Three New Pigment Protein Tyrosine Phosphatases Inhibitors from the Insect Parasite Fungus Cordyceps gracilioides: Terreusinone A, Pinophilin C and Cryptosporioptide A
Abstract
:1. Introduction
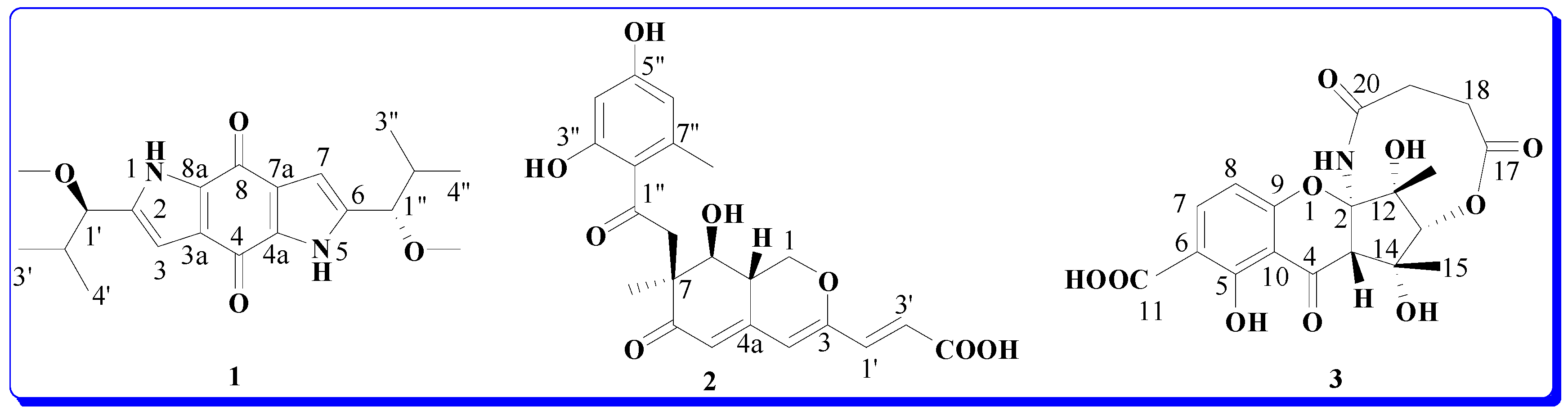
2. Results and Discussion
| Position | δH a (J in Hz) | δC b, mult | HMBC(H→C#) |
|---|---|---|---|
| 2 (6) | 139.1 (C) | ||
| 3 (7) | 6.42 (s) | 106.7 (CH) | 1' (1''), 8a(4a), 3a(7a), 2(6), 4(8) |
| 3a (7a) | 126.5 (C) | ||
| 4 (8) | 175.2 (C) | ||
| 4a (8a) | 132.7 (C) | ||
| 1' (1'') | 3.86 (d, J = 7.4) | 83.0 (CH) | 2(6),3(7),2' (2''),4' (4'') 1' (1'')-OCH3, 3' (3'') |
| 2' (2'') | 2.03 (m) | 33.4 (CH) | 2(6), 1' (1''), 3' (3''), 4' (4'') |
| 3' (3'') | 1.00 (d, J = 6.6) | 18.4 (CH3) | 1' (1''), 2' (2''), 4' (4'') |
| 4' (4'') | 0.80 (d, J = 6.6) | 18.0 (CH3) | 1' (1''), 2' (2''), 3' (3'') |
| 1' (1'')-OCH3 | 3.26 (s) | 56.8 (CH3) | 1' (1'') |


| Position | δH a (J in Hz) | δC b, Mult | HMBC(H→C#) |
|---|---|---|---|
| 1 | 4.81(dd, J = 10.7, 5.3) 3.81(dd, J = 13.6, 10.7) | 68.8 (CH2) | 8a, 4a, 3 |
| 3 | 157.0 (C) | ||
| 4 | 6.04 (s) | 109.6 (CH) | 8a, 5, 3, 1' |
| 4a | 149.2 (C) | ||
| 5 | 5.94 (d, J = 2.0) | 119.5 (CH) | 8a, 7, 4, |
| 6 | 194.2 (C) | ||
| 7 | 84.7 (C) | ||
| 7-Me | 1.67 (s) | 17.0 (CH3) | |
| 8 | 3.62 (d, J = 9.6) | 74.2 (CH) | 7-Me, 8a, 1, 7 |
| 8a | 3.25 (dddd, J = 13.6, 9.6, 5.3, 2.0) | 37.5 (CH) | 8, 4a |
| 1' | 7.05 (d, J = 15.4) | 137.1 (CH) | 4, 2', 3, 3' |
| 2' | 6.33 (d, J = 15.4) | 122.3 (CH) | 1', 3, 3' |
| 3' | 168.0 (COOH ) | ||
| 1'' | 169.8 (C) | ||
| 2' | 105.1 (C ) | ||
| 3'' | 163.9 (C) | ||
| 4' | 6.14 (d, J = 2.4) | 100.4 (CH) | 2'', 6'', 5'', 3'' |
| 5'' | 162.4 (C) | ||
| 6'' | 6.20 (d, J = 2.4) | 111.0 (CH) | 7''-Me, 2'', 4'', 5'' |
| 7'' | 143.1 (C) | ||
| 7''-Me | 23.0 (CH3) |
| Position | δH a (J in Hz) | δC b, mult | HMBC(H→C#) |
|---|---|---|---|
| 2 | - | 104.3 (C) | |
| 3 | 3.42 (s) | 55.8 (CH) | 2, 4, 11, 14, 15 |
| 4 | - | 187.8 (C) | |
| 5 | - | 159.0 (C) | |
| 6 | - | 117.1 (C) | |
| 7 | 7.40 (d, J = 8.4) | 140.5 (CH) | 5, 9, 10 |
| 8 | 6.42 (d, J = 8.4) | 108.2 (CH) | 4, 6, 9, 10 |
| 9 | - | 157.1 (C) | |
| 10 | - | 106.0 (C) | |
| 11 | - | 169.8 (COOH) | |
| 12 | - | 78.9 (C) | |
| 13 | 5.65 (s) | 72.5 (CH) | 2, 3, 4, 12, 14, 15, 16, 17 |
| 14 | - | 58.7 (C) | |
| 15 | 1.56 (s) | 18.1 (CH3) | 3, 13, 14, |
| 16 | 1.65 (s) | 28.4 (CH3) | 2, 12, 13 |
| 17 | - | 171.2 (C) | |
| 18 | 2.65 (m) | 28.8 (CH2) | 17 |
| 19 | 2.66 (m) | 28.8 (CH2) | 20 |
| 20 | - | 177.2 (C) | |
| 5-OH | 11.75 (s) | - | 5, 6, 10 |
| N–H | 14.03 (s) | - |
| Compounds | IC50 Values (µg/mL) | ||||
|---|---|---|---|---|---|
| PTP1B | SHP2 | CDC25B | LAR | SHP1 | |
| 1 | 12.5 | >50 | 4.1 | 10.6 | 5.6 |
| 2 | 6.8 | 8.0 | 4.5 | 4.7 | 3.4 |
| 3 | 7.3 | 5.7 | 7.6 | >50 | 4.9 |
| Sodium orthovanadate (positive control) | 38.3 | 122.2 | 0.93 | 163.0 | 4.3 |
3. Experimental Section
3.1. General Information
3.2. Fungal Material and Cultivation Conditions
3.3. Extraction and Isolation
3.4. Analytical Data
3.4.1. Terreusinone A (1)
3.4.2. Pinophilin C (2)
3.4.3. Cryptosporioptide A (3)
3.5. PTP Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Kittakoop, P.; Kirtikara, K.; Hywel-Jones, N.L.; Thebtaranonth, Y. Bioactive substances from insect pathogenic Fungi. Acc. Chem. Res. 2005, 38, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Tanticharoen, M.; Kongsaree, P.; Thebtaranonth, Y. Structures of Cordypyridones A–D, antimalarial N-hydroxy- and N-methoxy-2-pyridones from the insect pathogenic fungus Cordyceps nipponica. J. Org. Chem. 2001, 66, 4803–4808. [Google Scholar] [CrossRef] [PubMed]
- Kittakoop, P.; Punya, J.; Kongsaeree, P.; Lertwerawat, Y.; Jintasirikul, A.; Tanticharoen, M.; Thebtaranonth, Y. Bioactive naphthoquinones from Cordyceps unilateralis. Phytochemistry 1999, 52, 453–457. [Google Scholar] [CrossRef]
- Umeyama, A.; Takahashi, K.; Grudniewska, A.; Shimizu, M.; Hayashi, S.; Kato, M.; Okamoto, Y.; Suenaga, M.; Ban, S.; Kumada, T.; et al. In vitro antitrypanosomal activity of the cyclodepsipeptides, cardinalisamides A–C, from the insect pathogenic fungus Cordyceps cardinalis NBRC 103832. J. Antibiot. 2014, 67, 163–166. [Google Scholar]
- Grudniewska, A.; Hayashi, S.; Shimizu, M.; Kato, M.; Suenaga, M.; Imagawa, H.; Ito, T.; Asakawa, Y.; Ban, S.; Kumada, T.; et al. Opaliferin, a new polyketide from cultures of entomopathogenic fungus Cordyceps sp. NBRC 106954. Org. Lett. 2014, 16, 4695–4697. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.Z.; Li, C.R.; Chen, Y.Y.; Li, Z.Z. Cordyceps Gracilioides, a new record from China. Mycosystema 2001, 20, 273–274. [Google Scholar]
- Lee, S.M.; Li, X.F.; Jiang, H.; Cheng, J.G.; Seong, S.; Choi, H.D.; Son, B.W. Terreusinone, a novel UV-A protecting dipyrroloquinone from the marine algicolous fungus Aspergillus terreus. Tetrahedron Lett. 2003, 44, 7707–7710. [Google Scholar] [CrossRef]
- Myobatake, Y.; Takeuchi, T.; Kuramochi, K.; Kuriyama, I.; Ishido, T.; Hirano, K.; Sugawara, F.; Yoshida, H.; Mizushina, Y. Pinophilins A and B, inhibitors of mammalian A-, B-, and Y-family DNA polymerases and human cancer cell proliferation. J. Nat. Prod. 2012, 75, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Takeshi, I.; Nobuko, O.; Koohei, N.; Ken-ichi, K. Isolation and structures of new azaphilone derivatives, falconensins E–G, from Emericella falconensis and absolute configurations of falconensins A–G. Chem. Pharm. Bull. 1996, 44, 2213–2217. [Google Scholar] [CrossRef]
- Saleem, M.; Tousif, M.I.; Riaz, N.; Ahmed, I.; Schulz, B.; Ashraf, M.; Nasar, R.; Pescitelli, G.; Hussain, H.; Jabbar, A.; et al. Cryptosporioptide: A bioactive polyketide produced by an endophytic fungus Cryptosporiopsis sp. Phytochemistry 2013, 93, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Barr, A.J. Protein tyrosine phosphatases as drug targets: Strategies and challenges of inhibitor development. Future Med. Chem. 2010, 2, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sung, S.S.; Yip, M.L.; Lawrence, H.; Ren, Y.; Guida, W.C.; Sebti, S.M.; Lawrence, N.J.; Wu, J. Discovery of a novel Shp2 protein tyrosine phosphatase inhibitor. Mol. Pharmacol. 2006, 70, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chai, Q.; Li, Y.Y.; Shen, Q.; Ma, L.P.; Zhang, L.N.; Wang, X.; Sheng, L.; Li, J.Y.; Li, J.; et al. Discovery of novel PTP1B inhibitors with antihyperglycemic activity. Acta Pharmacol. Sin. 2010, 31, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.; Rudolph, J. Temperature dependence of binding and catalysis for the Cdc25B Phosphatase. Biophys. Chem. 2007, 125, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Hollfelder, F.; Gordon, S.J.; Chen, L.; Keng, Y.F.; Wu, L.; Herschlag, D.; Zhang, Z.Y. Impaired Transition State Complementarity in the Hydrolysis of O-Arylphosphorothioates by Protein-Tyrosine Phosphatases. Biochemistry 1999, 38, 12111–12123. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.M.; Ohva, J.L.; Bodega, G.; Chiloeches, A.; Lopez-Ruiz, P.; Prieto, J.C.; Susini, C.; Colas, B. Identification of a protein-tyrosine phosphatase (SHPl) different from that associated with acid phosphatase in rat prostate. FEBS Lett. 1997, 406, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.-Y.; Liu, L.-X.; Liu, T.; Chen, C.; Luo, D.-Q.; Shi, B.-Z. Three New Pigment Protein Tyrosine Phosphatases Inhibitors from the Insect Parasite Fungus Cordyceps gracilioides: Terreusinone A, Pinophilin C and Cryptosporioptide A. Molecules 2015, 20, 5825-5834. https://doi.org/10.3390/molecules20045825
Wei P-Y, Liu L-X, Liu T, Chen C, Luo D-Q, Shi B-Z. Three New Pigment Protein Tyrosine Phosphatases Inhibitors from the Insect Parasite Fungus Cordyceps gracilioides: Terreusinone A, Pinophilin C and Cryptosporioptide A. Molecules. 2015; 20(4):5825-5834. https://doi.org/10.3390/molecules20045825
Chicago/Turabian StyleWei, Pei-Yao, Lin-Xia Liu, Ting Liu, Chuan Chen, Du-Qiang Luo, and Bao-Zhong Shi. 2015. "Three New Pigment Protein Tyrosine Phosphatases Inhibitors from the Insect Parasite Fungus Cordyceps gracilioides: Terreusinone A, Pinophilin C and Cryptosporioptide A" Molecules 20, no. 4: 5825-5834. https://doi.org/10.3390/molecules20045825





