.044 |
| Tmin = 0.684, Tmax = 0.746 | θmax = 28.3° |
| 14240 measured reflections | Standard reflections: 0 |
| 3620 independent reflections | |
| Refinement | |
| R[F2 > 2σ(F2)] = 0.037 | 0 restraints |
| wR(F2) = 0.090 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | Δρmax = 0.37 e Å−3 |
| 3620 reflections | Δρmin = −0.24 e Å−3 |
| 197 parameters | |
| S1—C1 | 1.7280(14) | C7—H7 | 0.9500 |
| S1—C4 | 1.7510(15) | C8—C9 | 1.390(2) |
| N1—C1 | 1.3571(18) | C8—H8 | 0.9500 |
| N1—C5 | 1.4075(18) | C9—C10 | 1.383(2) |
| N1—H1 | 0.860(19) | C9—H9 | 0.9500 |
| O1—C15 | 1.2335(18) | C10—H10 | 0.9500 |
| C1—C2 | 1.413(2) | C12—C13 | 1.506(2) |
| O2—C11 | 1.2294(17) | C12—H12A | 0.9900 |
| D | H | A | D-H | H...A | D...A | D-H…A |
|---|---|---|---|---|---|---|
| N1 | H1 | O2 | 0.86(2) | 1.91(2) | 2.653(2) | 143(2) |
| C6 | H6 | S1 | 0.9500 | 2.46 | 3.158(2) | 130.00 |
| C14 | H14B | O3 | 0.9800 | 2.4200 | 2.793(2) | 102.00 |
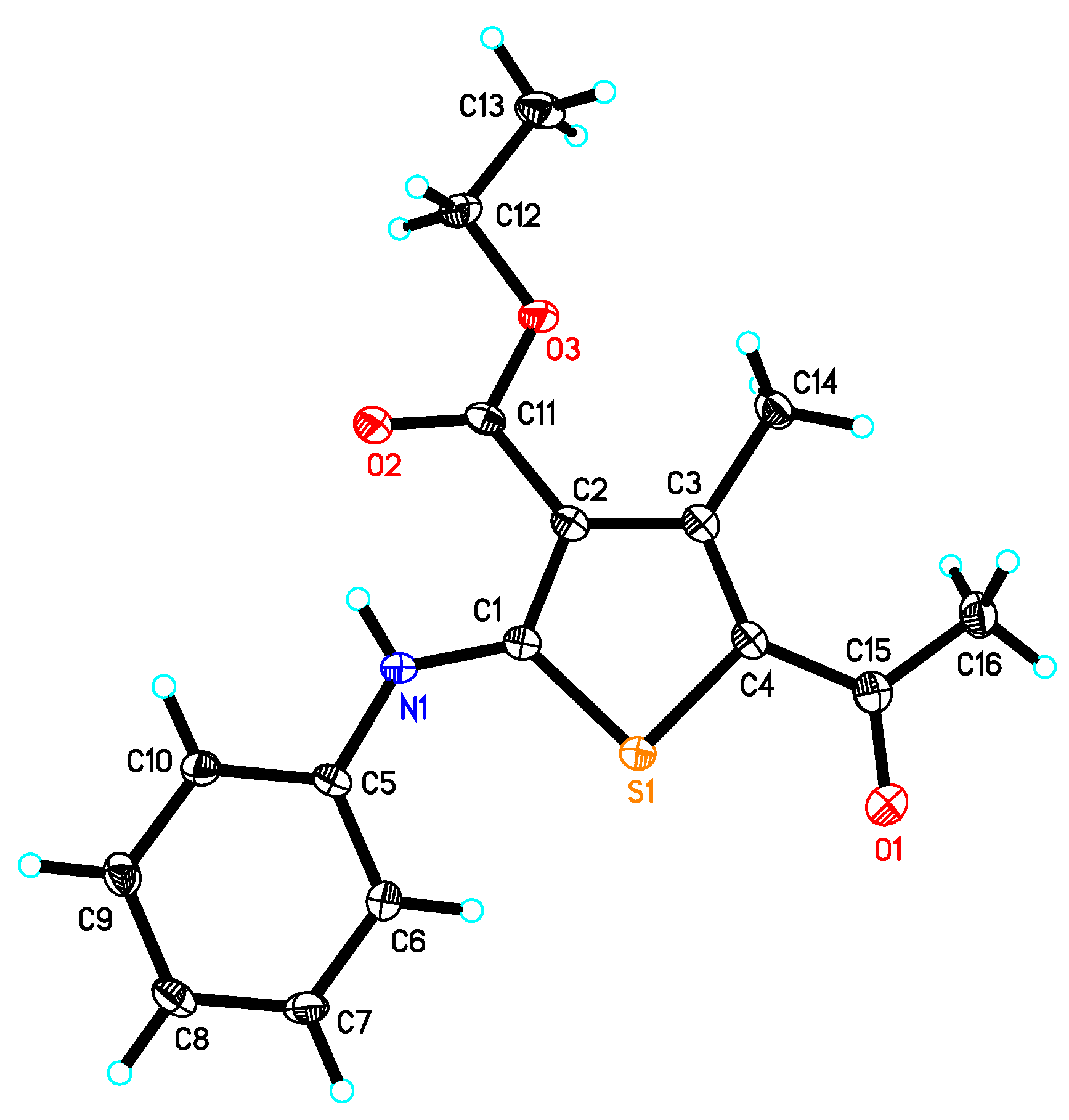
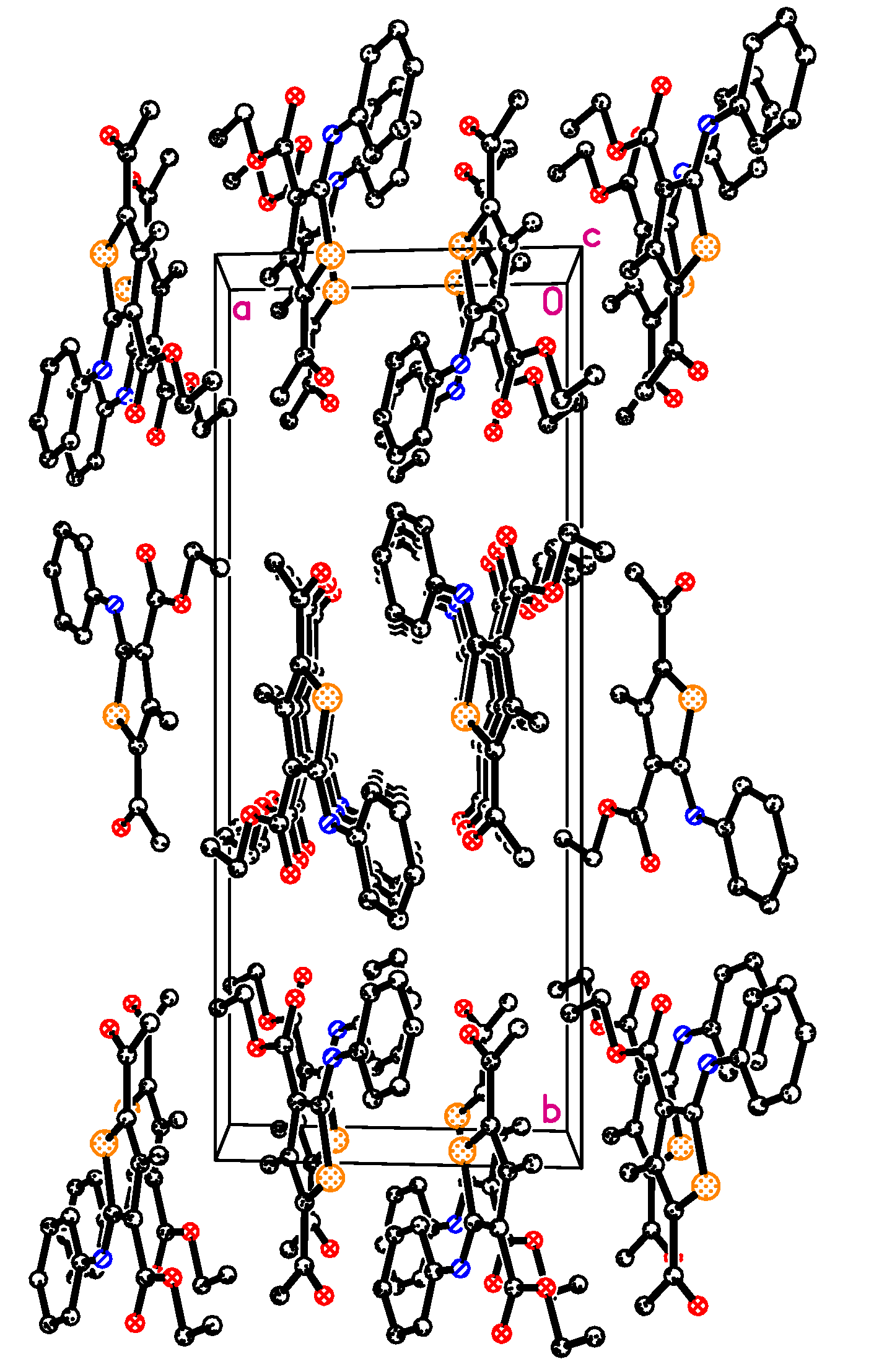
2.3. Antimicrobial Activity
| Compd. | Fungi [a] | Gram (+) Bacteria [b] | Gram (−) Bacteria [c] | |||||
|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (E) | (F) | (G) | (H) | |
| 2 | 13.8 ± 0.42 | 14.1 ± 0.35 | 13.2 ± 0.34 | 10.8 ± 0.22 | 13.7 ± 0.31 | 10.5 ± 0.32 | 11.7 ± 0.41 | 12.5 ± 0.48 |
| 3a | 23.8± 0.42 | 13.5 ± 0.29 | 16.7 ± 0.42 | 18.1 ± 0.41 | 20.8 ± 0.54 | 22.3 ± 0.64 | 15.3 ± 0.47 | 18.1 ± 0.62 |
| 3b | 15.9± 0.52 | 14.5 ± 0.34 | 16.4 ± 0.35 | 12.7 ± 0.37 | 12.2 ± 0.33 | 13.9 ± 0.52 | 11.8 ± 0.42 | 14.7 ± 0.50 |
| 3c | 24.3± 0.68 | 24.5 ± 0.64 | 25.8 ± 0.58 | 14.3 ± 0.58 | 16.3 ± 0.52 | 19.6 ± 0.58 | 12.5 ± 0.39 | 14.8 ± 0.47 |
| 4 | 23.7 ± 0.1 | 19.7 ± 0.2 | 28.7 ± 0.2 | 25.4 ± 0.1 | 23.8 ± 0.2 | 32.4 ± 0.3 | 17.3 ± 0.1 | 19.9 ± 0.3 |
| 6a | 13.9 ± 0.42 | 11.8 ± 0.31 | 13.7 ± 0.34 | 14.0 ± 0.29 | 16.9 ± 0.42 | 17.6 ± 0.31 | 12.9 ± 0.28 | 14.7 ± 0.4 |
| 6b | 20.6 ± 0.5 | 16.7 ± 0.33 | 22.4 ± 0.36 | 17.6 ± 0.58 | 18.3 ± 0.25 | 22.6 ± 0.44 | 19.3 ± 0.52 | 17.8 ± 0.44 |
| 6c | 16.8 ± 0.39 | 13.4 ± 0.58 | 19.6 ± 0.19 | 15.9 ± 0.44 | 16.7 ± 0.36 | 19.2 ± 0.27 | 13.3 ± 0.36 | 13.6 ± 0.36 |
| 9a | 22.3 ± 0.2 | 16.5 ± 0.25 | 25.8 ± 0.58 | 12.3 ± 0.35 | 19.5 ± 0.44 | 29.8 ± 0.58 | 12.3 ± 0.25 | 17.6 ± 0.19 |
| 9b | 20.6 ± 0.35 | 14.8 ± 0.34 | 21.5 ± 0.62 | 10.9 ± 0.18 | 17.8 ± 0.58 | 20.1 ± 0.39 | 10.9 ± 0.31 | 15.3 ± 0.32 |
| 9c | 21.7 ± 0.5 | 18.1 ± 0.32 | 20.7 ± 0.34 | 12.6 ± 0.37 | 18.7 ± 0.62 | 24.9 ± 0.46 | 13.8 ± 0.43 | 17.1 ± 0.52 |
| 9d | 17.8 ± 0.57 | 14.6 ± 0.64 | 18.0 ± 0.72 | 15.4 ± 0.36 | 16.9 ± 0.58 | 18.2 ± 0.44 | 11.8 ± 0.48 | 14.2 ± 0.42 |
| 9e | 19.1 ± 0.58 | 16.7 ± 0.48 | 14.9 ± 0.63 | 12.7 ± 0.44 | 16.8 ± 0.62 | 17.9 ± 0.48 | 18.1 ± 0.58 | 17.4 ± 0.47 |
| 9f | 23.7 ± 0.1 | 19.7 ± 0.2 | 28.7 ± 0.2 | 25.4 ± 0.1 | 23.8 ± 0.2 | 32.4 ± 0.3 | 12.9 ± 0.43 | 12.7 ± 0.56 |
| SD-1 [d] | 23.7 ± 0.1 | 19.7 ± 0.2 | 28.7 ± 0.2 | 25.4 ± 0.1 | - | - | - | - |
| SD-2 [e] | - | - | - | - | 23.8 ± 0.2 | 32.4 ± 0.3 | - | - |
| SD-3 [f] | - | - | - | - | - | - | 17.3 ± 0.1 | 19.9 ± 0.3 |
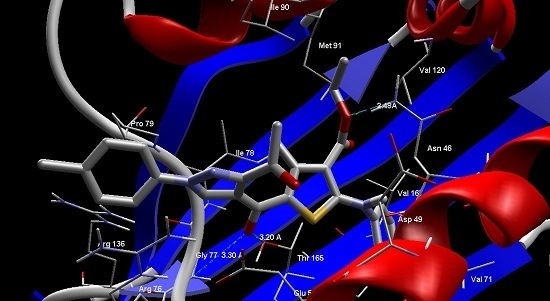
2.4. Molecular Modeling
| Ligand | E. Coli 24 kda Domain | Cytochrome p450 14α-Sterol Demethylase |
|---|---|---|
| MolDock Score | MolDock Score | |
| 2 | −129.864 | −146.394 |
| 4 | −140.52 | −144.834 |
| 3a | −140.548 | −157.378 |
| 3b | −149.314 | −147.109 |
| 3c | −140.293 | −139.61 |
| 6a | −141.739 | −153.137 |
| 6b | −146.186 | −167.821 |
| 6c | −144.798 | −175.468 |
| 9a | −148.469 | −215.797 |
| 9b | −167.672 | −221.17 |
| 9c | −160.583 | −213.93 |
| 9d | −165.572 | −190.91 |
| 9e | −168.035 | −204.502 |
| 9f | −169.884 | −167.873 |
| Reference | −175.052 | −136.776 |
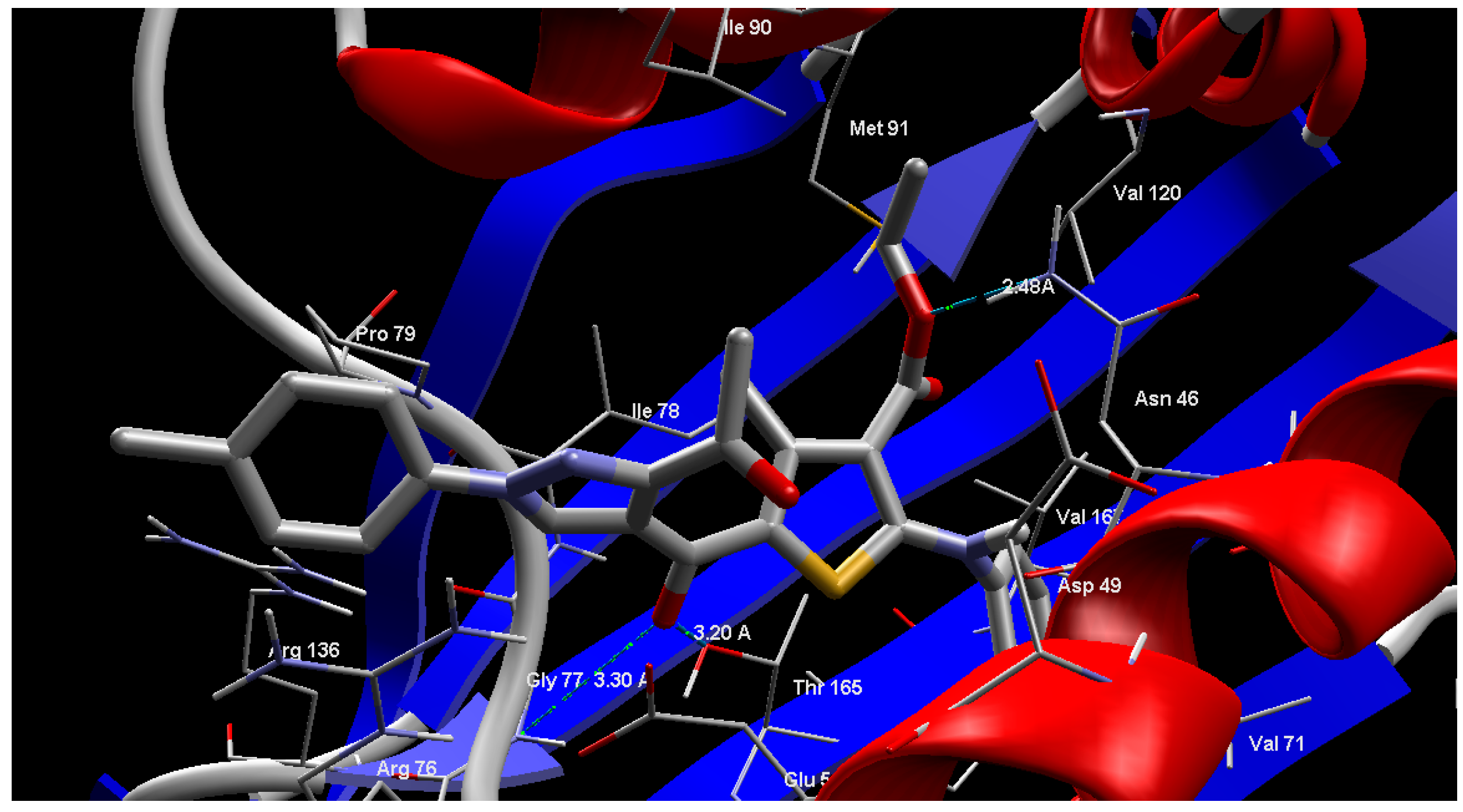
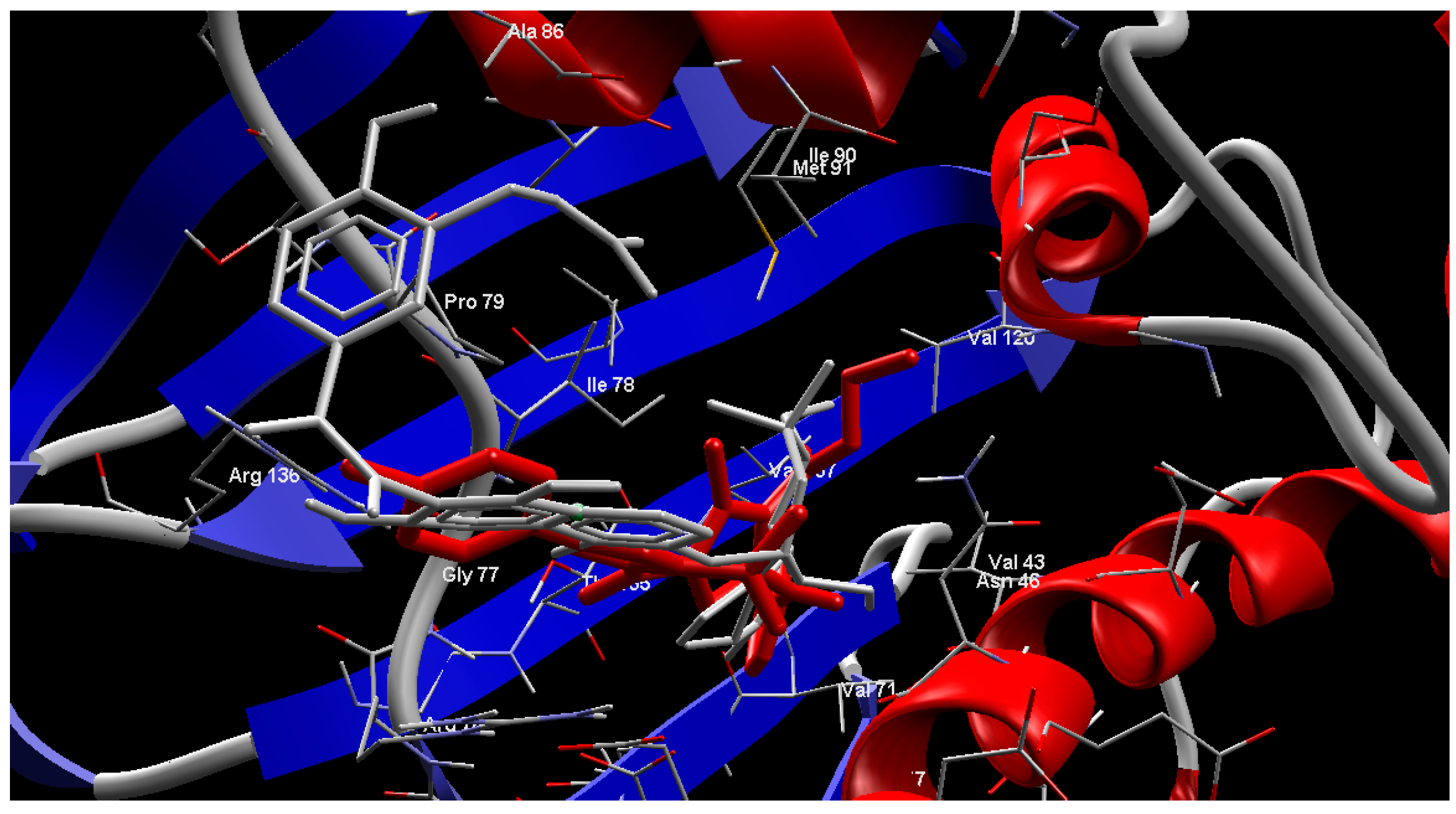
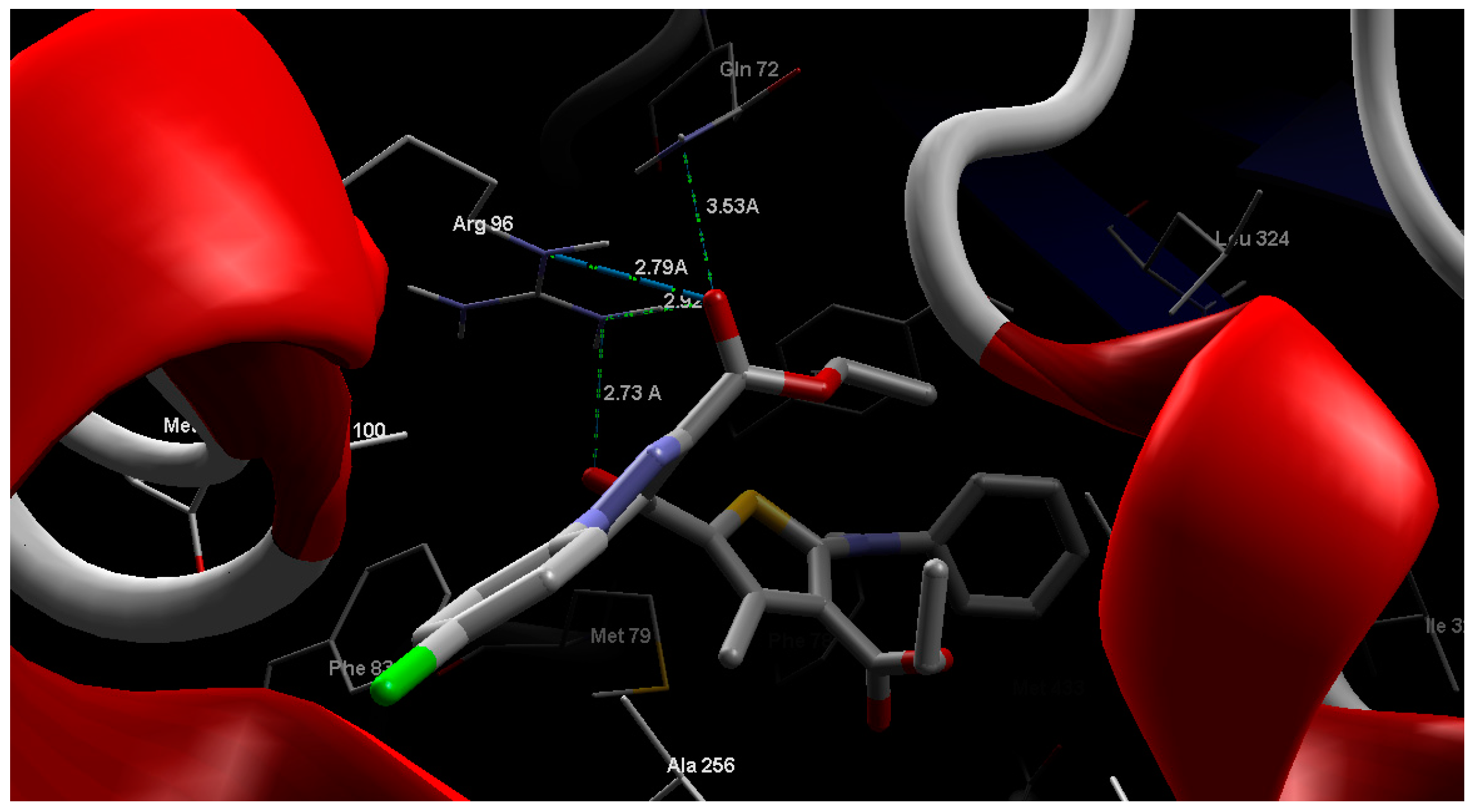
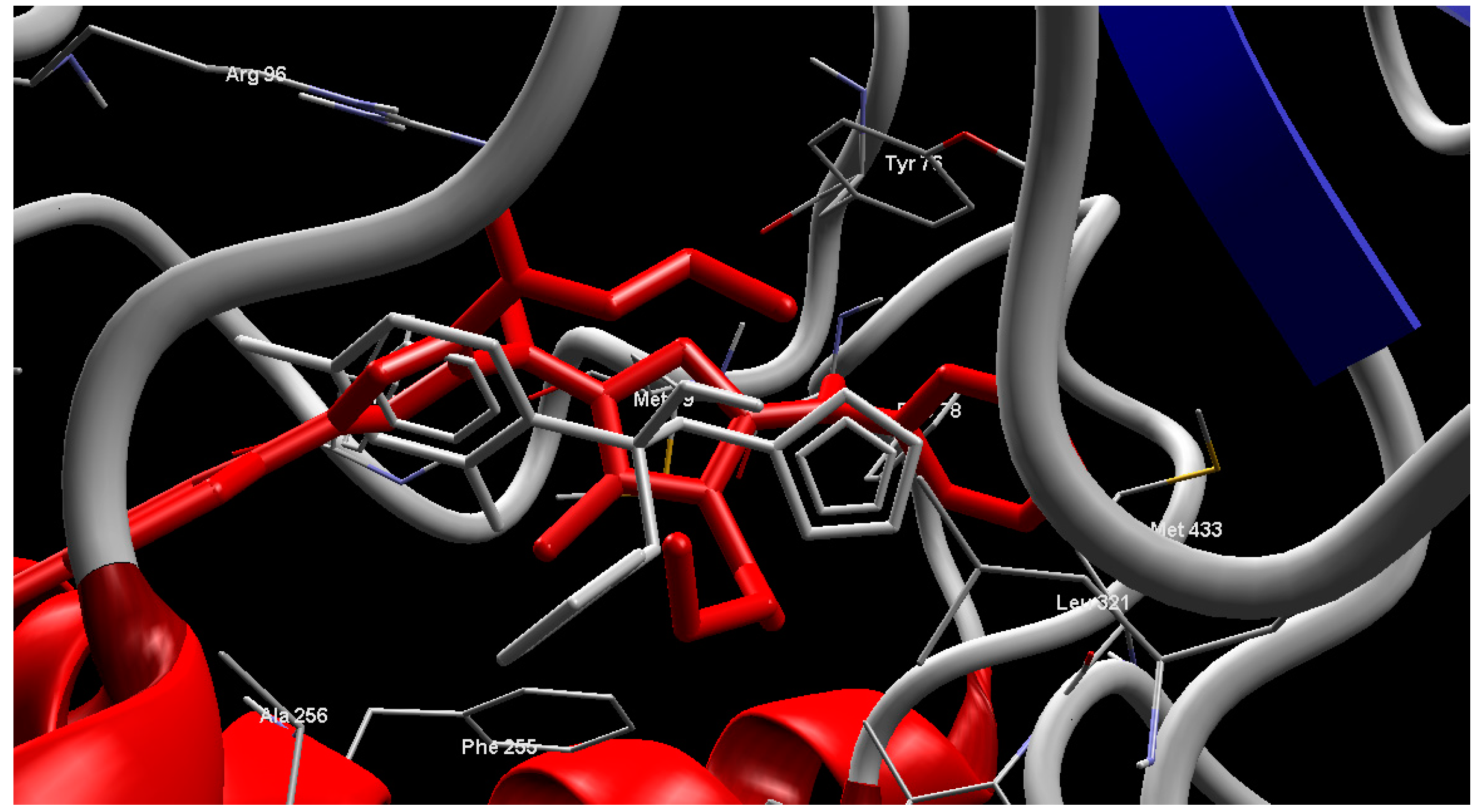
3. Experimental Section
3.1. General
3.2. Preparation of Ethyl 5-Acetyl-4-methyl-2-(phenylamino)thiophene-3-carboxylate (2)
3.3. General Preparation of Compounds 3a–c (GP1)
3.4. Preparation of (E)-Ethyl 5-(3-(dimethylamino)acryloyl)-4-methyl-2-(phenylamino)thiophene-3-carboxylate (4)
3.5. General Preparation of compounds 6a–c (GP2)
3.6. General Preparation of Compounds 9a–f (GP3)
3.7. Antifungal Activity of Compounds 2, 3a–c, 4, 6a–c and 9a–f
3.8. Antibacterial Activity of Compounds 2, 3a–c, 4, 6a–c and 9a–f
3.9. Molecular Modeling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ragavendran, J.V.; Sriram, D.; Patil, S.; Reddy, I.V.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide–GABA–anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Puthucode, R.N.; Smith, J.M.; Hetherington, M.; Wilson Quail, J.; Pugazhenthi, U.; Lechler, T.; Stables, J.P. Anticonvulsant activities of some arylsemicarbazones displaying potent oral activity in the maximal electroshock screen in rats accompanied by high protection indices. J. Med. Chem. 1996, 39, 3984–3997. [Google Scholar] [CrossRef] [PubMed]
- Polivka, Z.; Holubek, J.; Svatek, E.; Metys, J.; Protiva, M. Regio-and stereo-selection reaction of 1,3-dialkyl-substituted allyl anions with aldehydes via η3-allyltitanium compounds. J. Chem. Soc. Chem. Commun. 1984, 49, 621–623. [Google Scholar]
- Shank, R.P.; Doose, D.R.; Streeter, A.J.; Bialer, M. Plasma and whole blood pharmacokinetics of topiramate: The role of carbonic anhydrase. Epilepsy Res. 2005, 63, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Thirumurugan, R.; Kavya, R.; Samuel, J.S.; Stables, J.D. Sriram, 3-Chloro-2-methylphenyl-substituted semicarbazones: Synthesis and anticonvulsant activity. Eur. J. Med. Chem. 2004, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Kucukguzel, S.G.; Mazi, A.; Sahin, F.; Ozturk, S.; Stables, J. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003, 38, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, R.; Sriram, D.; Saxena, A.; Stables, J.; Yogeeswari, P. 2,4-Dimethoxyphenylsemicarbazones with anticonvulsant activity against three animal models of seizures: Synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2006, 14, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Anis, I.; Aziz-ur-Rehman; Malik, A.; Ahmed, Z.; Muhammad, P.; Shujaat, S.; Atta-ur-Rahman. Emodinol, β-Glucuronidase, inhibiting triterpine from Paeonia emodi. Nat. Prod. Res. 2003, 17, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Khan, A.; Farooq, U.; Kousar, F.; Khan, S.S.; Nawaz, S.A.; Abbasi, M.A.; Choudhary, M.I. Three new cholinesterase-inhibiting cis-clerodane diterpenoids from otostegia limbata. Chem. Pharm. Bull. 2005, 53, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Lapis, A.A.M.; Bernd, A.B.; Russowsky, D. Studies on the Eschenmoser coupling reaction and insights on its mechanism. Application in the synthesis of Norallosedamine and other alkaloids. Tetrahedron 2009, 65, 2484–2496. [Google Scholar] [CrossRef]
- Cvetovich, R.J.; Pipik, B.; Hartner, F.W.; Grabowski, E.J. Rapid synthesis of tetrahydro-4H-pyrazolo[1,5-a]diazepine-2-carboxylate. Tetrahedron Lett. 2003, 44, 5867–5870. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhang, X.N.; Mo, L.P.; Li, Y.X.; Ma, F.P. Catalyst-free synthesis of quinazoline derivatives using low melting sugar-urea-salt mixture as a solvent. Green. Chem. 2012, 14, 1502–1506. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Farooq, F. Heterocyclic Silica supported sodium hydrogen sulfate (NaHSO4–SiO2): A novel, green catalyst for synthesis of pyrazole and pyranyl pyridine derivatives under solvent-free condition via heterocyclic β-enaminones. J. Mol. Catal. A Chem. 2012, 363, 451–459. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Ahmed, N.; Farooq, F.; Khan, K. Highly efficient solvent-free synthesis of novel pyranyl pyridine derivatives via β-enaminones using ZnO nanoparticles. Tetrahedron Lett. 2013, 54, 3599–3604. [Google Scholar] [CrossRef]
- Li, M.Y.; Xu, H.W.; Fan, W.; Ye, Q.; Wang, X.; Jiang, B.; Wan, S.L.; Tu, S.J. New formal (3+3) cycloaddition of enaminones for forming tetracyclic indolo[2,3-b]quinolines under microwave irradiation. Tetrahedron 2014, 70, 1004–1010. [Google Scholar] [CrossRef]
- Abass, M.; Mostafa, B.B. Synthesis and evaluation of molluscicidal and derived from 4-hydroxyquinolinones: Part IX. Bioorg. Med. Chem. 2005, 13, 6133–6144. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.S.; Scott, K.R.; Gao, H.; Eddington, N.D. Effect of P-glycoprotein on the pharmacokinetics and tissue distribution of enaminone anticonvulsants: analysis by population and physiological approaches. J. Pharmacol. Exp. Ther. 2002, 302, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.S.; Scott, K.R.; Gao, H.; Raje, S.; Eddington, N.D. Influence of multidrug resistance (MDR) proteins at the blood-brain barrier on the transport and brain distribution of enaminone anticonvulsants. J. Pharm. Sci. 2001, 90, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Edafiogho, I.O.; Kombian, S.B.; Ananthalakshmi, K.V.; Salama, N.N.; Eddington, N.D.; Wilson, T.L.; Alexander, M.S.; Jackson, P.L.; Hanson, C.D.; Scott, K.R. Enaminones Exploring additional therapeutic activities. Pharm. Sci. 2007, 96, 2509–2531. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Barakat, A.; Yousuf, S.; Choudhary, M.I.; Frey, W.; Ben Hadda, T.; Mubarak, M.S. Substituted thieno[2,3-b]thiophenes and related congeners: Synthesis, β-glucuronidase inhibition activity, crystal structure, and POM analyses. Bioorg. Med. Chem. 2014, 22, 6715–6725. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Al-Majid, A.M.; Barakat, A.; Alshahrani, S.; Yamin, S. 1,1'-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as a building block in heterocyclic synthesis. Novel synthesis of some pyrazole and pyrimidine derivatives. Molecules 2011, 16, 6502–6511. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Al-Majid, A.M.; Alamary, A.S. Synthesis and chemical characterisation of some new diheteroaryl thienothiophene derivatives. Molecules 2011, 16, 7706–7714. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing Sulphur. Molecules 2009, 14, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, N.Y.; Barakat, A.; Al-Majid, A.M.; Choudhary, M.I. Synthesis of thieno[2,3-b]thiophene containing bis-heterocycles-novel pharmacophores. Int. J. Mol. Sci. 2013, 14, 5712–5722. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar]
- Smania, A.; Monache, F.D.; Smania, E.F.A.; Cuneo, R.S. Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Med. Mushrooms 1999, 1, 325–330. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank. Available online: http://www.rcsb.org/pdb (accessed on 1 March 2015).
- Molegro Virtual Docker (MVD 2013.6.0.0). Available online: http://www.molegro.com (accessed on 28 September 2013).
- Kerwin, S.M. ChemBioOffice Ultra 2010 suite. J. Am. Chem. Soc. 2010, 132, 2466–2467. [Google Scholar] [CrossRef] [PubMed]
- MarvinSketch, version 6.1.0. Available online: http://www.chemaxon.com/ (accessed on 2 October 2013).
- Lafitte, D.; Lamour, V.; Tsvetkov, P.O.; Makarov, A.A.; Klich, M.; Deprez, P.; Moras, D.; Briand, C.; Gilli, R. DNA gyrase interaction with coumarin-based inhibitors: The role of the hydroxybenzoate isopentenyl moiety and the 5'-methyl group of the noviose. Biochemistry 2002, 41, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Banfi, E.; Scialino, G.; Zampieri, D.; Mamolo, M.G.; Vio, L.; Ferrone, M.; Fermeglia, M.; Paneni, M.S.; Pricl, S. Antifungal and antimycobacterial activity of new imidazole and triazole derivatives. A combined experimental and computational approach. J. Antimicrob. Chemother. 2006, 58, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of compounds 2–9 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabkhot, Y.N.; Kaal, N.A.; Alterary, S.; Al-Showiman, S.S.; Barakat, A.; Ghabbour, H.A.; Frey, W. Synthesis, In-Vitro Antibacterial, Antifungal, and Molecular Modeling of Potent Anti-Microbial Agents with a Combined Pyrazole and Thiophene Pharmacophore. Molecules 2015, 20, 8712-8729. https://doi.org/10.3390/molecules20058712
Mabkhot YN, Kaal NA, Alterary S, Al-Showiman SS, Barakat A, Ghabbour HA, Frey W. Synthesis, In-Vitro Antibacterial, Antifungal, and Molecular Modeling of Potent Anti-Microbial Agents with a Combined Pyrazole and Thiophene Pharmacophore. Molecules. 2015; 20(5):8712-8729. https://doi.org/10.3390/molecules20058712
Chicago/Turabian StyleMabkhot, Yahia Nasser, Nahed Ahmed Kaal, Seham Alterary, Salim S. Al-Showiman, Assem Barakat, Hazem A. Ghabbour, and Wolfgang Frey. 2015. "Synthesis, In-Vitro Antibacterial, Antifungal, and Molecular Modeling of Potent Anti-Microbial Agents with a Combined Pyrazole and Thiophene Pharmacophore" Molecules 20, no. 5: 8712-8729. https://doi.org/10.3390/molecules20058712