Silver Nanopartical over AuFON Substrate for Enhanced Raman Readout and Their Application in Pesticide Monitoring
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication and Characterization of the AgNPs/AuFON Structure
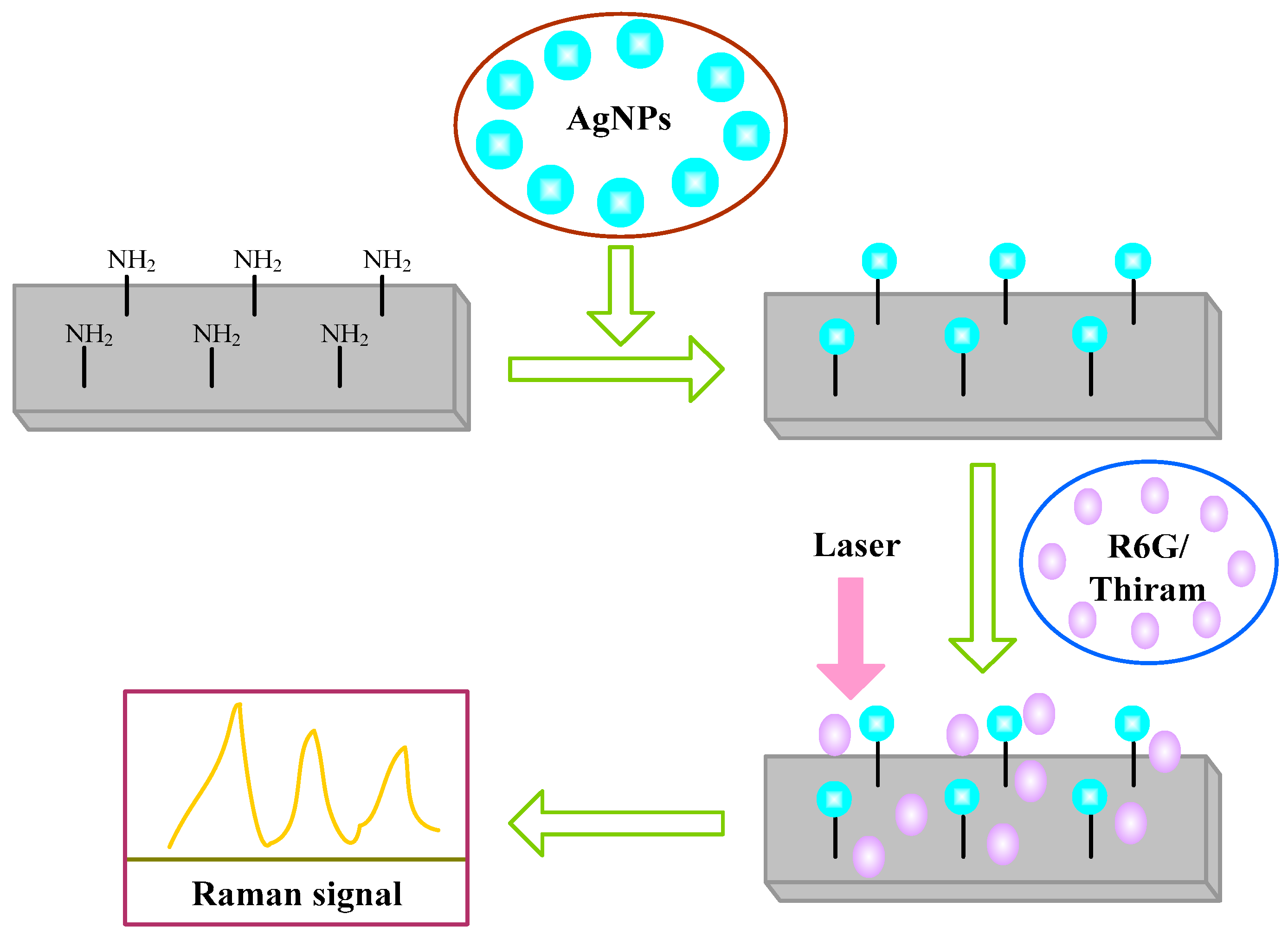
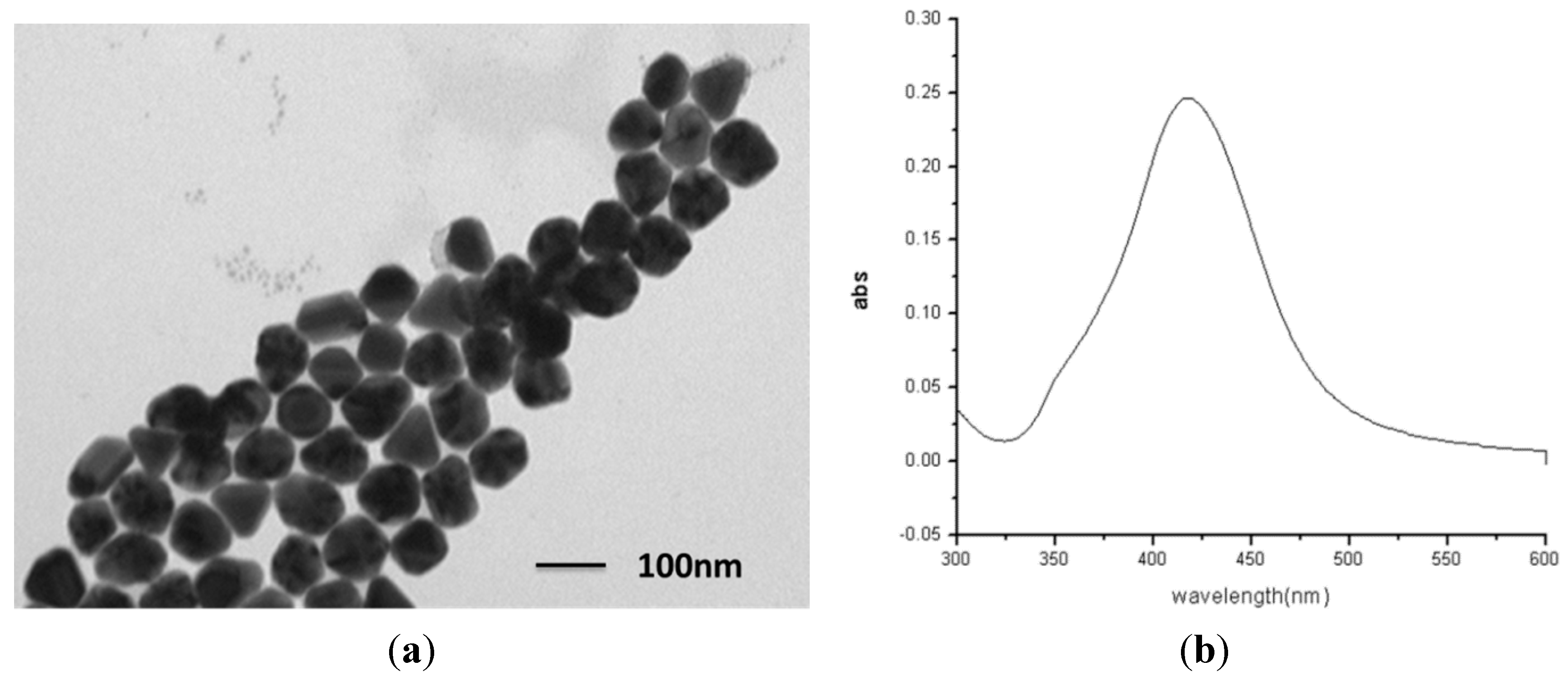
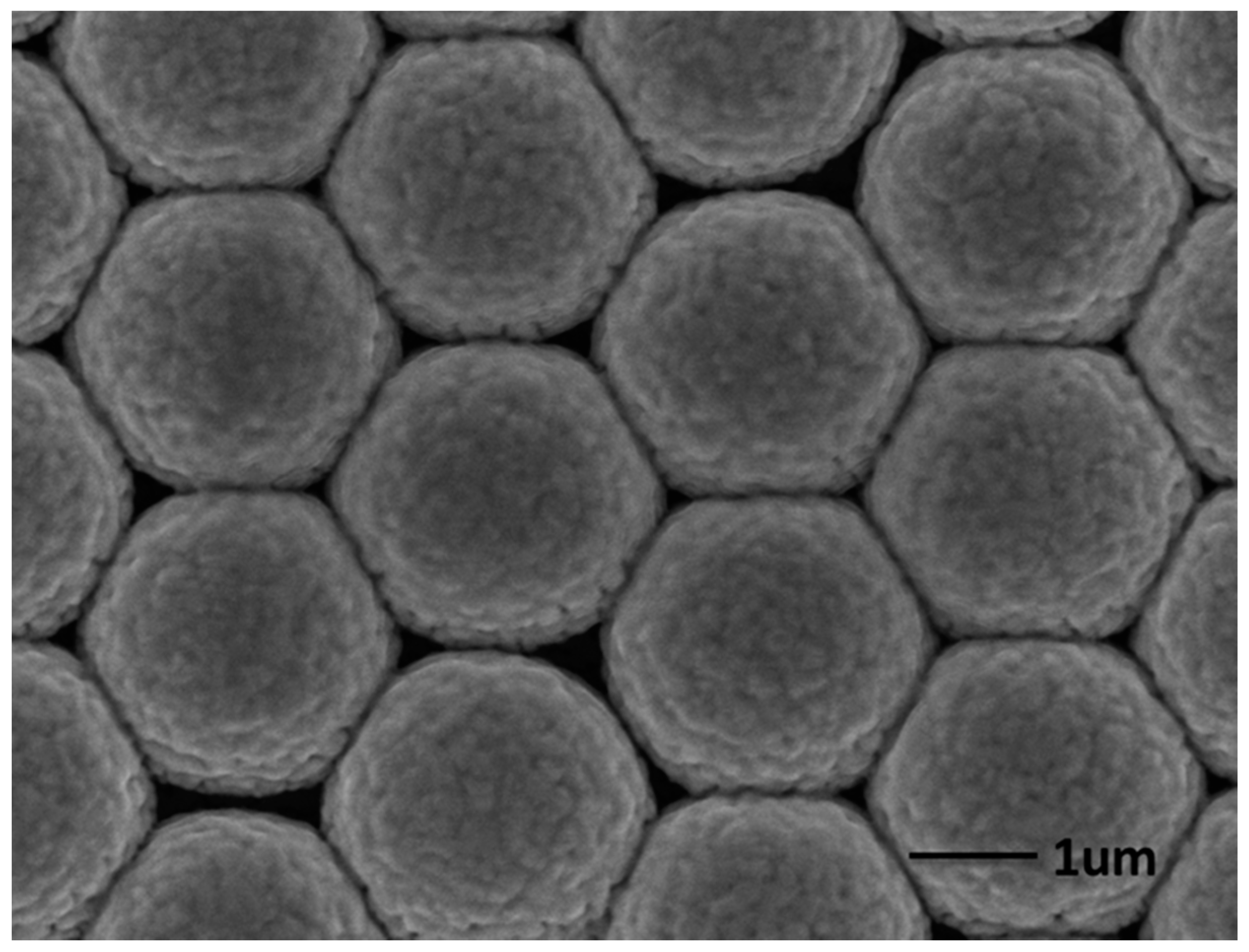
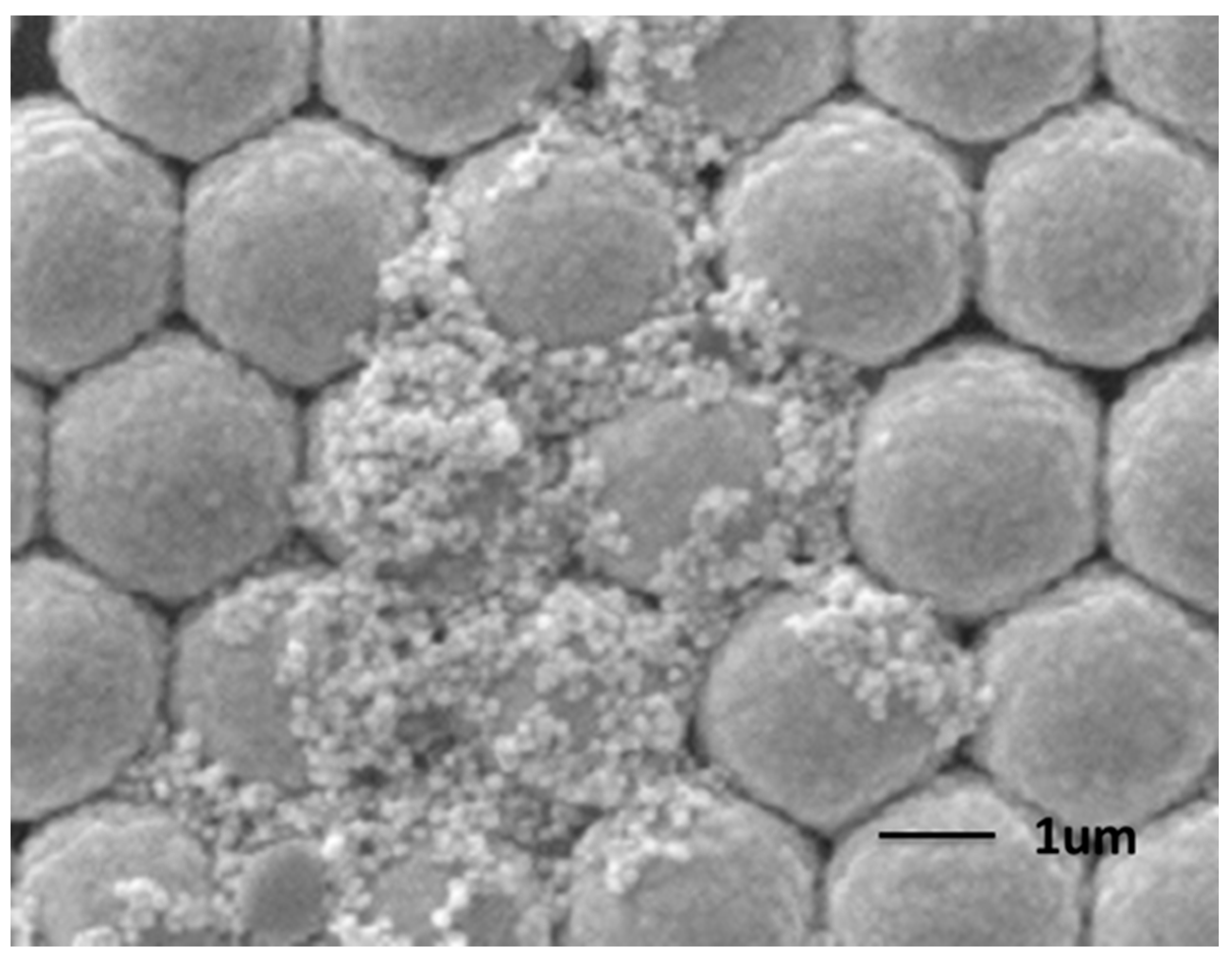

2.2. SERS Properties of the Ag NPs on AuFON as Substrate

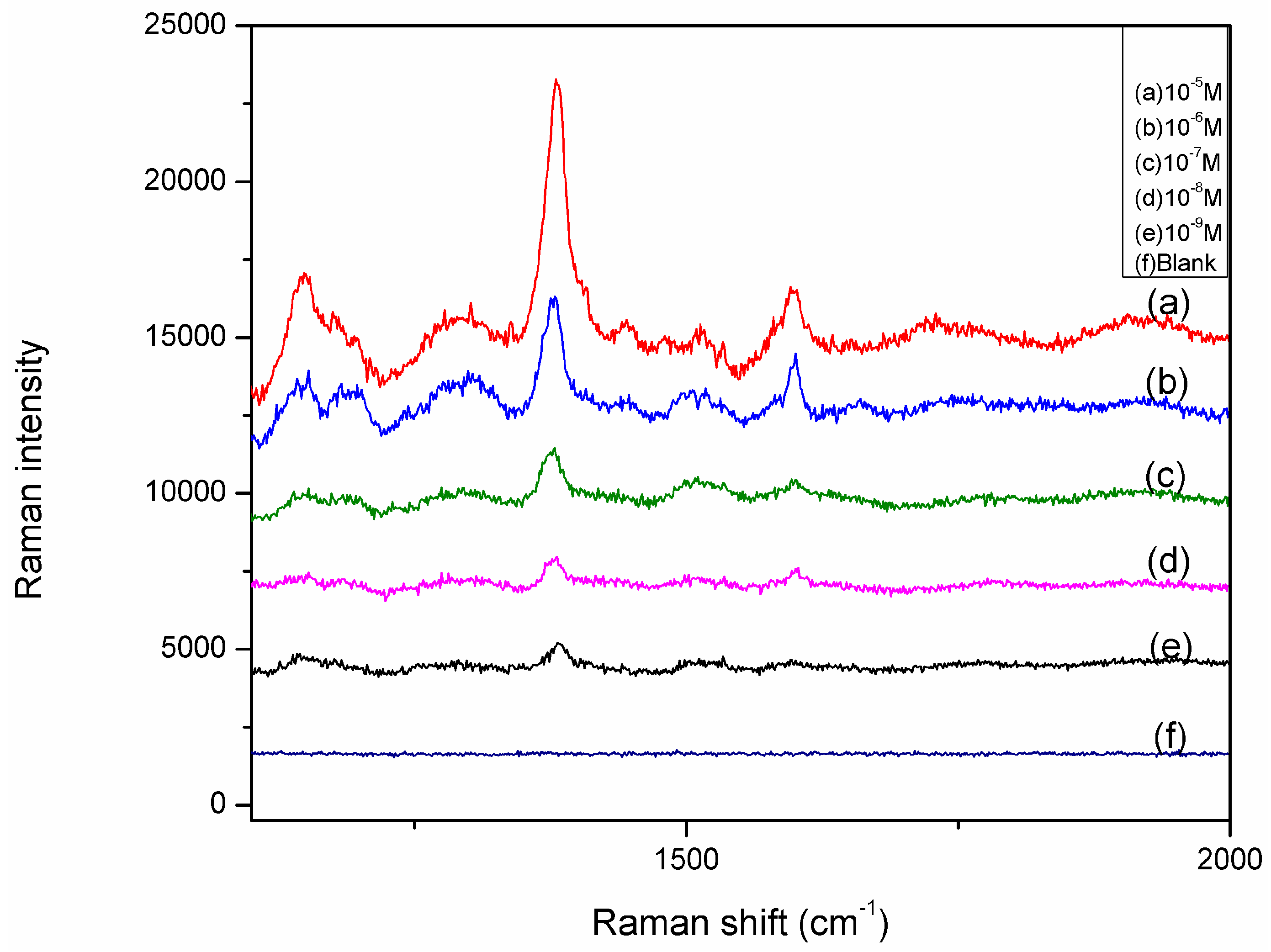
2.3. SERS Detection of Thiram


3. Experimental Section
3.1. Materials and Equipment
3.2. Silver Colloids Preparation
3.3. Fabrication the AgNPs/ AuFON Structure as Hybrid Substrate
3.4. Raman Detection of R6G in Water
3.5. Raman Detection Thiram in Spiked Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, P.; Liu, H.; Yang, L.; Liu, J. The time-resolved D-SERS vibrational spectra of pesticide thiram. Talanta 2013, 117, 39–44. [Google Scholar] [CrossRef]
- Isola, N.R.; Stokes, D.L.; Vo-Dinh, T. Surface enhanced Raman gene probe for HIV detection. Anal. Chem. 1998, 70, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Jin, R.; Mirkin, C. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Guo, L.; Wang, Z. Gold nanoparticle-based surface enhanced Raman scattering spectroscopic assay for the detection of protein-protein interactions. Anal. Sci. 2008, 24, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Grubisha, D.S.; Lipert, R.J.; Park, H.Y.; Driskell, J.; Porter, M.D. Femtomolar detection of prostate-specific antigen: An immunoassay based on surface-enhanced Raman scattering and immunogold labels. Anal. Chem. 2003, 75, 5936–5943. [Google Scholar] [CrossRef] [PubMed]
- Vongsvivut, J.; Robertson, E.G.; McNaughton, D. Surface-enhanced Raman spectroscopic analysis of fonofos pesticide adsorbed on silver andgold nanoparticles. J. Raman Spectrosc. 2010, 41, 1137–1148. [Google Scholar] [CrossRef]
- Seballos, L.; Zhang, J.Z.; Sutphen, R. Surface-enhanced Raman scattering detection of lysophosphatidic acid. Anal. Bioanal. Chem. 2005, 383, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Hiromoto, M.Y.K.; Begun, G.M.; Moody, R.L. Surface-Enhanced Raman Spectrometry for Trace Organic Analysis. Anal. Chem. 1984, 56, 1667–1670. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.; Ringe, E.; van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Dick, L.A.; McFarland, A.D.; Haynes, C.L.; van Duyne, R.P. Metal film over nanosphere (MFON) electrodes for surface-enhanced Raman spectroscopy (sers): Improvements in surface nanostructure stability and suppression of irreversible loss. J. Phys. Chem. B 2002, 106, 853–860. [Google Scholar] [CrossRef]
- Fang, Y.; Seong, N.-H.; Dlott, D.D. Measurement of the distribution of site enhancements in surface-enhanced Raman scattering. Science 2008, 321, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, Q.; Bai, B.; Fan, S. Reusable three-dimensional nanostructured substrates for surface-enhanced Raman scattering. Nanoscale Res. Lett. 2014, 9, 25. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, F.; Liu, H.; Yang, L.; Liu, J. Assembly of polymer-gold nanostructures with high reproducibility into a monolayer film SERS substrate with 5 nm gaps for pesticide trace detection. Analyst 2013, 138, 5832–5838. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Nanosensors based on viologen functionalized silver nanoparticles: few molecules surface-enhanced Raman spectroscopy detection of polycyclic aromatic hydrocarbons in interparticle hot spots. Anal. Chem. 2009, 81, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.M.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; van Duyne, R.P. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Bosnick, K.; Maillard, M.; Brus, L. Single Molecule Raman Spectroscopy at the Junctions of Large Ag Nanocrystals. J. Phys. Chem. B 2003, 107, 9964–9972. [Google Scholar] [CrossRef]
- Zamborini, F.P.; Bao, L.; Dasari, R. Nanoparticles in measurement science. Anal. Chem. 2012, 84, 541–576. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, H.; Osterloh, F.E. A Simple Large-Scale Synthesis of Nearly Monodisperse Gold and Silver Nanoparticles with Adjustable Sizes and with Exchangeable Surfactants. Chem. Mater. 2004, 16, 2509–2511. [Google Scholar] [CrossRef]
- Wang, J.F.; Wu, X.Z.; Xiao, R.; Dong, P.T.; Wang, C.G. Performance-Enhancing Methods for Au Film over Nanosphere Surface-Enhanced Raman Scattering Substrate and Melamine Detection Application. PLoS ONE 2014, 9, e97976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, C.; Zhang, Z. Graphene oxide embedded sandwich nanostructures for enhanced Raman readout and their applications in pesticide monitoring. Nanoscale 2013, 5, 3773–3779. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, P.; Stockburger, M. Surface-enhanced resonance Raman spectroscopy of Rhodamine 6G adsorbed on colloidal silver. J. Phys. Chem. 1984, 88, 5935–5944. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, K.-H.; Liu, Y.-C.; Yu, C.-C.; Wu, Y.-H. Surface-enhanced Raman scattering-active gold nanoparticles modified with a monolayer of silver film. Analyst 2012, 137, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Hwang, S.Y.; Lee, C.J.; Lee, M.S. SERS of Dithiocarbamate pesticides adsorbed on silver surface: Thiram. Bull. Korean Chem. Soc. 2002, 23, 1604–1610. [Google Scholar] [CrossRef]
- Li, P.; Dong, R.; Wu, Y.; Liu, H.; Kong, L.; Yang, L. Polystyrene/Ag nanoparticles as dynamic surface-enhanced Raman spectroscopy substrates for sensitive detection of organophosphorus pesticides. Talanta 2014, 127, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Zhang, X.; Jin, Y. Microwave-assisted green synthesis of silver nanoparticles by carboxymethyl cellulose sodium and silver nitrate. Mater. Chem. Phys. 2008, 108, 421–424. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006, 8, 34–38. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Xiao, R.; Zhang, X.; Wang, C.; Liu, Q.; Rong, Z.; Ye, L.; Chen, S. Silver Nanopartical over AuFON Substrate for Enhanced Raman Readout and Their Application in Pesticide Monitoring. Molecules 2015, 20, 6299-6309. https://doi.org/10.3390/molecules20046299
Guo K, Xiao R, Zhang X, Wang C, Liu Q, Rong Z, Ye L, Chen S. Silver Nanopartical over AuFON Substrate for Enhanced Raman Readout and Their Application in Pesticide Monitoring. Molecules. 2015; 20(4):6299-6309. https://doi.org/10.3390/molecules20046299
Chicago/Turabian StyleGuo, Kun, Rui Xiao, Xiaoye Zhang, Chaoguang Wang, Qiqi Liu, Zhen Rong, Lin Ye, and Suhong Chen. 2015. "Silver Nanopartical over AuFON Substrate for Enhanced Raman Readout and Their Application in Pesticide Monitoring" Molecules 20, no. 4: 6299-6309. https://doi.org/10.3390/molecules20046299




