Effect of Culture Conditions on Metabolite Production of Xylaria sp.
Abstract
:1. Introduction

2. Results and Discussion
2.1. Structure Determination
| Positionp | 6 | 7 | 8 | |||
|---|---|---|---|---|---|---|
| δH (mult., J in Hz) | δC (mult.) | δH (mult., J in Hz) | δC (mult.) | δH (mult., J in Hz) | δC (mult.) | |
| 2 | 161.6, qC | 163.3, qC | 164.1, qC | |||
| 3 | 5.96, d(2.0) | 93.5, CH | 5.54, d (2.2) | 87.5, CH | 5.52, d (2.2) | 87.7, CH |
| 4 | 169.5, qC | 171.0, qC | 171.5, qC | |||
| 5 | 6.91, d(2.0) | 104.6, CH | 6.10, d (2.2) | 97.7, CH | 6.05, d (2.2) | 99.9, CH |
| 6 | 153.9, qC | 167.8, qC | 166.0, qC | |||
| 7 | 193.0, qC | 4.22, dt (5.0, 7.6) | 68.9, CH | 2.44, t (7.5) | 33.1, CH2 | |
| 8 | 2.85, t (7.2) | 37.2, CH2 | 1.64, m/1.53, m | 34.7, CH2 | 1.55, dt (7.5) | 26.6, CH2 |
| 9 | 1.54, m | 23.0, CH2 | 1.30, m | 25.1, CH2 | 1.30, m | 25.3, CH2 |
| 10 | 1.28, m | 24.7, CH2 | 1.30, m | 24.7, CH2 | 1.42, m | 32.6, CH2 |
| 11 | 1.35, m | 38.7, CH2 | 1.30, m | 38.9, CH2 | 3.38, dd (6.4, 11.5) | 61.0, CH2 |
| 12 | 3.56, m | 65.6, CH | 3.54, m | 65.6, CH | ||
| 13 | 1.03, d (6.1) | 23.6, CH3 | 1.01, d (6.2) | 23.6, CH3 | ||
| 4-OCH3 | 3.88, s | 56.9, CH3 | 3.81, s | 56.3, CH3 | 3.79, s | 56.7, CH3 |
| 12-OH | 4.32, d (4.9) | 4.29, d (5.0) | ||||
| 7-OH | 5.58, d (5.4) | |||||
| 11-OH | 4.36, t (5.1) | |||||
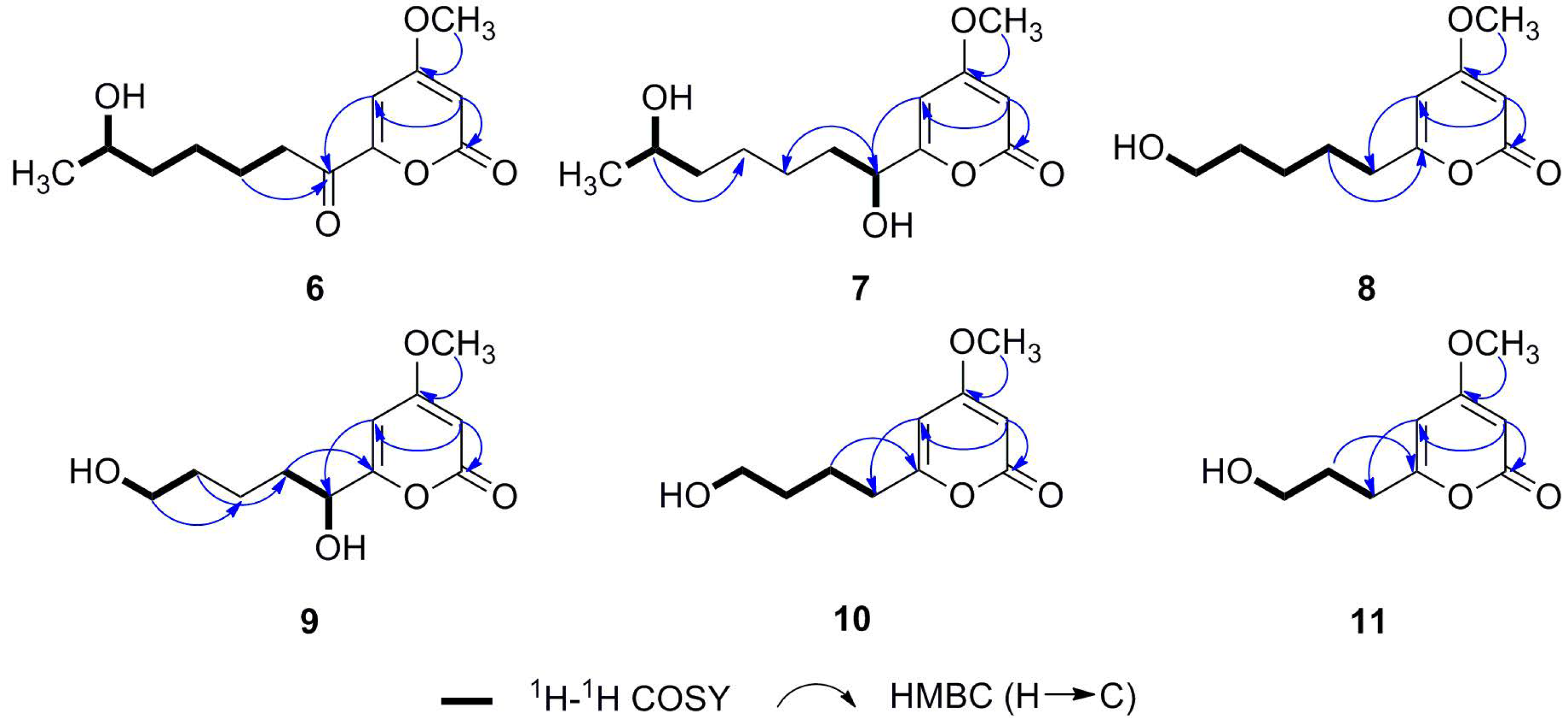
| Position | 9 | 10 | 11 | |||
|---|---|---|---|---|---|---|
| δH (mult., J in Hz) | δC (mult.) | δH (mult., J in Hz) | δC (mult.) | δH (mult., J in Hz) | δC (mult.) | |
| 2 | 163.8, qC | 164.2, qC | 164.1, qC | |||
| 3 | 5.55, d (2.0) | 88.1, CH | 5.53, d (2.2) | 87.7, CH | 5.52, d (2.2) | 87.7, CH |
| 4 | 171.5, qC | 171.5, qC | 171.5, qC | |||
| 5 | 6.11, d (2.0) | 98.3, CH | 6.06, d (2.2) | 99.9, CH | 6.04, d (2.2) | 99.8, CH |
| 6 | 168.3, qC | 166.0, qC | 166.0, qC | |||
| 7 | 4.23, brs | 69.5, CH | 2.46, t (7.4) | 32.9, CH2 | 2.48, t (7.8) | 30.0, CH2 |
| 8 | 1.65, m/1.54, m | 35.0, CH2 | 1.60, m | 23.3, CH2 | 1.70, m | 30.0, CH2 |
| 9 | 1.34, m | 21.7, CH2 | 1.45, m | 32.1, CH2 | 3.42, dd (6.0, 11.0) | 60.1, CH2 |
| 10 | 1.40, m | 32.8, CH2 | 3.42, dd (6.0, 11.0) | 60.7, CH2 | ||
| 11 | 3.37, dd (6.0, 11.1) | 61.1, CH2 | ||||
| 12 | ||||||
| 13 | ||||||
| 4-OCH3 | 3.81, s | 56.8, CH3 | 3.81, s | 56.7, CH3 | 3.79, s | 56.7, CH3 |
| 7-OH | 5.60, brs | |||||
| 11-OH | 4.35, t (5.0) | |||||
| 10-OH | 4.43, t (5.0) | |||||
| 9-OH | 4.55, t (5.0) | |||||
2.2. Cytotoxic Properties
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HepG2 | 25 | 59 | 45 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| Caski | 29 | 63 | 53 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Isolation and Identification of the Strain
3.3. Seed and Mass Cultures of the Strain
3.4. Extraction and Isolation of Compounds
3.5. Cytotoxicity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, C.; Scherlach, K. Triggering Cryptic Natural Product Biosynthesis in Microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Macdonald, C.A. Drug Discovery from Uncultivable Microorganisms. Drug Discov. Today 2010, 15, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Zhang, J.Z.; Luo, D.Q. The Taxonomy, Biology and Chemistry of the Fungal Pestalotiopsis genus. Nat. Prod. Rep. 2012, 29, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Paranagama, P.A.; Wijeratne, E.M.K.; Gunatilake, A.A.L. Uncovering Biosynthetic Potential of Plant-Associated Fungi: Effect of Culture Conditions on Metabolite Production by Paraphaeosphaeria quadriseptata and Chaetomium chiversii. J. Nat. Prod. 2007, 70, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Deng, Z.S.; Guo, Z.Y.; Tu, X.; Wang, J.Z.; Zou, K. Pestalafuranones F–J, Five New Furanone Analogues from the Endophytic Fungus Nigrospora sp. BM-2. Molecules 2014, 19, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.B.; Walker, M.; Zeeck, A. Structure and Biosynthesis of Mutolide, a Novel Macrolide from a UV Mutant of the Fungus F-24'707. Eur. J. Org. Chem. 2000, 8, 1451–1456. [Google Scholar] [CrossRef]
- Ola, A.R.B.; Thomy, D.; Lai, D.W.; Brötz-Oesterhelt, H.; Proksch, P. Inducing Secondary Metabolite Production by the Endophytic Fungus Fusarium tricinctum through Coculture with Bacillus subtilis. J. Nat. Prod. 2013, 76, 2094–2099. [Google Scholar] [PubMed]
- Udwary, D.W.; Zeiqler, L.; Asolkar, R.N.; Singan, V.; Lapidus, A.; Fenical, W.; Jensen, P.R.; Moore, B.S. Genome Sequencing Reveals Complex Secondary Metabolome in the Marine Actinomycete Salinispora tropica. Proc. Natl. Acad. Sci. USA 2007, 104, 10376–10381. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, Q.Q.; Zhu, W.M.; Cui, C.B.; Fan, G.T.; Fang, Y.C.; Zhu, J.T.; Liu, H.B. 10-Phenyl-[12]-cytochalasins Z7, Z8 and Z9 from the Marine-Derived Fungus Spicariaelegans. J. Nat. Prod. 2006, 69, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Zhang, J.; Hu, S.; Zhang, Z.J.; Zhu, C.J.; Ng, S.W.; Tan, R.X. Ardeemins and Cytochalasins from Aspergillu sterreus Resding in Artemisia annua. Planta Med. 2010, 76, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, J.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in Ginkgo biloba, and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3734–3741. [Google Scholar] [CrossRef] [PubMed]
- McGlacken, G.P.; Fairlamb, I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterization and biological acitivity. Nat. Prod. Rep. 2005, 22, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Lee, H.-S.; Lee, J.S.; Shin, J.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Yun, J.; Kang, J.S. Violapyrones H and I, New Cytotoxic Compounds Isolated from Streptomyces sp. Associated with the Marine Starfish Acanthaster planci. Mar. Drugs 2014, 12, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.M.; Meng, L.; Li, C.S.; Gao, S.S.; Shang, Z.; Proksch, P.; Huang, C.G.; Wang, B.G. Nigerapyrones A-H, α-Pyrone Derivatives from the Marine Mangrove-Derived Endophytic Fungus Aspergillus niger MA-132. J. Nat. Prod. 2011, 74, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Jiang, Y.; Cao, Y.; Liu, J.; Zhang, D.; Chen, X.; Han, L.; Jiang, C.L.; Huang, X.S. Violapyrones A-G, α-Pyrone Derivatives from Streptomyces violascens Isolated from Hylobates hoolock Feces. J. Nat. Prod. 2013, 76, 2126–2130. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Sun, Q.Q.; Qin, J.C.; Pescitelli, G.; Gao, J.M. Characterization of cytochalasins from the endophytic Xylaria sp. and their biological functions. J. Agric. Food Chem. 2014, 62, 10962–10969. [Google Scholar] [CrossRef]
- Isaka, M.; Srisanoh, U.; Sappan, M.; Kongthong, S.; Srikitikulchai, P. Eremophilane and eudesmane sesquiterpenoids and a pimarane diterpenoid from the wood-decay fungus Xylaria sp. BCC 5484. Phytochem. Lett. 2012, 5, 78–82. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Y.; Wang, J.J.; Pang, J.Y.; Huang, C.H.; Wu, X.Y.; She, Z.G.; Vrijmoed, L.L.P.; Jones, E.B.G.; Lin, Y.C. Benzofuran Derivatives from the Mangrove Endophytic Fungus Xylaria sp. (#2508). J. Nat. Prod. 2008, 71, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Healy, P.C.; Hocking, A.; Tran-Dinh, N.; Pitt, J.I.; Shivas, R.G.; Mitchell, J.K.; Kotiw, M.; Davis, R.A. Xanthones from a microfungus of the genus Xylaria. Phytochemistry 2004, 65, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dai, H.Q.; Bao, L.; Ren, B.; Lu, J.C.; Luo, Y.M.; Guo, L.D.; Zhang, L.X.; Liu, H.W. Isolation and Structural Elucidation of Proline-Containing Cyclopentapeptides from an Endolichenic Xylaria sp. J. Nat. Prod. 2011, 74, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.S. The 13C-NMR spectrum of a pyrone metabolite of aspergillus melleus. Biosynthetic incorporation of singly and doubly labelled [13C]-acetate. Tetrahedron Lett. 1975, 16, 175–178. [Google Scholar] [CrossRef]
- Terry, D.C.; Gerald, A.P. Pyrones. VIII. Biosynthetic investigations of the fungal metabolite phacidin. Can. J. Chem. 1982, 60, 133–137. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Khamthong, N.; Sukpondma, Y.; Pakawatchai, C.; Phongpaichit, S.; Sakayaroj, J.; Kirtikara, K. An [11]Cytochalasin Derivative from the Marine-Derived Fungus Xylaria sp. PSU-F100. Chem. Pharm. Bull. 2009, 57, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors because bioactivity tests of those compounds are going on.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Deng, Z.; Guo, Z.; Peng, Y.; Huang, N.; He, H.; Tu, X.; Zou, K. Effect of Culture Conditions on Metabolite Production of Xylaria sp. Molecules 2015, 20, 7940-7950. https://doi.org/10.3390/molecules20057940
Zhang H, Deng Z, Guo Z, Peng Y, Huang N, He H, Tu X, Zou K. Effect of Culture Conditions on Metabolite Production of Xylaria sp. Molecules. 2015; 20(5):7940-7950. https://doi.org/10.3390/molecules20057940
Chicago/Turabian StyleZhang, Hongqi, Zhangshuang Deng, Zhiyong Guo, Yan Peng, Nianyu Huang, Haibo He, Xuan Tu, and Kun Zou. 2015. "Effect of Culture Conditions on Metabolite Production of Xylaria sp." Molecules 20, no. 5: 7940-7950. https://doi.org/10.3390/molecules20057940





