Biological Activities and Cytotoxicity of Diterpenes from Copaifera spp. Oleoresins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Tyrosinase
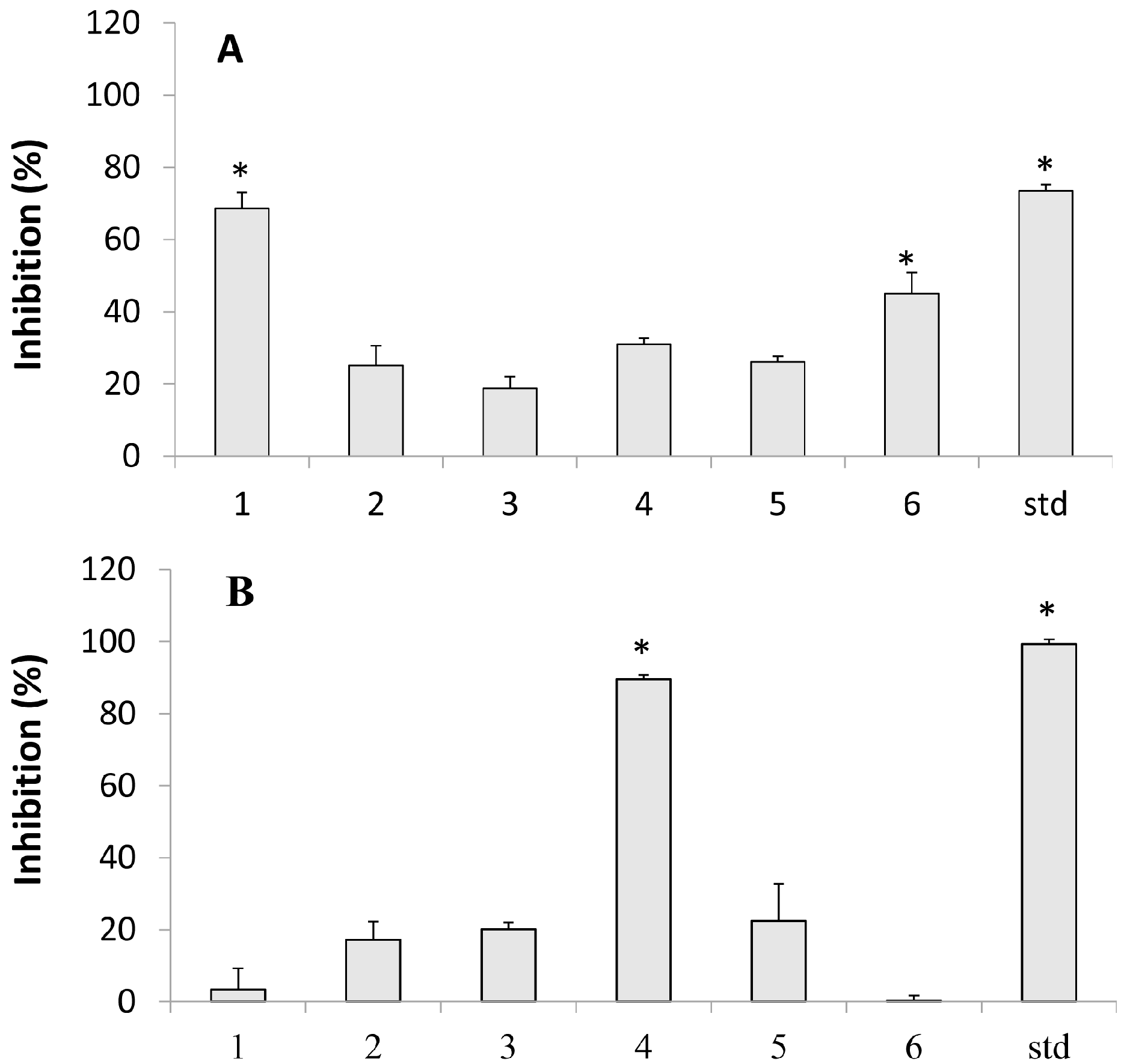
2.2. Lipoxygenase Inhibition
2.3. Hemolytic Activity
2.4. Cytotoxicity
| Kolavic Acid 15 Methyl Ester | Copalic Acid | Hardwickiic Acid | Kaurenoic Acid | 3-Hydroxy-Copalic Acid | 3-Acetoxy-Copalic Acid | |
|---|---|---|---|---|---|---|
| AGP01 | 17.4 ± 6.7 | NT | NT | 28.1 ± 4.3 | 8.5 ± 13.1 | 13.1 ± 5.3 |
| HCT-116 | ≤0.01 | ≤0.01 | ≤0.01 | 0.27 ± 4.3 | 4.7 ± 5.6 | ≤0.01 |
| NIHOVCAR | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | 2.0 ± 3.5 |
| SF295 | ≤0.01 | ≤0.01 | ≤0.01 | 28.0 ± 6.5 | 20.5 ± 5.0 | 17.7 ± 0.9 |
| MCF7 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 |
| SKMELL28 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 |
2.5. Inhibition of Nitric Oxide Production
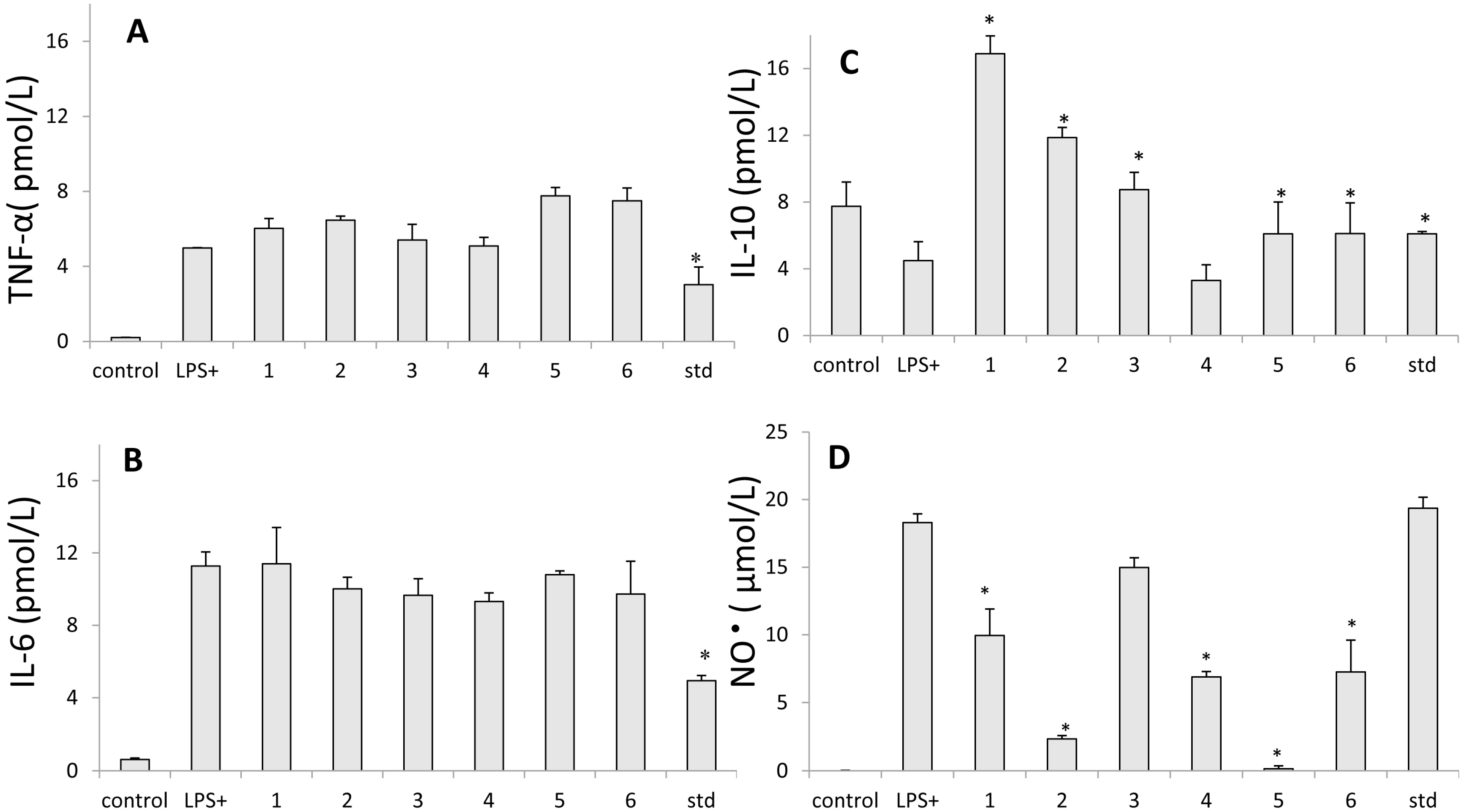
2.6. Assay for Cytokine Production
3. Materials and Methods
3.1. Experimental Procedures
3.2. Plant Material
3.3. Extraction, Isolation and Structural Elucidation

3.4. Isolated Compounds Data
3.5. Cell Culture
3.6. Hemolysis Test
3.7. NO• Production Assay
3.8. Measurement of Cytokines
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Veiga, V.F., Jr.; Pinto, A.C. O Gênero Copaifera L. Quím. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Leandro, L.M.; Vargas, F.S.; Barbosa, P.S.C.; Neves, J.K.O.; Silva, J.A.; Veiga-Junior, V.F. Review: Chemistry and Biological Activities of Terpenoids from Copaiba (Copaifera spp.) Oleoresins. Molecules 2012, 17, 3866–3889. [Google Scholar] [CrossRef]
- Gelmini, F.; Beretta, G.; Anselmi, C.; Centini, M.; Magnic, P.; Ruscica, M.; Cavalchini, A.; Facino, R.M. GC–MS profiling of the phytochemical constituents of the oleoresin from Copaifera langsdorffii Desf. and a preliminary in vivo evaluation of its antipsoriatic effect. Int. J. Pharm. 2013, 440, 170–178. [Google Scholar]
- Veiga, V.F., Jr.; Patittucci, M.L.; Pinto, A.C. Controle de autenticidade de óleos de copaíbas comerciais por cromatografia gasosa de alta resolução. Quím. Nova 1997, 20, 612–615. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; Wiley: Chichester, UK, 2002. [Google Scholar]
- Samuelsson, G.; Bohlin, L. Drugs of Natural Origin: A Treatise of Pharmacognosy, 6th ed.; Swedish Pharmaceutical Press: Stockholm, Sweden, 2010. [Google Scholar]
- Veiga, V.F., Jr.; Zunino, L.; Patitucci, M.L.; Pinto, A.C.; Calixto, J.B. The inhibition of paw oedema formation caused by the oleoresin of Copaifera multijuga Hayne and its fractions. J. Pharm. Pharmacol. 2006, 58, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.V.H.; Moreira, R.J.; Tavares, M.L.C.; Carballo, M.C.S.; Carneiro, T.X.; Santos, A.A.S. Efeito do óleo de copaíba nos níveis séricos de uréia e creatinina em ratos submetidos à síndrome de isquemia e reperfusão renal. Acta Cir. Bras. 2005, 20, 243–246. [Google Scholar] [PubMed]
- Veiga, V.F., Jr.; Zunino, L.; Calixto, J.B.; Patitucci, M.L.; Pinto, A.C. Phytochemical and antioedematogenic studies of commercial copaiba oils available in Brazil. Phytother. Res. 2001, 15, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.M.; Pereira, N.A.; Paulo, L.G. Antinflamatory activity of copaifera spp. balsam. Rev. Bras. Farm. 1992, 73, 53–56. [Google Scholar]
- Ohsaki, A.; Yana, L.T.; Itoa, S.; Edatsugib, H.; Iwatab, D.; Komoda, Y. The Isolation and in vivo Potent Antitumor Activity of Clerodane Diterpenoid from the Oleoresin of the Brazilian Medicinal Plant, Copaifera Langsdorfii Desfon. Bioorg. Med. Chem. Lett. 1994, 4, 2889–2892. [Google Scholar] [CrossRef]
- Yokochi, N.; Morita, T.; Yagi, T. Inhibition of diphenolase activity of tyrosinase by vitamin B6 compounds. J. Agric. Food Chem. 2003, 51, 2733–2736. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, E.; Decker, H. Tyrosinases from crustaceans form hexamers. Biochem. J. 2003, 371, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Seiberg, M.; Paine, C.; Sharlow, E.; Andrade-Gordon, P.; Costanzo, M.; Eisinger, M.; Shapiro, S.S. The protease-activated receptor 2 regulates pigmentation via keratinocyte-melanocyte interactions. Exp. Cell Res. 2000, 254, 25–32. [Google Scholar] [CrossRef]
- Perluigi, M.; De Marco, F.; Foppoli, C.; Coccia, R.; Blarzinho, C.; Marcante, M.L.; Cini, C. Tyrosinase protects human melanocytes from ROS-generating compounds. Biochem. Biophys. Res. Commun. 2003, 305, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, P.S.; Gilchrest, B.A. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J. Cell Physiol. 1987, 133, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.; Chang, W.L.; Lu, T.M. Antityrosinase and Antioxidant Effects ofent-Kaurane Diterpenes from Leaves of Broussonetia papyrifera. J. Nat. Prod. 2008, 71, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.V.M.; Ohara, M.T.; Vila, M.M.D.C.; Gonçalves, M.M. In vitro evaluation of copaiba oil as a kojic acid skin enhancer. Braz. J. Pharm. Sci. 2010, 46, 363–370. [Google Scholar] [CrossRef]
- Tae, J.H.; Isao, K. Lipoxygenase Inhibitory Activity of Anacardic Acids. J. Agric. Food Chem. 2005, 53, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Masuoka, N.; Xiao, P.; Haraguchi, H. Antioxidant activity of docecyl gallate. J. Agric. Food Chem. 2002, 50, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Honn, K.V. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell. Mol. Life Sci. 2002, 59, 799–807. [Google Scholar] [CrossRef]
- Aziz, R.; Malik, A.; Riaz, N.; Nawaz, H.R.; Ahmad, H.; Nawaz, S.A.; Choudhary, M.I. Lipoxygenase inhibitory constituents from Periploca aphylla. J. Nat. Prod. 2004, 67, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Nisar, M.; Zarrelli, A.; Di Fabio, G.; Gul, F.; Gilani, S.N.; Shah, M.R.; Khan, A.Z.; Samiullah; Amim, H. Molecular insights to explore abietane diterpenes as new LOX inhibitors. Med. Chem. Res. 2013, 22, 5809–5813. [Google Scholar] [CrossRef]
- Abe, M.; Ozawa, Y.; Uda, Y.; Morimitsu, Y.; Nakamura, Y.; Osawa, T. A novel labdane-type trialdehyde from myoga (Zingiber mioga Roscoe) that potently inhibits human platelet aggregation and human 5-lipoxygenase. Biosci. Biotechnol. Biochem. 2006, 70, 2494–2500. [Google Scholar] [CrossRef] [PubMed]
- Costa-Lotufo, L.V.; Cunha, G.M.A.; Farias, P.A.M.; Viana, G.S.B.; Cunha, K.M.A.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Gramosa, N.V.; Rao, V.S.N. The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from Copaifera langsdorffii oleo-resin. Toxicon 2002, 40, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Leite, S.N.; Frade, M.A.C. Cytotoxicity and wound healing properties of Copaifera langsdorffii oleoresin in rabbits. Int. J. Nat. Prod. Sci. 2013, 3, 10–20. [Google Scholar]
- Zhao, G.; Jung, J.H.; Smith, D.L.; Wood, K.V.; Mclaughlin, J.L. Cytotoxic clerodane diterpenes from Polyalthia longfolia. Planta Med. 1991, 57, 380. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Mancebo-Aracil, J.; Tangarife-Castanõ, V.; Agudelo-Goméz, L.; Zapata, B.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of (+)-labdadienedial, derivatives and precursors from (+)-sclareolide. Eur. J. Med. Chem. 2010, 45, 4403–4408. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Sarkar, J.; Sinhaa, S. Cytotoxic clerodane diterpenoids from the leaves of Polyalthia longifolia. Nat. Prod. Res. 2010, 24, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.F.; Cheng, Y.D.; Shen, C.R.; Cherng, J.Y. A molecular pharmacology study into the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J. Nat. Med. 2010, 64, 330–335. [Google Scholar]
- Choi, H.; Ahn, S.; Lee, B.G.; Chang, I.; Hwang, J.S. Inhibition of skin pigmentation by an extract of Lepidium apetalum and its possible implication in IL-6 mediated signaling. Pigment Cell Res. 2005, 18, 439–446. [Google Scholar] [PubMed]
- Veiga, V.F., Jr.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne-A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.; Portella, N.A.; Ramos, M.F.; De, S.; Siani, A.C.; Saraiva, E.M. Trans-β-Caryophyllene: An Effective antileishmanial compound found in commercial copaiba oil (Copaifera spp.). Evid. Based Complement. Altern. Med. 2013, 2013, 761323. [Google Scholar]
- Girón, N.; Través, P.G.; Rodríguez, B.; Fontal, R.L.; Boscá, L.; Hortelano, S.; Heras, B.L. Suppression of inflammatory responses by labdane-type diterpenoids. Toxicol. Appl. Pharmacol. 2008, 228, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Szliska, E.; Mertas, A.; Czuba, Z.P.; Król, W. Inhibition of inflammatory response by Artepillin C in activated RAW264.7 macrophages. Evid. Based Complement. Altern. Med. 2013, 2013, 735176. [Google Scholar]
- Diaz-Viciedo, R.; Hortelano, S.; Giron, N.; Masso, J.M.; Rodriguez, B.; Villar, A.; Heras, B.L. Modulation of inflammatory responses by diterpene acids from Helianthus annuus L. Biochem. Biophys. Res. Commun. 2008, 369, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, A.; Heras, B.L.; Hortelano, S.; Rodriguez, B.; Villar, A.; Bosca, L. Inhibition of the Nuclear FactorkB (NF-kB) Pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-kβ-inducing kinase activity and on the coordinate activation of erk and p38 MAPK. J. Biol. Chem. 2001, 276, 15854–15860. [Google Scholar]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Ling, T.; Chowdhury, C.; Chao, T.H.; Bahjat, F.R.; Lloyd, G.K.; Moldawer, L.L.; Palladinob, M.A.; Theodorakis, E.A. Synthesis of a novel family of diterpenes and their evaluation as anti-inflammatory agents. Bioorg. Med. Chem. 2003, 13, 3217–3221. [Google Scholar] [CrossRef]
- Kuwata, H.; Watanabe, Y.; Miyoshi, H.; Yamamoto, M.; Kaisho, T.; Takeda, K.; Akira, S. IL-10–inducible Bcl-3 negatively regulates LPS-induced TNF-α production in macrophages. Blood 2003, 102, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Lee, J.Y.; Uemura, T.; Ezaki, Y.; Takahashi, N.; Kawada, T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008, 369, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.H.M.; Rogero, M.M.; Arêas, J.A.G. Mecanismos de ação de compostos bioativos dos alimentos no contexto de processos inflamatórios relacionados à obesidade. Arq. Bras. Endocrinol. Metabol. 2009, 53, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.; Watkinson, A.R.; Peres, C.A. Determinants of yield in a non-timber forest product: Copaifera oleoresin in Amazonian extractive reserves. For. Ecol. Manag. 2011, 261, 255–264. [Google Scholar] [CrossRef]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43. [Google Scholar] [CrossRef]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N nitrates] in biological fluids. Anal. Biochem. 1982, 26, 131–138. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De S. Vargas, F.; D. O. de Almeida, P.; Aranha, E.S.P.; De A. Boleti, A.P.; Newton, P.; De Vasconcellos, M.C.; Junior, V.F.V.; Lima, E.S. Biological Activities and Cytotoxicity of Diterpenes from Copaifera spp. Oleoresins. Molecules 2015, 20, 6194-6210. https://doi.org/10.3390/molecules20046194
De S. Vargas F, D. O. de Almeida P, Aranha ESP, De A. Boleti AP, Newton P, De Vasconcellos MC, Junior VFV, Lima ES. Biological Activities and Cytotoxicity of Diterpenes from Copaifera spp. Oleoresins. Molecules. 2015; 20(4):6194-6210. https://doi.org/10.3390/molecules20046194
Chicago/Turabian StyleDe S. Vargas, Fabiano, Patrícia D. O. de Almeida, Elenn Suzany P. Aranha, Ana Paula De A. Boleti, Peter Newton, Marne C. De Vasconcellos, Valdir F. Veiga Junior, and Emerson S. Lima. 2015. "Biological Activities and Cytotoxicity of Diterpenes from Copaifera spp. Oleoresins" Molecules 20, no. 4: 6194-6210. https://doi.org/10.3390/molecules20046194






