Thapsigargin—From Thapsia L. to Mipsagargin
Abstract
:1. The Genus Thapsia
1.1. Traditional Use and Description
1.2. Phylogeny of Thapsia L.

1.3. Sesquiterpenoids in Thapsia

| Species | Compound | R1 | R2 | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| T. garganica L. | Thapsigargin | 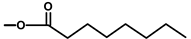 | 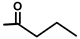 | [8] | ||||
| Thapsigargicin | 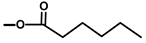 | 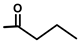 | [8] | |||||
| Thapsivillosin C-E | See below | [15] | ||||||
| Thapsivillosin I | 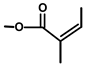 |  | [13] | |||||
| Thapsivillosin J | 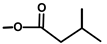 |  | [13] | |||||
| Thapsivillosin L | 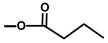 | 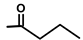 | [16] | |||||
| Nortrilobolid | H | 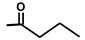 | [17] | |||||
| Trilobolid | H | 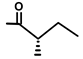 | [13,17] | |||||
| not named | 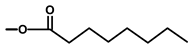 | H | [18] | |||||
| not named | 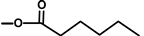 | H | [18] | |||||
| T. gymnesica Rosselló & A. Pujadas | Thapsigargin | See above | [7] | |||||
| Thapsigargicin | See above | [7] | ||||||
| Nortrilobolid | See above | [7] | ||||||
| T. villosa L. | Thapsivillosin A | 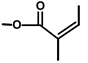 | 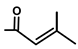 | [19] | ||||
| Thapsivillosin B | 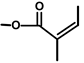 |  | [13] | |||||
| Thapsivillosin C | 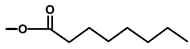 | 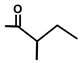 | [13] | |||||
| Thapsivillosin D | 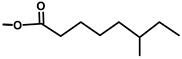 | 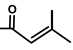 | [13] | |||||
| Thapsivillosin E | 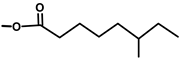 | 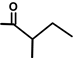 | [13] | |||||
| Thapsivillosin F | H | 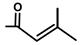 | [13] | |||||
| Thapsivillosin G | 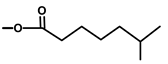 | 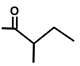 | [13] | |||||
| Thapsivillosin H | 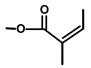 or or 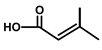 Exact positions undefined | [13] | ||||||
| T. villosa L. | Thapsivillosin K | 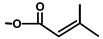 | 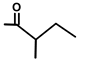 | [13] | ||||
| Thapsitranstagin | 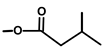 | 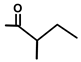 | [13,20] | |||||
| Trilobolide | See above | [19] | ||||||
| T. transtagana Brot. | Thapsitranstagin |  | 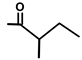 | [13,21] | ||||
| Thapsivillosin B | See above | [13,21] | ||||||
| Trilobolid | See above | [13,21] | ||||||
| Thapsivillosin K | See above | [13,21] | ||||||
| T. smittii Simonsen, Rønsted, Weitzel and Spalik | Thapsivillosin A, B, H | See above | [19] | |||||
2. Thapsigargin and Guaianolide Biosynthesis in Thapsia
2.1. The Biosynthesis of Sesquiterpene Lactones
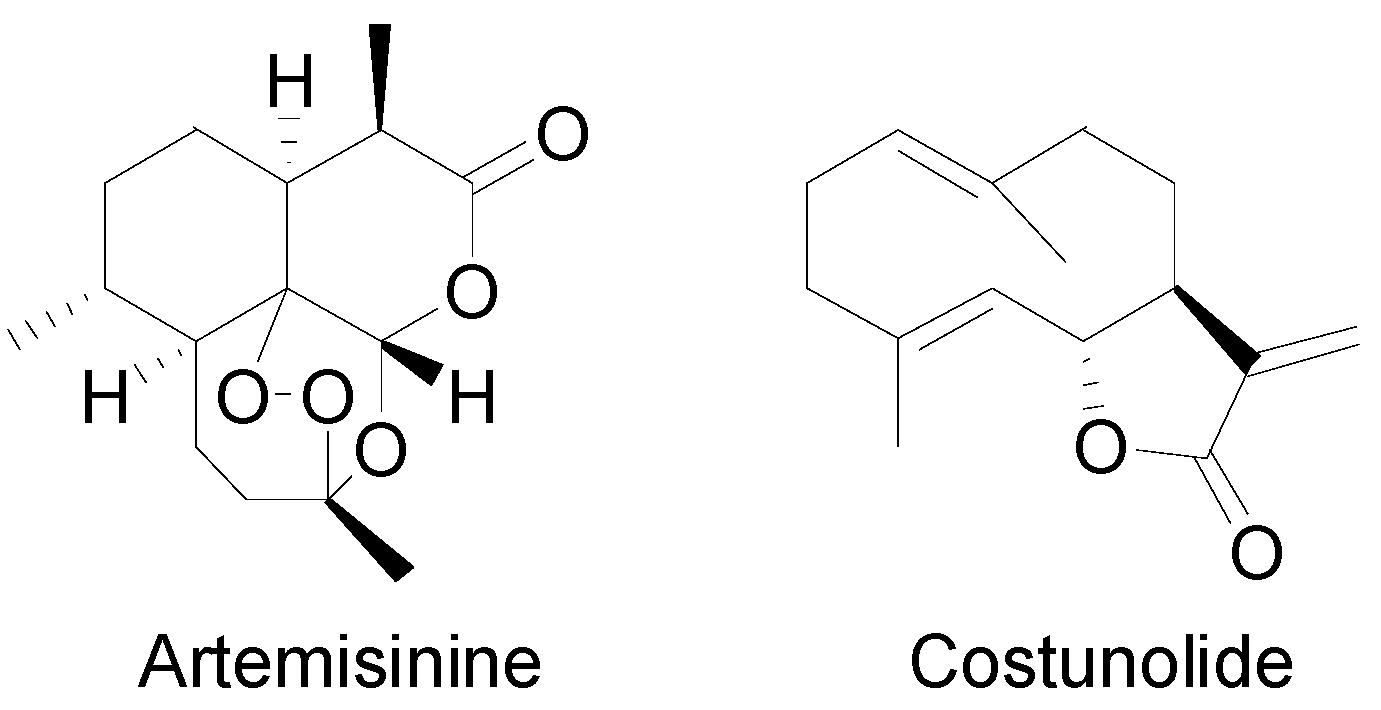
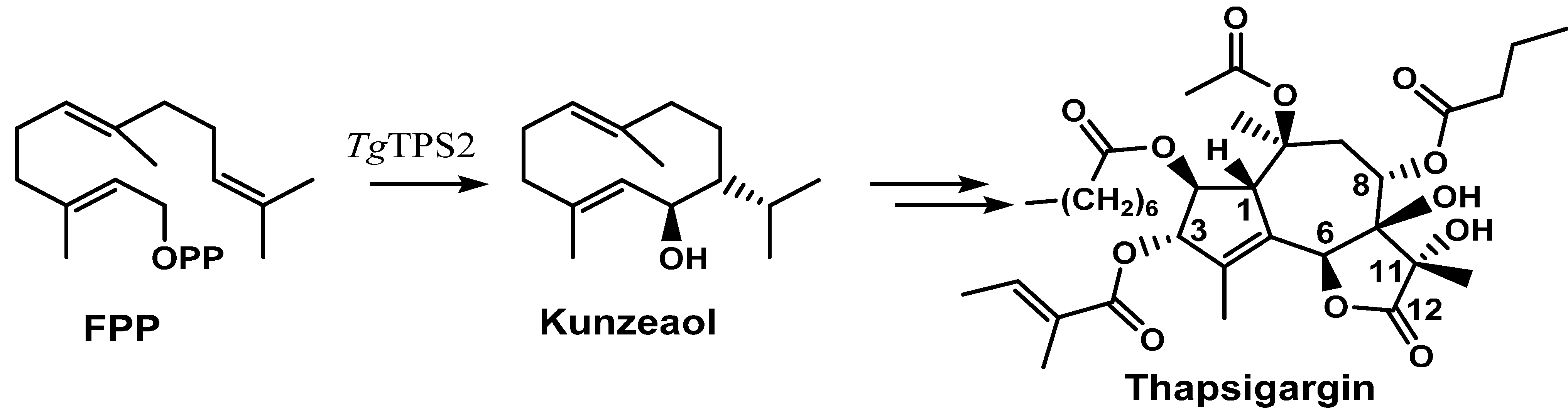
2.2. Thapsigargin Biosynthesis
3. Pharmacology of Thapsigargin
4. Production Platforms for Thapsigargin
4.1. Agricultural Production
4.2. Organ Cultures for Thapsigargin Production
4.3. Production in Heterologous Hosts
4.4. Chemical Synthesis of Thapsigargin
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gerard, J. The Herball or Generall Historie of Plantes; Imprinted at London by John Norton: London, UK, 1597. [Google Scholar]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle: Médecine Arabe Ancienne et Savoirs Populaires; Ibis Press: Paris, France, 1997. [Google Scholar]
- Abderrahim, O.; Martin, G.J.; Abdelaziz, A. Botanical identification and ethno-medicinal uses of some underground part of medicinal plants collected and traded in Marrakech region. J. Med. Plants Res. 2013, 7, 2165–2169. [Google Scholar]
- Bertherand, E.-L. Notice sur la résine de Thapsia garganica et son emploi comme emplatre révulsif. J. Méd. Chir. Pharmacol. 1857, 24, 273–274. [Google Scholar]
- Ouarghidi, A.; Powell, B.; Martin, G.; de Boer, H.; Abbad, A. Species Substitution in Medicinal Roots and Possible Implications for Toxicity of Herbal Remedies in Morocco. Econ. Bot. 2012, 66, 370–382. [Google Scholar] [CrossRef]
- Rasmussen, U. Farmakognostiske Undersøgelser af Slægten Thapsia L. Ph.D. Thesis, Danmarks Farmaceutiske Højskole, Copenhagen, Danmarks, 1979. [Google Scholar]
- Christensen, S.B.; Andersen, A.; Smitt, U.W. Sesquiterpenoids from Thapsia species and medicinal chemistry of the thapsigargins. Fortschr. Chem. Org. Naturst. 1997, 71, 129–167. [Google Scholar] [PubMed]
- Rasmussen, U.; Christensen, S.B.; Sandberg, F. Thapsigargine and thapsigargicine, two new histamine liberators from Thapsia garganica L. Acta Pharm. Suec. 1978, 15, 133–140. [Google Scholar] [PubMed]
- Thastrup, O.; Cullen, P.J.; Drobak, B.K.; Hanley, M.R.; Dawson, A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA 1990, 87, 2466–2470. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, C.; Rønsted, N.; Simonsen, H.T. Resurrecting deadly carrots. Towards a revision of Thapsia L. (Apiaceae) based on phylogenetic analysis of nrITS sequences and chemical profiles. Bot. J. Linn. Soc. 2014, 174, 620–636. [Google Scholar]
- Pujades-Salvà, A.J.; Rosselló, J.A.; Thapsia, L. Flora Ibérica (Vol. X): Araliaceae—Umbelliferae; Castroviejo, S., Aedo, C., Cirujano, S., Laínz, M., Montserrat, P., Morales, R., Muñoz Garmendia, F., Navarro, C., Paiva, J., Soriano, C., Eds.; Real Jardín Botánico, C.S.I.C: Madrid, Spain, 2003; Volume 10, pp. 401–410. [Google Scholar]
- Bajaj, Y.P.S. Medicinal and Aromatic Plants VIII; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995; Volume 8. [Google Scholar]
- Smitt, U.W.; Jäger, A.K.; Adsersen, A.; Gudiksen, L. Comparative studies in phytochemistry and fruit anatomy of Thapsia garganica and T.transtagana, Apiaceae (Umbelliferae). Bot. J. Linn. Soc. 1995, 117, 281–292. [Google Scholar]
- Drew, D.P.; Krichau, N.; Reichwald, K.; Simonsen, H.T. Guaianolides in Apiaceae: Perspectives on pharmacology and biosynthesis. Phytochem. Rev. 2009, 8, 581–599. [Google Scholar] [CrossRef]
- Christensen, S.B.; Norup, E.; Rasmussen, U. Chemistry and structure-activity relationship of the histamine secretagogue thapsigargin and related compounds. In Natural Products and Drug Development: Proceedings of the Alfred Benzon Symposium 20; Krogsgaard-Larsen, P., Christensen, S.B., Kofod, H., Eds.; Munksgaard International Publishers Ltd.: Copenhagen, Denmark, 1984; Volume 20, pp. 405–416. [Google Scholar]
- Liu, H.Z.; Jensen, K.G.; Tran, L.M.; Chen, M.; Zhai, L.; Olsen, C.E.; Søhoel, H.; Denmeade, S.R.; Isaacs, J.T.; Christensen, S.B. Cytotoxic phenylpropanoids and an additional thapsigargin analogue isolated from Thapsia garganica. Phytochemistry 2006, 67, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Smitt, U.W.; Christensen, S.B. Nortrilobolide, a new potent guaianolide secretagogue from Thapsia garganica. Planta Med. 1991, 57, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Falsone, G.; Haddad, H.; Wendisch, D. Sesquiterpenelactone triesters with unusual structures from Thapsia garganica L. (umbelliferae). Arch. Pharm. 1986, 319, 372–379. [Google Scholar] [CrossRef]
- Avato, P.; Trabace, G.; Smitt, U.W. Essential oils from fruits of three types of Thapsia villosa. Phytochemistry 1996, 43, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Rubal, J.J.; Guerra, F.M.; Moreno-Dorado, F.J.; Jorge, Z.D.; Massanet, G.M.; Søhoel, H.; Smitt, U.W.; Frydenvang, K.; Christensen, S.B.; Nielsen, C.; et al. Sesquiterpenes from Thapsia nitida var. meridionalis and Thapsia nitida var. nitida. J. Nat. Prod. 2006, 69, 1566–1571. [Google Scholar]
- Rubal, J.J.; Moreno-Dorado, F.J.; Guerra, F.M.; Jorge, Z.D.; Saouf, A.; Akssira, M.; Mellouki, F.; Romero-Garrido, R.; Massanet, G.M. A pyran-2-one and four meroterpenoids from Thapsia transtagana and their implication in the biosynthesis of transtaganolides. Phytochemistry 2007, 68, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, H.T.; Weitzel, C.; Christensen, S.B. Guaianolide sesquiterpenoids—Their pharmacology and biosynthesis. In Handbook of Natural Products—Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Merillon, J.M., Eds.; Springer-Verlag: Berlin, Germany, 2013; pp. 3069–3098. [Google Scholar]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M.; Knani, M.; Simonin, P.; Sutter, B.; Sahm, H. Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 1993, 295, 517–524. [Google Scholar] [PubMed]
- Gerber, E.; Hemmerlin, A.; Hartmann, M.; Heintz, D.; Hartmann, M.A.; Mutterer, J.; Rodriguez-Concepcion, M.; Boronat, A.; van Dorsselaer, A.; Rohmer, M.; et al. The plastidial 2-C-methyl-d-erythritol 4-phosphate pathway provides the isoprenyl moiety for protein geranylgeranylation in tobacco BY-2 cells. Plant Cell 2009, 21, 285–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, K.; Kasahara, H.; Yamaguchi, S.; Kawaide, H.; Kamiya, Y.; Nojiri, H.; Yamane, H. Genetic evidence for the role of isopentenyl diphosphate isomerases in the mevalonate pathway and plant development in Arabidopsis. Plant Cell Physiol. 2008, 49, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Bick, J.A.; Lange, B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 2003, 415, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Mosandl, A.; Wüst, M. Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): Metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 2005, 66, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hemmerlin, A.; Hoeffler, J.F.; Meyer, O.; Tritsch, D.; Kagan, I.A.; Grosdemange-Billiard, C.; Rohmer, M.; Bach, T.J. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J. Biol. Chem. 2003, 278, 26666–26676. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, C.; Simonsen, H.T. Cytochrome P450-enzymes involved in the biosynthesis of mono- and sesquiterpenes. Phytochem. Rev. 2015, 14, 7–24. [Google Scholar] [CrossRef]
- Olofsson, L.; Engstrom, A.; Lundgren, A.; Brodelius, P. Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.-M.; Wang, B.; Rydén, A.-M.; Woittiez, L.; van Herpen, T.; Verstappen, F.W.A.; Ruyter-Spira, C.; Beekwilder, J.; Bouwmeester, H.J.; van der Krol, A. The metabolite chemotype of Nicotiana benthamiana transiently expressing artemisinin biosynthetic pathway genes is a function of CYP71AV1 type and relative gene dosage. New Phytol. 2013, 199, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.; de Vos, R.C.; Beekwilder, J.; van der Krol, S.; Bouwmeester, H.J. Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS ONE 2011, 6, e23255. [Google Scholar] [CrossRef] [PubMed]
- Pickel, B.; Drew, D.P.; Manczak, T.; Weitzel, C.; Simonsen, H.T.; Ro, D.K. Identification and characterization of a kunzeaol synthase from Thapsia garganica: Implications for the biosynthesis of the pharmaceutical thapsigargin. Biochem. J. 2012, 448, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Andersen, A.; Kromann, H.; Treiman, M.; Tombal, B.; Denmeade, S.; Isaacs, J.T. Thapsigargin analogues for targeting programmed death of androgen-independent prostate cancer cells. Bioorg. Med. Chem. 1999, 7, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Genspera Genspera, Inc. Available online: http://www.genspera.com (accessed on 17 February 2015).
- Ikram, N.K.B.K.; Zhan, X.; Pan, X.; King, B.C.; Simonsen, H.T. Stable Heterologous Expression of Biologically Active Terpenoids in Green Plant Cells. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, H.T.; Drew, D.P.; Lunde, C. Perspectives on Using Physcomitrella Patens as an Alternative Production Platform for Thapsigargin and Other Terpenoid Drug Candidates. Perspect. Med. Chem. 2009, 3, 1–6. [Google Scholar]
- Ikezawa, N.; Göpfert, J.C.; Nguyen, D.T.; Kim, S.-U.; O’Maille, P.E.; Spring, O.; Ro, D.-K. Lettuce Costunolide Synthase (CYP71BL2) and Its Homolog (CYP71BL1) from Sunflower Catalyze Distinct Regio- and Stereoselective Hydroxylations in Sesquiterpene Lactone Metabolism. J. Biol. Chem. 2011, 286, 21601–21611. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Inesi, G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J. Biol. Chem. 1991, 266, 13503–13506. [Google Scholar] [PubMed]
- Søhoel, H.; Jensen, A.M.L.; Møller, J.V.; Nissen, P.; Denmeade, S.R.; Isaacs, J.T.; Olsen, C.E.; Christensen, S.B. Natural products as starting materials for development of second-generation SERCA inhibitors targeted towards prostate cancer cells. Bioorg. Med. Chem. 2006, 14, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.T.; Paulsen, E.S.; Sehgal, P.; Moller, J.V.; Nissen, P.; Denmeade, S.R.; Isaacs, J.T.; Dionne, C.A.; Christensen, S.B. Targeting thapsigargin towards tumors. Steroids 2014, 97, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Mhaka, A.M.; Rosen, D.M.; Brennen, W.N.; Dalrymple, S.; Dach, I.; Olesen, C.; Gurel, B.; Demarzo, A.M.; Wilding, G.; et al. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Jakobsen, C.M.; Denmeade, S.R.; Isaacs, J.T.; Gady, A.; Olsen, C.E.; Christensen, S.B. Design, synthesis, and pharmacological evaluation of thapsigargin analogues for targeting apoptosis to prostatic cancer cells. J. Med. Chem. 2001, 44, 4696–4703. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Rosen, D.M.; Wang, H.; Isaacs, J.T.; Denmeade, S.R. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J. Natl. Cancer I 2012, 104, 1320–1334. [Google Scholar] [CrossRef]
- Chandran, S.S.; Nan, A.; Rosen, D.M.; Ghandehari, H.; Denmeade, S.R. A prostate-specific antigen-activated N-(2-hydroxypropyl) methacrylamide copolymer prodrug as dual-targeted therapy for prostate cancer. Mol. Cancer Ther. 2007, 6, 2928–2937. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.X.; Tsai, Y. Injectable Cancer Compositions. Patent WO/2014/062587, 24 April 2014. [Google Scholar]
- Isaacs, J.T.; Denmeade, S.R. Prostate Specific Antigen; Immobilization; Antiproliferative Agents; Drug Delivery; Contain Cleavage Sites Specifically Cleaved by Prostate Specific Membrane Antigen (PSMA); Useful for Substantially Inhibiting the Non-Specific Toxicity of a Variety of Therapeutic Drugs. US 12/323,380, 25 June 2009. [Google Scholar]
- Denmeade, S.R.; Isaacs, J.T. Tumor Activated Prodrugs. PCT/US2006/048680, 18 September 2008. [Google Scholar]
- Mahalingam, D.; Cetnar, J.; Wilding, G.; Denmeade, S.; Sarantopoulos, J.; Kurman, M.; Carducci, M. A first-in-human phase 1 clinical study of G-202, a thapsigargin-based Prostate-Specific Membrane Antigen (PSMA) activated prodrug, in patients with advanced solid tumors. Mol. Cancer Ther. 2013, 12, B244–B244. [Google Scholar] [CrossRef]
- Makunga, N.P.; Jager, A.K.; van Staden, J. Micropropagation of Thapsia garganica—A medicinal plant. Plant Cell Rep. 2003, 21, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Skytte, D.M.; Denmeade, S.R.; Dionne, C.; Moller, J.V.; Nissen, P.; Isaacs, J.T. A Trojan horse in drug development: Targeting of thapsigargins towards prostate cancer cells. Anti-Cancer Agents Med. Chem. 2009, 9, 276–294. [Google Scholar] [CrossRef]
- Jäger, A.K.; Schottländer, B.; Smitt, U.W.; Nyman, U. Somatic embryogenesis in cell cultures of Thapsia garganica. Plant Cell Rep. 1993, 12, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, A.; Grougnet, R.; Cachet, X.; Meriane, D.; Ardisson, J.; Boutefnouchet, S.; Deguin, B. Large scale purification of the SERCA inhibitor Thapsigargin from Thapsia garganica L. roots using centrifugal partition chromatography. J. Chromatogr. B 2013, 926, 16–20. [Google Scholar] [CrossRef]
- Makunga, N.P.; Jäger, A.K.; van Staden, J. Improved in vitro rooting and hyperhydricity in regenerating tissues of Thapsia garganica L. Plant Cell Tissue Organ Cult. 2006, 86, 77–86. [Google Scholar] [CrossRef]
- Fett-Neto, A.G. Plant Secondary Metabolism Engineering; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Makunga, N.P.; Jäger, A.K.; van Staden, J. An improved system for the in vitro regeneration of Thapsia garganica via direct organogenesis–influence of auxins and cytokinins. Plant Cell Tissue Organ Cult. 2005, 82, 271–280. [Google Scholar] [CrossRef]
- Pan, X.-W. Metabolic Engineering of the Diterpnoid Sclareol in the Moss Physcomitrella Patens. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2014. [Google Scholar]
- Anterola, A.; Shanle, E.; Perroud, P.-F.; Quatrano, R. Production of taxa-4(5),11(12)-diene by transgenic Physcomitrella patens. Transgenic Res. 2009, 18, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.; Cove, D. The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens. Mol. Gen. Genet. 1977, 154, 87–95. [Google Scholar] [CrossRef]
- Schaefer, D.G. A newmoss genetics: Targeted mutagenesis in Physcomitrella patens. Annu. Rev. Plant Biol. 2002, 53, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Frank, W.; Decker, E.L.; Reski, R. Molecular tools to study Physcomitrella patens. Plant Biol. 2005, 7, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Andersen, A.; Poulsen, J.-C.J.; Treiman, M. Derivatives of thapsigargin as probes of its binding site on endoplasmic reticulum Ca2+ ATPase: Stereoselectivity and important functional groups. FEBS Lett. 1993, 335, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Søhoel, H.; Liljefors, T.; Ley, S.V.; Oliver, S.F.; Antonello, A.; Smith, M.D.; Olsen, C.E.; Isaacs, J.T.; Christensen, S.B. Total synthesis of two novel subpicomolar sarco/endoplasmatic reticulum Ca2+-ATPase inhibitors designed by an analysis of the binding site of thapsigargin. J. Med. Chem. 2005, 48, 7005–7011. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.P.; Tait, M.M.; Ball, M.; Ley, S.V. Design and total synthesis of unnatural analogues of the sub-nanomolar SERCA inhibitor thapsigargin. Org. Biomol. Chem. 2007, 5, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Marín-Barrios, R.; García-Cabeza, A.L.; Moreno-Dorado, F.J.; Guerra, F.M.; Massanet, G.M. Acyloxylation of Cyclic Enones: Synthesis of Densely Oxygenated Guaianolides. J. Org. Chem. 2014, 79, 6501–6509. [Google Scholar] [CrossRef] [PubMed]
- Kaliappan, K.P.; Nandurdikar, R.S. A facile domino metathetic route to a thapsigargin skeleton. Org. Biomol. Chem. 2005, 3, 3613–3614. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Antonello, A.; Balskus, E.P.; Booth, D.T.; Christensen, S.B.; Cleator, E.; Gold, H.; Högenauer, K.; Hünger, U.; Myers, R.M.; et al. Synthesis of the thapsigargins. Proc. Natl. Acad. Sci. USA 2004, 101, 12073–12078. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.; Andrews, S.P.; Wierschem, F.; Cleator, E.; Smith, M.D.; Ley, S.V. Total Synthesis of Thapsigargin, a Potent SERCA Pump Inhibitor. Org. Lett. 2007, 9, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Manzano, F.L.; Guerra, F.M.; Moreno-Dorado, F.J.; Jorge, Z.D.; Massanet, G.M. Toward the Synthesis of Thapsigargin: Enantioselective Synthesis of 7, 11-Dihydroxyguaianolides. Org. Lett. 2006, 8, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Tap, A.; Jouanneau, M.; Galvani, G.; Sorin, G.; Lannou, M.-I.; Férézou, J.-P.; Ardisson, J. Asymmetric synthesis of a highly functionalized enantioenriched system close to thapsigargin framework. Org. Biomol. Chem. 2012, 10, 8140–8146. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, T.B.; López, C.Q.; Manczak, T.; Martinez, K.; Simonsen, H.T. Thapsigargin—From Thapsia L. to Mipsagargin. Molecules 2015, 20, 6113-6127. https://doi.org/10.3390/molecules20046113
Andersen TB, López CQ, Manczak T, Martinez K, Simonsen HT. Thapsigargin—From Thapsia L. to Mipsagargin. Molecules. 2015; 20(4):6113-6127. https://doi.org/10.3390/molecules20046113
Chicago/Turabian StyleAndersen, Trine Bundgaard, Carmen Quiñonero López, Tom Manczak, Karen Martinez, and Henrik Toft Simonsen. 2015. "Thapsigargin—From Thapsia L. to Mipsagargin" Molecules 20, no. 4: 6113-6127. https://doi.org/10.3390/molecules20046113





