Formulations, Hemolytic and Pharmacokinetic Studies on Saikosaponin a and Saikosaponin d Compound Liposomes
Abstract
:1. Introduction
2. Results and Discussion
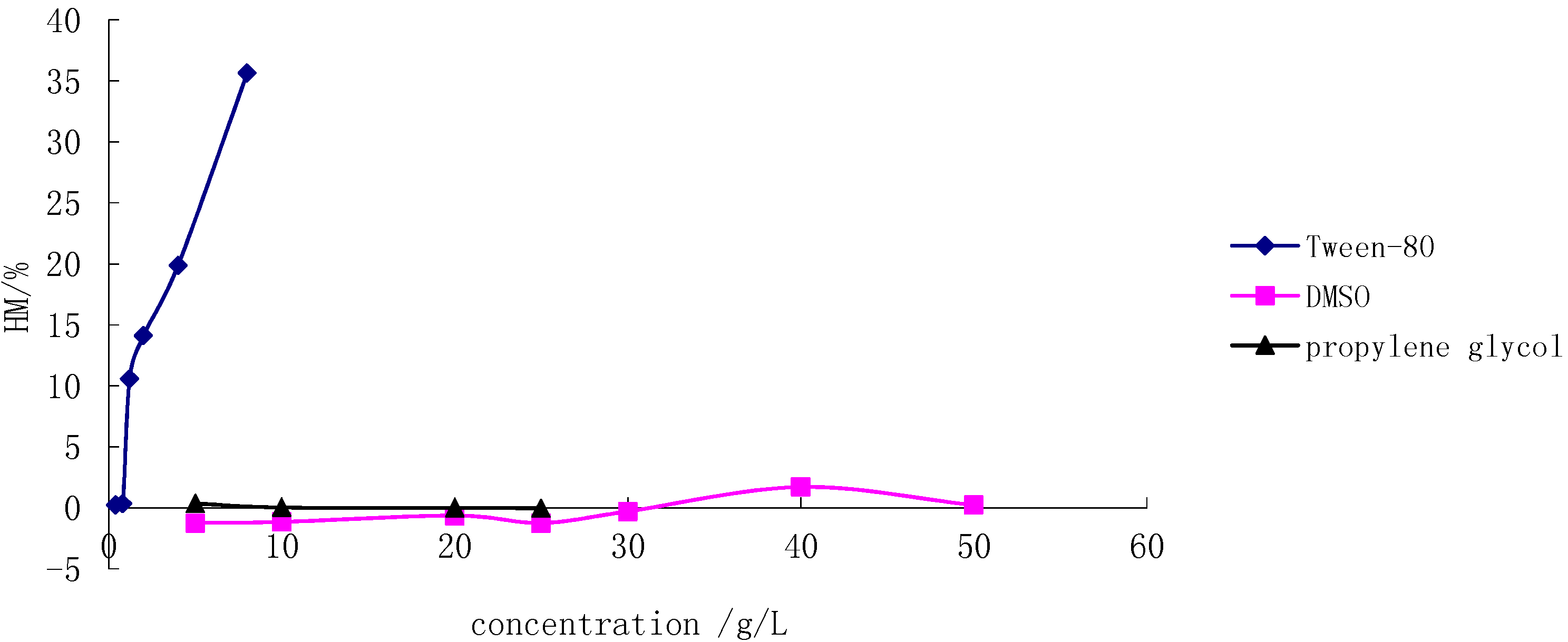
2.1. Hemolysis Test in Japanese Big Ear Rabbit RBCs
| Demulsifier | The Amount Added (mL) | The Rate of Change of Absorbance (%) |
|---|---|---|
| 10% X-triton | 0.1 | 0.94 |
| isopropyl alcohol | 0.7 | 61.06 |
| absolute ethyl alcohol | 2.0 | 30.15 |
2.2. Design of Experiments
2.2.1. Screening Study
2.2.2. Optimisation Study
| Variable No. | Factors | Unit | Lower Level(-1) | Higher Level(+1) |
|---|---|---|---|---|
| X1 | EPC/SSa-SSd | g/g | 10 | 40 |
| X2 | EPC/Chol | g/g | 4 | 10 |
| X3 | Water bath temperature | °C | 40 | 70 |
| X4 | pH of different PBS | - | 6.5 a | 7.4 b |
| X5 | Ultrasound time | min | 10 | 20 |
| Standard No. | Run | Process Factors | Response Values | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | EESSa% | EESSd% | HM% | MD (nm) | PDI | |||||||||||
| 1 | 1 | 1 | 1 | −1 | 1 | 1 | 74.24 | 77.72 | 85.99 | 251 | 0.246 | |||||||||
| 2 | 7 | 1 | −1 | −1 | −1 | 1 | 65.87 | 66.97 | 48.28 | 167 | 0.213 | |||||||||
| 3 | 6 | −1 | −1 | −1 | 1 | −1 | 51.79 | 53.98 | 0.88 | 325 | 0.314 | |||||||||
| 4 | 12 | −1 | −1 | −1 | −1 | −1 | 42.34 | 47.36 | 7.26 | 222 | 0.271 | |||||||||
| 5 | 10 | −1 | 1 | 1 | 1 | −1 | 45.16 | 49.76 | 90.72 | 411 | 0.334 | |||||||||
| 6 | 8 | 1 | 1 | −1 | −1 | −1 | 76.56 | 76.39 | 84.19 | 280 | 0.417 | |||||||||
| 7 | 11 | 1 | −1 | 1 | 1 | 1 | 68.81 | 67.79 | 29.78 | 158 | 0.231 | |||||||||
| 8 | 4 | −1 | 1 | −1 | 1 | 1 | 53.94 | 56.28 | 93.27 | 228 | 0.256 | |||||||||
| 9 | 9 | 1 | 1 | 1 | −1 | −1 | 59.32 | 60.81 | 90.32 | 198 | 0.279 | |||||||||
| 10 | 2 | −1 | 1 | 1 | −1 | 1 | 39.62 | 40.49 | 88.93 | 248 | 0.246 | |||||||||
| 11 | 3 | 1 | −1 | 1 | 1 | −1 | 70.57 | 73.15 | 6.12 | 294 | 0.338 | |||||||||
| 12 | 5 | −1 | −1 | 1 | −1 | 1 | 36.59 | 40.44 | 3.31 | 316 | 0.226 | |||||||||
| Souce | EESSa% | EESSd% | HM% | MD (nm) | PDI | |||||||||||||||
| F | p -value | F | p -value | F | p -value | F | p -value | F | p -value | |||||||||||
| model | 33.34 | 0.0003 | 45.79 | 0.0001 | 18.09 | 0.0015 | 1.57 | 0.2975 | 3.03 | 0.1051 | ||||||||||
| X1 | 139.45 | <0.0001 | 182.92 | <0.0001 | 1.64 | 0.2475 | 3.25 | 0.1216 | 0.27 | 0.6233 | ||||||||||
| X2 | 1.08 | 0.3378 | 1.40 | 0.2816 | 86.43 | <0.0001 | 0.37 | 0.5678 | 1.55 | 0.2601 | ||||||||||
| X3 | 13.06 | 0.0112 | 21.63 | 0.0035 | 0.051 | 0.8281 | 0.46 | 0.5220 | 0.18 | 0.6867 | ||||||||||
| X4 | 12.80 | 0.0117 | 21.59 | 0.0035 | 0.11 | 0.7530 | 1.14 | 0.3264 | 0.20 | 0.6683 | ||||||||||
| X5 | 0.29 | 0.6090 | 1.40 | 0.2816 | 2.21 | 0.1873 | 2.64 | 0.1556 | 12.93 | 0.0114 | ||||||||||
| Variable No. | Factors | Unit | Lower Level(−1) | Higher Level(+1) |
|---|---|---|---|---|
| X1 | EPC/SSa-SSd | g/g | 10 | 40 |
| X2 | EPC/Chol | g/g | 4 | 10 |
| X3 | Water bath temperature | °C | 40 | 70 |
| Fixed | pH of PBS | - | 7.4 | |
| Fixed | Ultrasound time | min | 15 | |
| Standard No. | Run | Process Factors | Response Values | |||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | EESSa% | EESSd% | HM% | |||
| 1 | 9 | 10 | 4 | 55 | 56.85 | 57.60 | 35.46 | |
| 2 | 6 | 40 | 4 | 55 | 77.65 | 75.20 | 61.53 | |
| 3 | 1 | 10 | 10 | 55 | 43.98 | 48.44 | 70.33 | |
| 4 | 11 | 40 | 10 | 55 | 74.70 | 80.98 | 92.09 | |
| 5 | 16 | 10 | 7 | 40 | 54.26 | 55.73 | 69.03 | |
| 6 | 3 | 40 | 7 | 40 | 80.70 | 81.08 | 75.94 | |
| 7 | 2 | 10 | 7 | 70 | 40.61 | 42.00 | 42.45 | |
| 8 | 7 | 40 | 7 | 70 | 50.88 | 55.68 | 69.63 | |
| 9 | 10 | 25 | 4 | 40 | 79.15 | 83.85 | 31.2 | |
| 10 | 17 | 25 | 10 | 40 | 68.43 | 69.12 | 58.11 | |
| 11 | 5 | 25 | 4 | 70 | 50.83 | 58.23 | 3.65 | |
| 12 | 15 | 25 | 10 | 70 | 44.37 | 47.27 | 49.33 | |
| 13 | 12 | 25 | 7 | 55 | 76.46 | 81.10 | 38.87 | |
| 14 | 8 | 25 | 7 | 55 | 81.40 | 80.57 | 37.94 | |
| 15 | 4 | 25 | 7 | 55 | 77.93 | 79.22 | 40.8 | |
| 16 | 14 | 25 | 7 | 55 | 80.13 | 85.30 | 41.1 | |
| 17 | 13 | 25 | 7 | 55 | 79.69 | 79.03 | 38.37 | |
| Response | Source | Sum of Squares | df | Mean Square | f-Value | p-value | Model |
|---|---|---|---|---|---|---|---|
| EESSa% | Model | 3677.45 | 7 | 525.35 | 57.88 | <0.0001 | significant |
| X1 | 972.84 | 1 | 972.84 | 107.19 | <0.0001 | ||
| X2 | 136.20 | 1 | 136.20 | 15.01 | 0.0038 | ||
| X3 | 1148.71 | 1 | 1148.71 | 126.56 | <0.0001 | ||
| X1X3 | 65.32 | 1 | 65.32 | 7.20 | 0.0251 | ||
| X21 | 417.37 | 1 | 417.37 | 45.99 | <0.0001 | ||
| X22 | 145.16 | 1 | 145.16 | 15.99 | 0.0031 | ||
| X23 | 663.62 | 1 | 663.62 | 73.12 | <0.0001 | ||
| Residual | 81.69 | 9 | 9.08 | ||||
| Lack of Fit | 66.65 | 5 | 13.33 | 3.55 | 0.1219 | not significant | |
| Pure Error | 15.04 | 4 | 3.76 | ||||
| Cor Total | 3759.14 | 16 | |||||
| EESSd% | Model | 3307.86 | 6 | 551.31 | 26.83 | <0.0001 | significant |
| X1 | 993.77 | 1 | 993.77 | 48.36 | <0.0001 | ||
| X2 | 105.70 | 1 | 105.7 | 5.14 | 0.0467 | ||
| X3 | 937.44 | 1 | 937.44 | 45.62 | <0.0001 | ||
| X21 | 485.75 | 1 | 485.75 | 23.64 | 0.0007 | ||
| X22 | 94.94 | 1 | 94.94 | 4.62 | 0.0571 | ||
| X23 | 574.32 | 1 | 574.32 | 27.95 | 0.0004 | ||
| Residual | 205.49 | 10 | 20.55 | ||||
| Lack of Fit | 179.73 | 6 | 29.95 | 4.65 | 0.0793 | not significant | |
| Pure Error | 25.76 | 4 | 6.44 | ||||
| Cor Total | 3513.35 | 16 | |||||
| HM% | Model | 7063.03 | 6 | 1177.17 | 150.97 | <0.0001 | significant |
| X1 | 838.86 | 1 | 838.86 | 107.58 | <0.0001 | ||
| X2 | 2381.19 | 1 | 2381.19 | 305.37 | <0.0001 | ||
| X3 | 598.93 | 1 | 598.93 | 76.81 | <0.0001 | ||
| X1X3 | 102.72 | 1 | 102.72 | 13.17 | 0.0046 | ||
| X2X3 | 88.08 | 1 | 88.08 | 11.30 | 0.0072 | ||
| X21 | 3053.26 | 1 | 3053.26 | 391.56 | <0.0001 | ||
| Residual | 77.98 | 10 | 7.80 | ||||
| Lack of Fit | 69.65 | 6 | 11.61 | 5.58 | 0.0590 | not significant | |
| Pure Error | 8.32 | 4 | 2.08 | ||||
| Cor Total | 7141.00 | 16 |
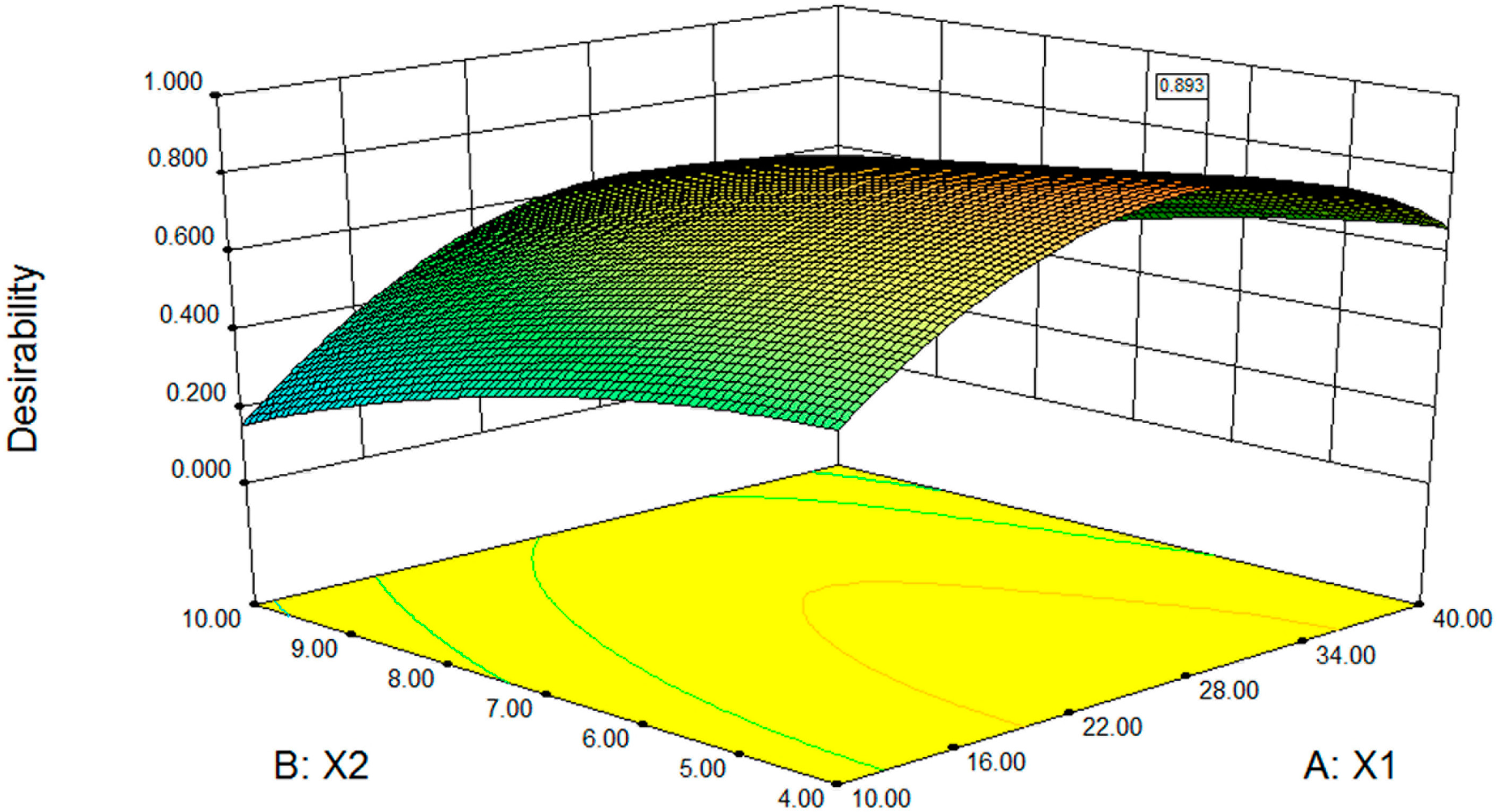
2.2.3. Desirability Optimisation
| Name | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| the EPC/SSa-SSd ratio (g/g) | Is in a rang | 10 | 40 |
| The EPC/Chol ratio (g/g) | Is in a rang | 4 | 10 |
| the water bath temperature (°C) | Is in a rang | 40 | 70 |
| Entrapment efficiency of SSa (%) | Maximize | 40.61 | 81.40 |
| Entrapment efficiency of SSd (%) | Maximize | 42.00 | 85.30 |
| The hemolysis (%) | Minimize | 3.65 | 92.09 |
2.2.4. Verification
| Dependent Variable | Predicted Value | Observed Value | Bias */% |
|---|---|---|---|
| EESSa/% (Y1) | 81.40 | 79.87 | 1.88 |
| EESSd/% (Y2) | 83.43 | 86.19 | −3.31 |
| HM/% (Y3) | 26.37 | 25.16 | 4.59 |
2.3. Pharmacokinetic Studies of SSa-SSd-Lip
2.3.1. Method Validation
Matrix Effect and Recovery
| Compounds | Nominal Concentration (ng/mL) | Recovery (%) | RSD (%) | IS Normalized Matrix Factor (%) | RSD (%) |
|---|---|---|---|---|---|
| SSa | 5.00 | 92.3 | 12.3 | 0.97 | 9.4 |
| 300 | 93.5 | 5.7 | 0.94 | 8.7 | |
| 1500 | 92.7 | 6.9 | 0.92 | 3.6 | |
| SSd | 5.00 | 91.6 | 13.4 | 0.96 | 11.2 |
| 300 | 95.4 | 7.2 | 0.89 | 8.3 | |
| 1500 | 93.5 | 8.0 | 0.93 | 5.0 | |
| IS | 1000 | 92.9 | 7.7 |
Linearity, Sensitivity, Carryover and Specificity

Precision and Accuracy
Dilution Integrity
| Statistical Variable | SSa | SSd | ||||||
|---|---|---|---|---|---|---|---|---|
| Nominal concentration (ng/mL) | 5.00 | 300 | 1500 | 15,000 | 5.00 | 300 | 1500 | 15,000 |
| Mean(ng/mL) | 4.87 | 307 | 1469 | 16,327 | 4.78 | 296 | 1488 | 15,460 |
| RSD (%) | 4.3 | 5.2 | 7.1 | 8.5 | 5.9 | 4.6 | 4.5 | 7.4 |
| Accuracy (%) | 97.4 | 102.3 | 97.9 | 108.8 | 95.6 | 98.7 | 99.2 | 103.1 |
| RE (%) | −2.6 | 2.3 | −2.1 | 8.8 | −4.4 | −1.3 | −0.8 | 3.1 |
| Statistical Variable | SSa | SSd | ||||||
|---|---|---|---|---|---|---|---|---|
| Nominal concentration(ng/mL) | 5.00 | 300 | 1500 | 15,000 | 5.00 | 300 | 1500 | 15,000 |
| Mean(ng/mL) | 4.91 | 286 | 1537 | 14269 | 5.14 | 293 | 1490 | 15,540 |
| RSD (%) | 5.0 | 5.3 | 5.5 | 7.1 | 3.6 | 4.8 | 5.2 | 6.9 |
| Accuracy (%) | 98.2 | 95.3 | 102.5 | 95.1 | 102.8 | 97.7 | 99.3 | 103.6 |
| RE (%) | −1.8 | −4.7 | 2.5 | −4.9 | 2.8 | −2.3 | −0.7 | 3.6 |
Stability
2.3.2. Pharmacokinetic Study
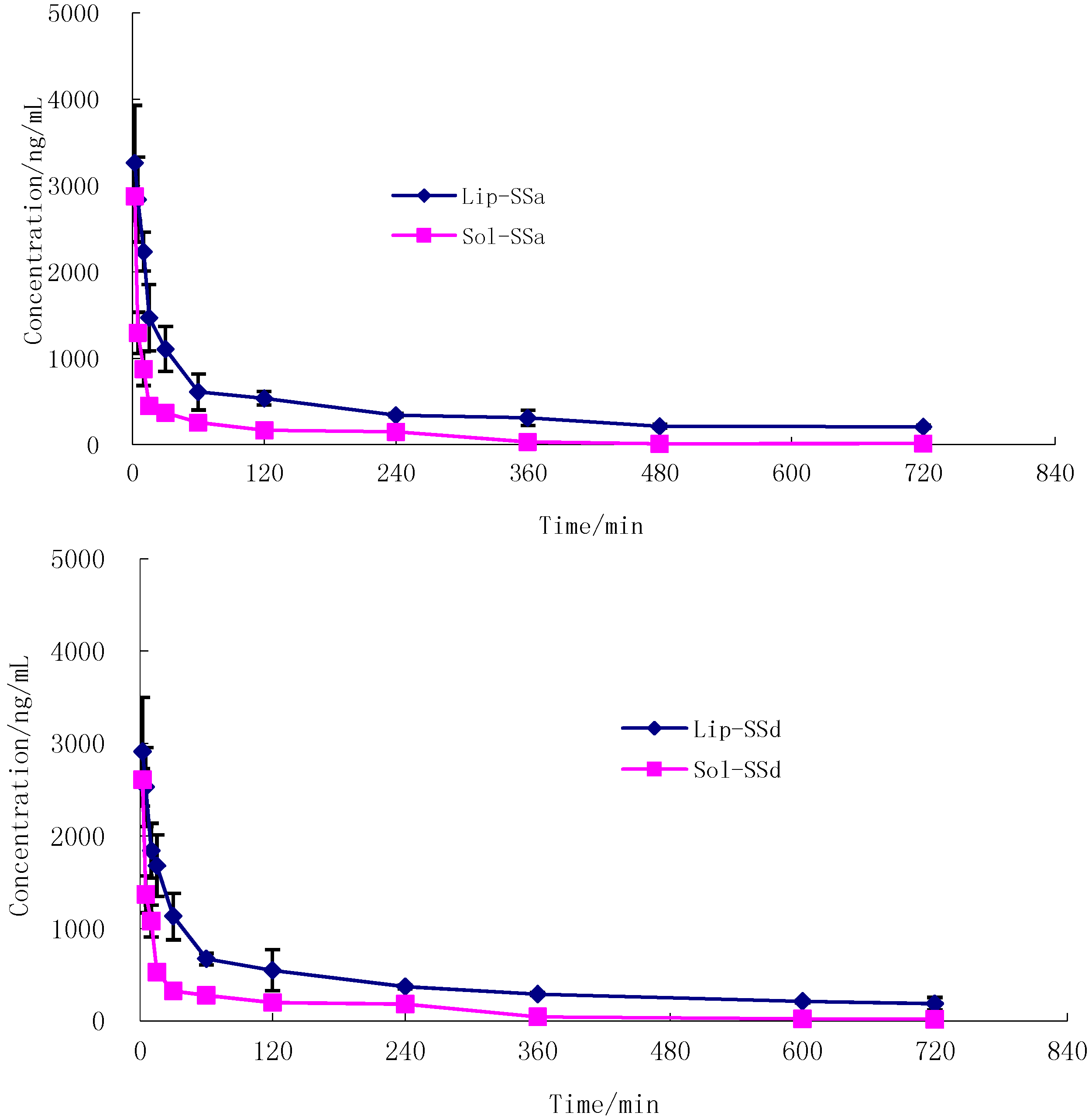
| Parameter | SSa-SSd-Liposome | SSa-SSd-Solution | ||
|---|---|---|---|---|
| SSa | SSd | SSa | SSd | |
| T1/2β (min) | 399.8 ± 23.7 | 346.8 ± 34.1 | 127.9 ± 22.4 | 140.3 ± 19.8 |
| MRT (min) | 233.9 ± 53.2 | 228.2 ± 35.9 | 126.1 ± 17.9 | 141.5 ± 11.5 |
| Vc (mL/kg) | 1404.7 ± 87.2 | 1301.3 ± 74.8 | 2050.9 ± 328.2 | 2015.2 ± 268.0 |
| CL (mL·mg/min) | 2.4 ± 0.37 | 2.6 ± 0.33 | 11.3 ± 2.58 | 10.0 ± 3.76 |
| AUC0–t (μg·min/mL) | 291.0 ± 67.5 | 290.8 ± 86.9 | 87.3 ± 20.1 | 96.7 ± 15.4 |
| AUC0–∞ (μg·min/mL) | 410.6 ± 79.3 | 384.5 ± 103.4 | 90.0 ± 18.3 | 100.4 ± 37.4 |
3. Experimental Section
3.1. Materials
3.2. Preparation of Liposomes
3.3. Hemolysis Assay
3.3.1. The Choice of Co-Solvent
3.3.2. The Choice of Emulsion-Breaker
3.4. Determination of Entrapment Efficiency (EE%)
3.5. Particle Size
3.6. Design of Experiments
3.6.1. Screening Study
3.6.2. Optimisation Study
3.7. Pharmacokinetic Studies of SSa-SSd-Lip
3.7.1. Chromatographic Conditions
3.7.2. Mass Spectrometric Conditions
3.7.3. Preparation of Stock and Working Solutions, Calibration Standards and Quality Control Samples
3.7.4. Sample Preparation
3.7.5. Bioanalytical Method Validation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pharmacopoeia of the People’s Republic of China; Ministry of Public Health of the People’s Republic of China: Beijing, China, 2010; Volume 1, p. 263.
- Huang, W.; Sun, R. Progress researches on the pharmacology and toxicology of different chemical component of saikosaponins. Pharmacol. Clin. Chin. Mater. Med. 2010, 26, 71–74. [Google Scholar]
- Wan, F.; Guo, J.Z.; Li, X.; Li, P.; Chen, Y.W.; Li, K.M.; Wang, C.; Zhang, Y.; Li, J.; Liu, Y. Study on effects of Saikosaponin-d on TPA, PAI, MDA and NO in Rats with Liver Fibrosis. China Pharm. 2007, 18, 1847–1849. [Google Scholar]
- He, Y.; Hu, Z.F.; Li, P.; Xiao, C.; Chen, Y.W.; Li, K.M.; Guo, J.Z.; Pan, L.; Xiong, J.P. Experimental study of saikosaponin-d (SSd) on lipid peroxidation of hepatic fibrosis on rat. China J. Chin. Mater. Med. 2008, 33, 915–919. [Google Scholar]
- Guo, J.Z.; Wan, F.; Li, X.; Li, P.; He, Y.; Hu, Z.F.; Li, K.M.; Li, H. Study of the influence about the lipoperoxidation and zine, calcium content on Saikosaponin-d against liver fibrosis in rats. Pharmacol. Clin. Chin. Mater. Med. 2009, 25, 11–13. [Google Scholar]
- Motoo, Y.; Sawabu, N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994, 86, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Ng, L.T.; Liu, L.T.; Shieh, D.E.; Lin, C.C. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003, 69, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Feng, C.H.; Luo, X.J.; Hu, P.Y.; Xiao, Z.Q.; Wang, Y.S. Hemolytic effects of extraction of Saikosaponins in vitro. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 166–169. [Google Scholar]
- Ding, W.X.; Qi, X.R.; Chen, Y.W.; Li, K.M.; Li, P. Cholesteryl hemisuccinate as liposomal membrane stabilizer and its use in the preparation of saikosaponin-d liposomes. Acta Pharm. Sin. 2005, 40, 623–627. [Google Scholar]
- Portney, N.G.; Ozkan, M. Nano-oncology: Drug delivery, imaging, and sensing. Anal. Bioanal. Chem. 2006, 384, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Hernández, L.; Fleire, L.; González-Alvarez, I.; Montoya, A.; Ballesteros, M.P.; Dea-Ayuela, M.A.; Mirób, M.; Bolás-Fernándezd, F.; Torradoa, J.J. Hemolytic and pharmacokinetic studies of liposomal and particulate amphotericin B formulations. Int. J. Pharm. 2013, 447, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Guo, D.; Zheng, X.L.; Sun, P.; Xu, L.Y.; Chen, J.M. Preliminary study on sea cucumber saponin-nobiliside A liposome and its hemolytic activity. Acta Pharm. Sin. 2008, 43, 214–220. [Google Scholar]
- Dong, X.H.; Li, L.; Shi, L.; Zhang, L.; Lu, Y.; Deng, Y.H. Study on hemolysis of the cucurbitacin B galactosylated liver-targeting liposomes in vitro. Chin. Pharm. J. 2011, 46, 194–197. [Google Scholar]
- Shi, Y.J.; Wu, P.J.; Wei, P. Optimization on preparation of Hawthorn fruit total flavonoids-phospholipid complex using Plackett-Burman Design, Central Composite Design and Response Surface Methodology. J. Chin. Med. Mater. 2010, 33, 437–441. [Google Scholar]
- Ducat, E.; Brion, M.; Lecomte, F.; Evrard, B.; Piel, G. The experimental design as practical approach to develop and optimize a formulation of peptide-loaded liposomes. Am. Assoc. Pharm. Sci. 2010, 11, 966–975. [Google Scholar]
- Yue, P.F.; Zheng, Q.; Wu, B.; Hu, P.Y.; Wu, Z.F.; Yang, M. Application of Plackett-Burman design and Box-Behnken design to achieve process optimization for Geniposide submicron emulsion. J. Dispers. Sci. Technol. 2012, 33, 213–222. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds SSa and SSd are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-S.; Hu, P.-Y.; Li, D.-X.; He, M.-Z.; Rao, X.-Y.; Luo, X.-J.; Wang, Y.-S.; Wang, Y.-R. Formulations, Hemolytic and Pharmacokinetic Studies on Saikosaponin a and Saikosaponin d Compound Liposomes. Molecules 2015, 20, 5889-5907. https://doi.org/10.3390/molecules20045889
Zhang G-S, Hu P-Y, Li D-X, He M-Z, Rao X-Y, Luo X-J, Wang Y-S, Wang Y-R. Formulations, Hemolytic and Pharmacokinetic Studies on Saikosaponin a and Saikosaponin d Compound Liposomes. Molecules. 2015; 20(4):5889-5907. https://doi.org/10.3390/molecules20045889
Chicago/Turabian StyleZhang, Guo-Song, Peng-Yi Hu, Dong-Xun Li, Ming-Zhen He, Xiao-Yong Rao, Xiao-Jian Luo, Yue-Sheng Wang, and Yu-Rong Wang. 2015. "Formulations, Hemolytic and Pharmacokinetic Studies on Saikosaponin a and Saikosaponin d Compound Liposomes" Molecules 20, no. 4: 5889-5907. https://doi.org/10.3390/molecules20045889





