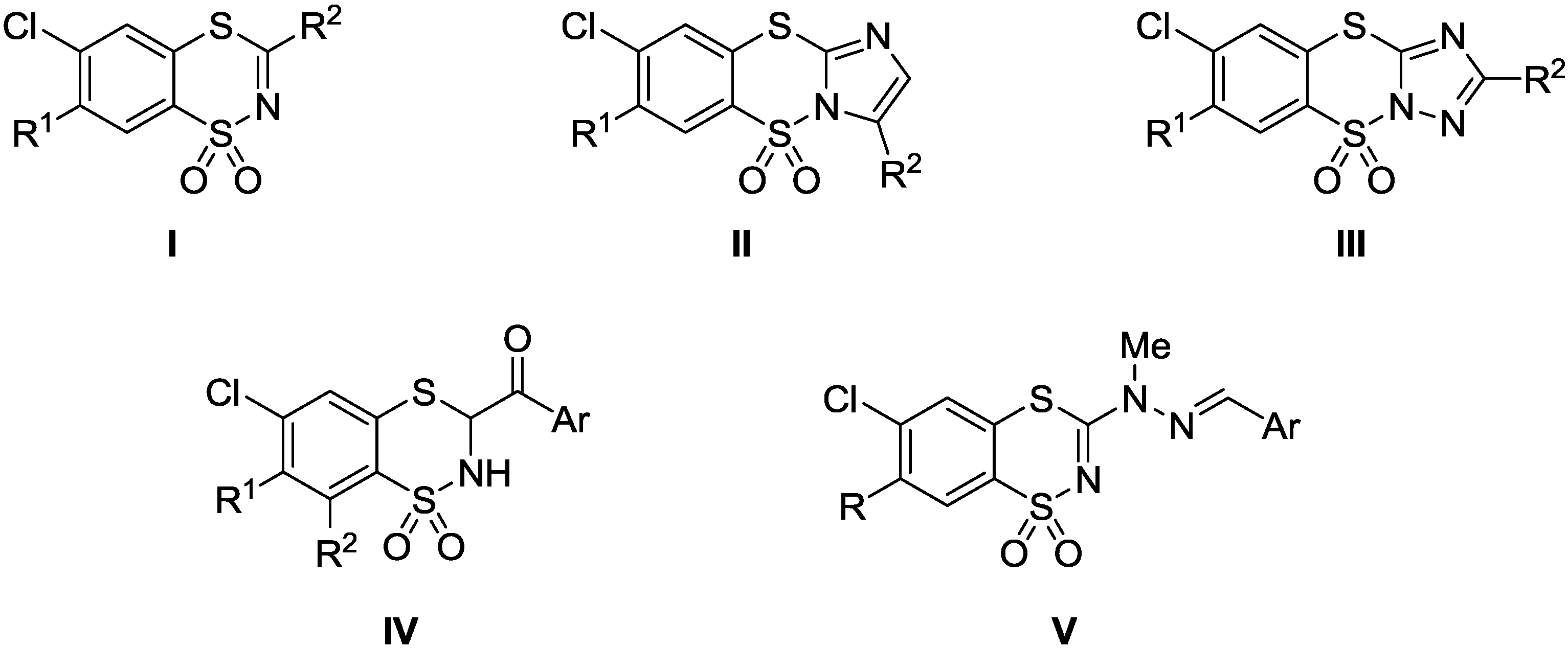
Synthesis and QSAR Study of Novel 6-Chloro-3-(2-Arylmethylene-1-methylhydrazino)-1,4,2-benzodithiazine 1,1-Dioxide Derivatives with Anticancer Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxic Activity
| Compounds | IC50 [µM] | ||
|---|---|---|---|
| MCF-7 | HCT-116 | HeLa | |
| 2 | 250 ± 7 | 280 ± 1 | 125 ± 7 |
| 4 | 155 ± 2 | 240 ± 5 | 99 ± 4 |
| 6 | 45 ± 0.5 | 31 ± 1 | 66 ± 2 |
| 7 | 270 ± 13 | 150 ± 4 | >>100 a |
| 8 | 84 ± 3 | 38 ± 1 | 80 ± 1 |
| 9 | 95 ± 1 | 24 ± 1 | 32 ± 2 |
| 10 | 73 ± 2 | 39 ± 1 | 64 ± 3 |
| 11 | 70 ± 3 | 38 ± 1 | 75 ± 3 |
| 12 | 63 ± 1 | 84 ± 1 | 100 ± 3 |
| 13 | 140 ± 1 | 115 ± 2 | 100 ± 1 |
| 14 | 15 ± 0.5 | 16 ± 0.5 | 135 ± 9 |
| 15 | 105 ± 2 | 70 ± 2 | 170 ± 5 |
| 16 | 150 ± 3 | 23 ± 0.5 | 10 ± 0.5 |
| 17 | 83 ± 1 | 74 ± 1 | 62 ± 1 |
| 18 | 165 ± 6 | 260 ± 3 | 140 ± 7 |
| Cisplatin | 3.0 ± 0.1 | 3.8 ± 0.2 | 2.2 ± 0.1 |
2.3. Quantitative Structure-Activity Relationships (QSARs) of Cytotoxic Activity
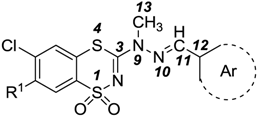
| Compd | CPK Area (Å2) | CPK Volume (Å3) | PSA (Å2) | MW (amu) | CFD (HBD) | CFD (HBA) | Angle (C3,N9,N10) | Angle (N9,N10,C11) | Dihedral (C3,N9,N10,C11) | Dihedral (N9,N10,C11,C12) | Distance (S4,C12) | Distance (S1,C12) | LogP | Hardness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 378.32 | 351.11 | 89.6827 | 411.890 | 2 | 8 | 119.59 | 115.50 | 69.29 | −179.26 | 6.053 | 5.332 | 4.8599 | 0.199531 |
| 8 | 389.58 | 362.09 | 69.9921 | 474.787 | 1 | 7 | 119.89 | 116.30 | 65.86 | −179.35 | 6.053 | 5.325 | 6.0783 | 0.197595 |
| 9 | 395.72 | 366.01 | 109.1826 | 440.888 | 1 | 10 | 120.39 | 117.25 | 61.89 | −179.55 | 6.052 | 5.320 | 5.2833 | 0.201345 |
| 10 | 417.97 | 388.93 | 74.8555 | 504.813 | 1 | 8 | 119.77 | 116.17 | 66.77 | −179.57 | 6.055 | 5.335 | 5.9519 | 0.195736 |
| 11 | 402.80 | 375.17 | 68.4649 | 509.232 | 1 | 7 | 119.73 | 116.36 | 65.77 | −179.05 | 6.050 | 5.317 | 6.6365 | 0.197948 |
| 12 | 346.63 | 318.50 | 63.7233 | 368.869 | 1 | 7 | 121.27 | 117.32 | 59.23 | 179.13 | 6.044 | 5.315 | 2.9324 | 0.190920 |
| 13 | 370.32 | 337.44 | 99.2438 | 414.850 | 0 | 10 | 120.74 | 118.17 | 58.08 | −179.47 | 6.027 | 5.308 | 3.6225 | 0.193076 |
| 14 | 377.41 | 345.96 | 91.1344 | 430.917 | 0 | 10 | 121.28 | 118.67 | 55.91 | 179.99 | 6.045 | 5.329 | 4.3443 | 0.186695 |
| 15 | 411.87 | 380.93 | 110.6991 | 455.899 | 2 | 9 | 119.50 | 115.35 | 69.58 | −179.19 | 6.053 | 5.334 | 4.1929 | 0.188761 |
| 16 | 411.80 | 380.88 | 110.6926 | 455.899 | 2 | 9 | 119.90 | 116.04 | 66.55 | −179.41 | 6.058 | 5.337 | 4.1929 | 0.184929 |
| 17 | 370.21 | 345.35 | 85.0143 | 406.874 | 1 | 8 | 119.29 | 115.11 | 63.61 | −177.70 | 6.017 | 5.199 | 4.7956 | 0.185247 |
| 18 | 371.53 | 338.82 | 114.5075 | 425.833 | 0 | 11 | 120.30 | 117.52 | 60.87 | −179.34 | 6.027 | 5.303 | 3.1687 | 0.194908 |
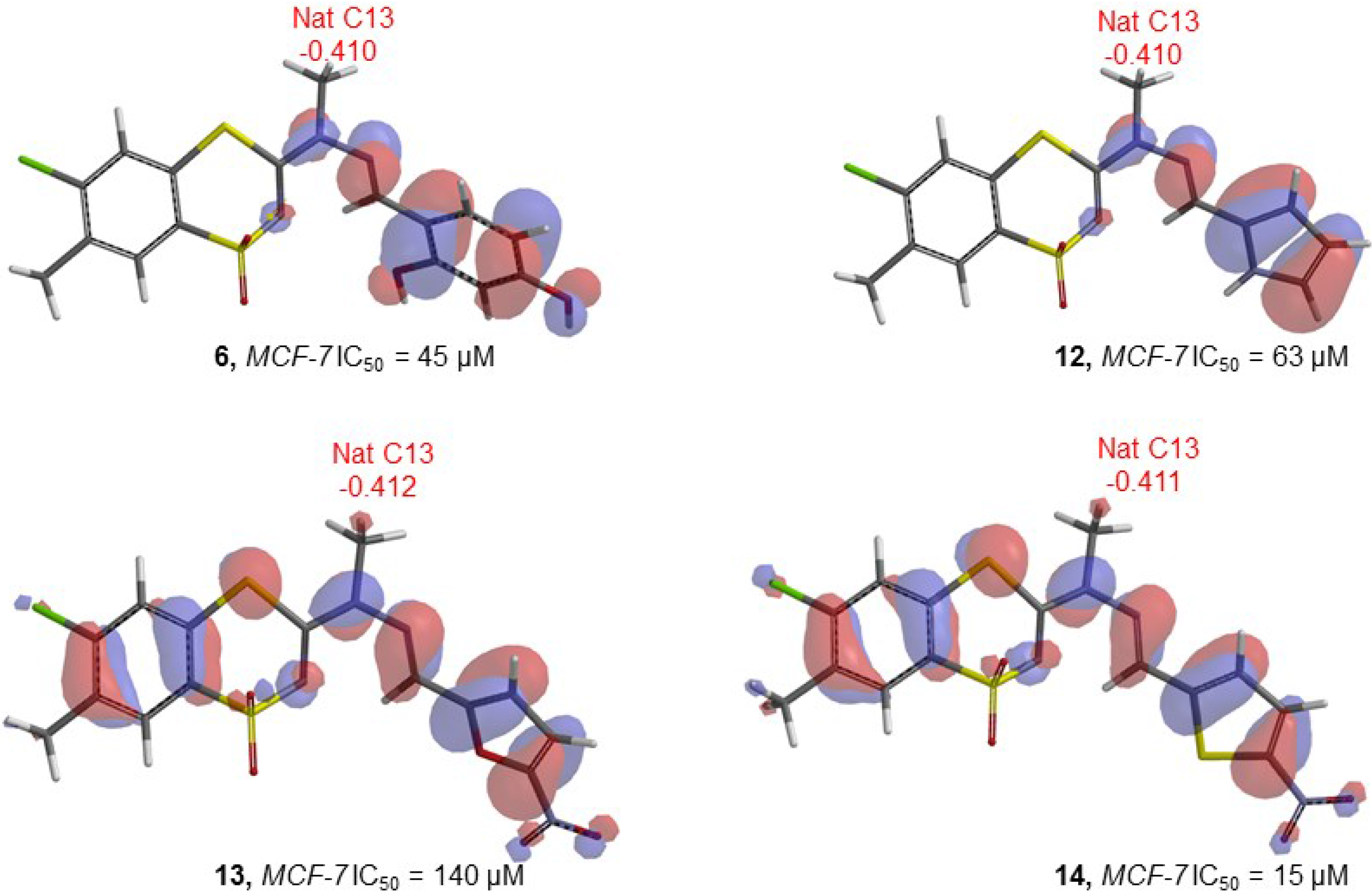
| Compd | E | E HOMO | E LUMO | LUMO-HOMO | Elect (C13) | Mull (C13) | Nat (C13) | Elect (C11) | Mull (C11) | Nat (C11) | Elect (N10) | Mull (N10) | Nat (N10) | Electronegativity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (kcal/mol) | ||||||||||||||
| 6 | −1462850 | −194.26 | 56.16 | 250.42 | −0.579 | −0.303 | −0.410 | 0.330 | 0.224 | 0.226 | −0.384 | −0.352 | −0.376 | 0.110038 |
| 8 | −3029531 | −201.90 | 46.09 | 247.99 | −0.608 | −0.306 | −0.411 | 0.299 | 0.217 | 0.213 | −0.365 | −0.338 | −0.355 | 0.124147 |
| 9 | −1543557 | −214.66 | 38.03 | 252.69 | −0.588 | −0.308 | −0.411 | 0.253 | 0.215 | 0.203 | −0.338 | −0.338 | −0.350 | 0.140737 |
| 10 | −3100993 | −196.45 | 49.20 | 245.65 | −0.587 | −0.306 | −0.411 | 0.297 | 0.217 | 0.213 | −0.355 | −0.337 | −0.353 | 0.117330 |
| 11 | −3317493 | −208.48 | 39.95 | 248.43 | −0.582 | −0.307 | −0.411 | 0.301 | 0.218 | 0.209 | −0.350 | −0.334 | −0.349 | 0.134281 |
| 12 | −1355166 | −186.14 | 53.47 | 239.61 | −0.662 | −0.306 | −0.410 | 0.175 | 0.157 | 0.183 | −0.384 | −0.327 | −0.351 | 0.105707 |
| 13 | −1495275 | −218.12 | 24.20 | 242.32 | −0.609 | −0.311 | −0.412 | 0.143 | 0.131 | 0.141 | −0.376 | −0.297 | −0.313 | 0.154513 |
| 14 | −1697748 | −218.76 | 15.55 | 234.31 | −0.602 | −0.311 | −0.411 | 0.189 | 0.175 | 0.163 | −0.340 | −0.301 | −0.317 | 0.161921 |
| 15 | −1580568 | −195.25 | 41.65 | 236.90 | −0.595 | −0.304 | −0.411 | 0.350 | 0.225 | 0.228 | −0.386 | −0.351 | −0.377 | 0.122384 |
| 16 | −1580564 | −191.14 | 40.95 | 232.09 | −0.574 | −0.307 | −0.411 | 0.347 | 0.212 | 0.215 | −0.375 | −0.334 | −0.355 | 0.119665 |
| 17 | −1448937 | −206.78 | 25.71 | 232.49 | −0.575 | −0.307 | −0.411 | 0.326 | 0.158 | 0.208 | −0.399 | −0.310 | −0.350 | 0.144282 |
| 18 | −1528335 | −224.84 | 19.78 | 244.62 | −0.595 | −0.313 | −0.413 | 0.150 | 0.139 | 0.150 | −0.383 | −0.296 | −0.316 | 0.163393 |
| Cell Line | Equation | N | R | s | Rcv | RMSECV | F | p |
|---|---|---|---|---|---|---|---|---|
| MCF-7 | IC50 = −72361.3 (Nat C13) + 2.8 (E HOMO) − 29088.3 | 12 | 0.874 | 23.52 | 0.750 | 28.66 | 14.592 | 0.001499 |
| HCT-116 | IC50 = −45749.1 (Nat C13) − 937.3 (Elect N10) − 19099.8 | 10 | 0.902 | 14.97 | 0.715 | 21.54 | 15.303 | 0.002782 |
| Compounds | IC50 [µM] | |||
|---|---|---|---|---|
| MCF-7 | HCT-116 | |||
| Observed | Predicted | Observed | Predicted | |
| 6 | 45 ± 0.5 | 41 | 31 ± 1 | 17 |
| 8 | 84 ± 3 | 92 | 38 ± 1 | 45 |
| 9 | 95 ± 1 | 57 | 24 ± 1 | 20 |
| 10 | 73 ± 2 | 108 | 39 ± 1 | 36 |
| 11 | 70 ± 3 | 74 | 38 ± 1 | 31 |
| 12 | 63 ± 1 | 64 | 84 ± 1 | - a |
| 13 | 140 ± 1 | 120 | 115 ± 2 | 101 |
| 14 | 15 ± 0.5 | 46 | 16 ± 0.5 | 22 |
| 15 | 105 ± 2 | 111 | 70 ± 2 | 65 |
| 16 | 150 ± 3 | 122 | 23 ± 0.5 | 54 |
| 17 | 83 ± 1 | 79 | 74 ± 1 | 77 |
| 18 | 165 ± 6 | 174 | 260 ± 3 | - a |
3. Experimental Section
3.1. General Information
3.2. Chemistry
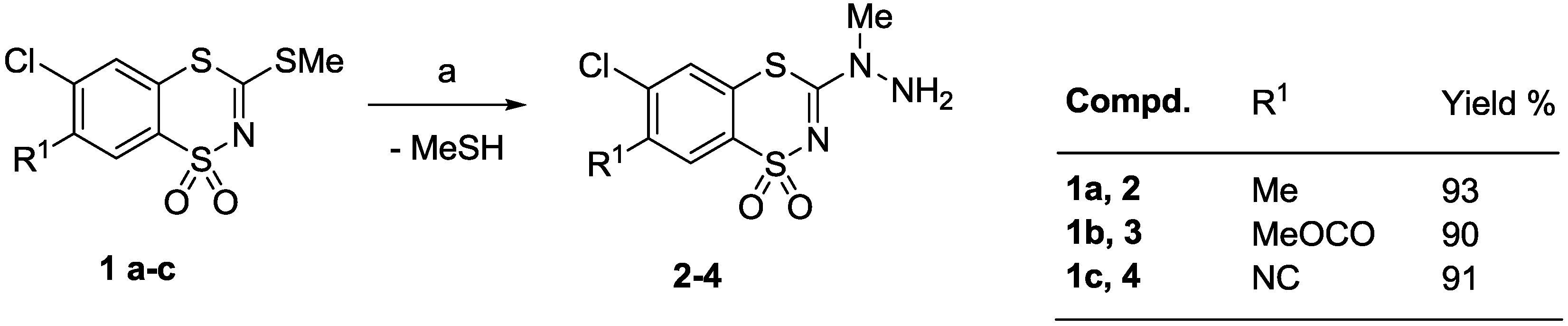
3.2.1. Procedure for the Preparation of N-Methyl-N-(6-chloro-7-R1-1,1-dioxo-1,4,2-benzodithiazin-3-yl)hydrazines 2–4
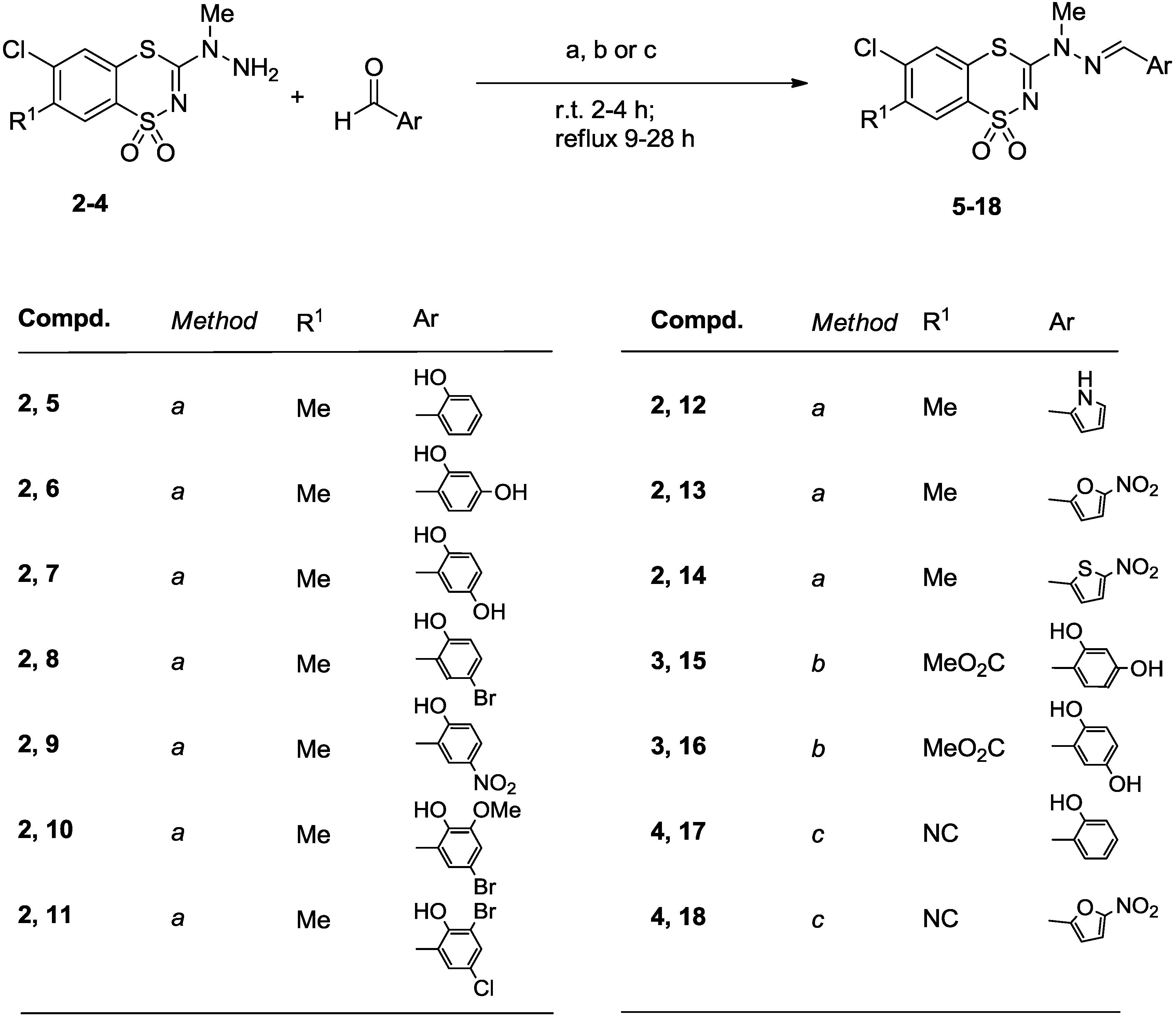
3.2.2. General Procedure for the Preparation of 6-Chloro-7-methyl-3-(2-arylmethylene-1-methyl-hydrazino)-1,1-dioxo-1,4,2-benzodithiazines 5–14
3.2.3. Procedure for the Preparation of Methyl 6-Chloro-3-[2-(2,4-dihydroxybenzylidene)-1-methyl-hydrazino]-1,1-dioxo-1,4,2-benzodithiazine-7-carboxylates 15, 16
3.2.4. Procedure for the Preparation of 6-Chloro-7-cyano-3-(2-arylmethylene-1-methylhydrazino)-1,1-dioxo-1,4,2-benzodithiazines 17, 18
3.3. Cell Culture and Cell Viability Assay
3.4. Molecular Modeling Methodology/Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brzozowski, Z.; Sławiński, J.; Angielski, S.; Szczepańska-Konkel, M. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. III Synthesis and diuretic properties of 3-(R,R1-phenyl)amino-6-chloro-7-methyl-1,1-dioxo- 1,4,2-benzodithiazines. Acta Pol. Pharm. 1985, 42, 313–318. [Google Scholar]
- Brzozowski, Z.; Sławiński, J. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. IV Synthesis of some novel N-substituted 3-amino-6-chloro-7-methyl-1,1-dioxo-1,4,2-benzodithiazines. Acta Pol. Pharm. 1985, 42, 319–325. [Google Scholar]
- Brzozowski, Z.; Sławiński, J.; Gajewski, F.; Angielski, S.; Hoppe, A. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. V Synthesis and diuretic properties of some novel 3-phenylamino-7-carboxy-1,1-dioxo-1,4,2-benzodithiazines. Acta Pol. Pharm. 1985, 42, 413–420. [Google Scholar]
- Brzozowski, Z.; Sławiński, J. Preparation Method for Novel 1,1-Dioxo-3-mercapto-1,4,2-benzodithiazines (Sposób otrzymywania nowych 1,1-diokso-3-merkapto-1,4,2-benzoditiazyn). Polish Patent 134567; issued 20 May 1986,
- Brzozowski, Z.; Sławiński, J.; Gajewski, F.; Angielski, S.; Hoppe, A.; Janiec, W.; Piekarska, T. Preparation Method for Novel 3-Amino-1,1-dioxo-1,4,2-benzodithiazines (Sposób otrzymywania nowych 3-amino-1,1-diokso-1,4,2-benzoditiazyn). Polish Patent 140677; issued 30 April 1988,
- Brzozowski, Z.; Sławiński, J.; Gajewski, F.; Pomarnacka, E.; Janiec, W.; Piekarska, T. Preparation Method for Novel 3-Amino-1,1-dioxo-1,4,2-benzodithiazine derivatives (Sposób otrzymywania pochodnych 3-amino-1,1-diokso-1,4,2-benzoditiazyny). Polish Patent 141834; issued 30 November 1988,
- Brzozowski, Z.; Sławiński, J.; Janiec, W.; Cegieła, U.; Śliwiński, L.; Sedlak, I. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. XII Synthesis and pharmacological properties of some 6-chloro-3-carboxyalkylamino-7-methyl-1,1-dioxo-1,4,2-benzodithiazines. Acta Pol. Pharm. Drug Res. 1992, 49, 75–79. [Google Scholar]
- Brzozowski, Z.; Sączewski, F.; Gdaniec, M. Synthesis, structural characterization and in vitro antitumor activity of novel 6-chloro-1,1-dioxo-1,4,2-benzodithiazie derivatives. Bioorg. Med. Chem. 2003, 11, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Gdaniec, M. Synthesis, structural characterization and in vitro antitumor activity of 4-dimethylaminopyridinium (6-chloro-1,1-dioxo-1,4,2-benzodithiazin-3-yl)methanides. Eur. J. Med. Chem. 2003, 38, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Sławiński, J.; Bednarski, P.J.; Grünert, R.; Gdaniec, M. Synthesis, structural characterization, and in vitro antitumor activity of novel N-(6-chloro-1,1-dioxo-1,4,2-benzodithiazin-3-yl)arylsulfonamides. Bioorg. Med. Chem. 2007, 15, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F. A new type of mixed anhydride and its applications to the synthesis of 7-substituted 8-chloro-5,5-dioxoimidazo[1,2-b][1,4,2]benzodithiazines with in vitro antitumor activity. J. Med. Chem. 2002, 45, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Pomarnacka, E.; Gdaniec, M. Synthesis and anticancer activity of 2-amino-8-chloro-5,5-dioxo[1,2,4]triazolo[2,3-b][1,4,2]benzodithiazine derivatives. Bioorg. Med. Chem. 2003, 11, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Neamati, N. Synthesis, antitumor and anti-HIV activities of benzodithiazine-dioxides. Bioorg. Med. Chem. 2006, 14, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Sanchez, T.; Kuo, C.L.; Gdaniec, M.; Neamati, N. Synthesis, antiviral, and anti-HIV-1 integrase activities of 3-aroyl-1,1-dioxo-1,4,2-benzodithiazines. Bioorg. Med. Chem. 2004, 12, 3663–3672. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Sączewski, F.; Sławiński, J.; Sanchez, T.; Neamati, N. Synthesis and anti-HIV-1 integrase activities of 3-aroyl-2,3-dihydro-1,1-dioxo-1,4,2-benzodithiazines. Eur. J. Med. Chem. 2009, 44, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, H. Quantitative structure-activity relationship (QSAR) and molecular modeling in cancer research. J. Cancer Res. Clin. Oncol. 1990, 116, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Schmidt, E.W. Hydrazines and Its Derivatives: Preparation, Properties, Applications, 2nd ed.; VCH: Weinheim, Germany, 2001. [Google Scholar]
- Nigst, T.A.; Ammer, J.; Mayr, H. Ambident reactivities of methylhydrazines. Angew. Chem. Int. Ed. 2012, 51, 1353–1356. [Google Scholar] [CrossRef]
- Spartan ’08; Wavefunction, Inc.: Irvine, CA, USA, 2009.
- Statistica 10; StatSoft. Inc.: Tulsa, OK, USA, 2011.
- Ghose, A.K.; Pritchett, A.; Crippen, G.M. Atomic Physicochemical Parameters for Three Dimensional Structure Directed Quantitative Structure-Activity Relationships III: Modeling Hydrophobic Interactions. J. Comput. Chem. 1988, 9, 80–90. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Sławiński, J. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. II Synthesis of some 3-mercapto-1,1-dioxo-1,4,2-benzodithiazine derivatives. Acta Pol. Pharm. 1984, 41, 133–139. [Google Scholar]
- Brzozowski, Z.; Sławiński, J. 1,1-Dioxo-1,4,2-benzodithiazine derivatives. I Synthesis of some 7-carboxy-3-mercapto-1,1-dioxo-1,4,2-benzodithiazine derivatives. Acta Pol. Pharm. 1984, 41, 5–13. [Google Scholar]
- Brzozowski, Z. Derivatives of 2-mercaptobenzenesulphonamide XIV. On the products of selective alcoholysis and hydrolysis of 3-methyltio-1,4,2-benzodithiazine 1,1-dioxide derivatives. Acta Pol. Pharm. Drug Res. 1994, 51, 69–76. [Google Scholar]
- Sample Availability: Samples of the compounds 1–18 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławiński, J.; Żołnowska, B.; Brzozowski, Z.; Kawiak, A.; Belka, M.; Bączek, T. Synthesis and QSAR Study of Novel 6-Chloro-3-(2-Arylmethylene-1-methylhydrazino)-1,4,2-benzodithiazine 1,1-Dioxide Derivatives with Anticancer Activity. Molecules 2015, 20, 5754-5770. https://doi.org/10.3390/molecules20045754
Sławiński J, Żołnowska B, Brzozowski Z, Kawiak A, Belka M, Bączek T. Synthesis and QSAR Study of Novel 6-Chloro-3-(2-Arylmethylene-1-methylhydrazino)-1,4,2-benzodithiazine 1,1-Dioxide Derivatives with Anticancer Activity. Molecules. 2015; 20(4):5754-5770. https://doi.org/10.3390/molecules20045754
Chicago/Turabian StyleSławiński, Jarosław, Beata Żołnowska, Zdzisław Brzozowski, Anna Kawiak, Mariusz Belka, and Tomasz Bączek. 2015. "Synthesis and QSAR Study of Novel 6-Chloro-3-(2-Arylmethylene-1-methylhydrazino)-1,4,2-benzodithiazine 1,1-Dioxide Derivatives with Anticancer Activity" Molecules 20, no. 4: 5754-5770. https://doi.org/10.3390/molecules20045754