Anti-Ageing Effects of Sonchus oleraceus L. (pūhā) Leaf Extracts on H2O2-Induced Cell Senescence
Abstract
:1. Introduction
2. Results and Discussion
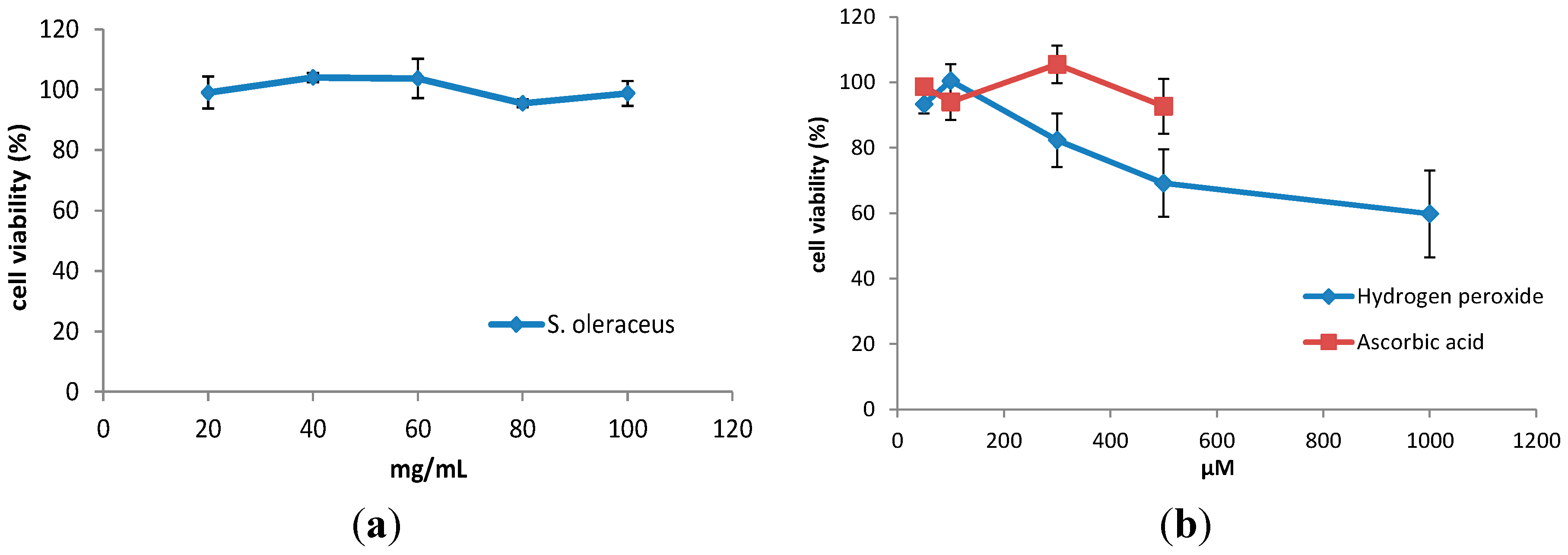
2.1. Cell Viability
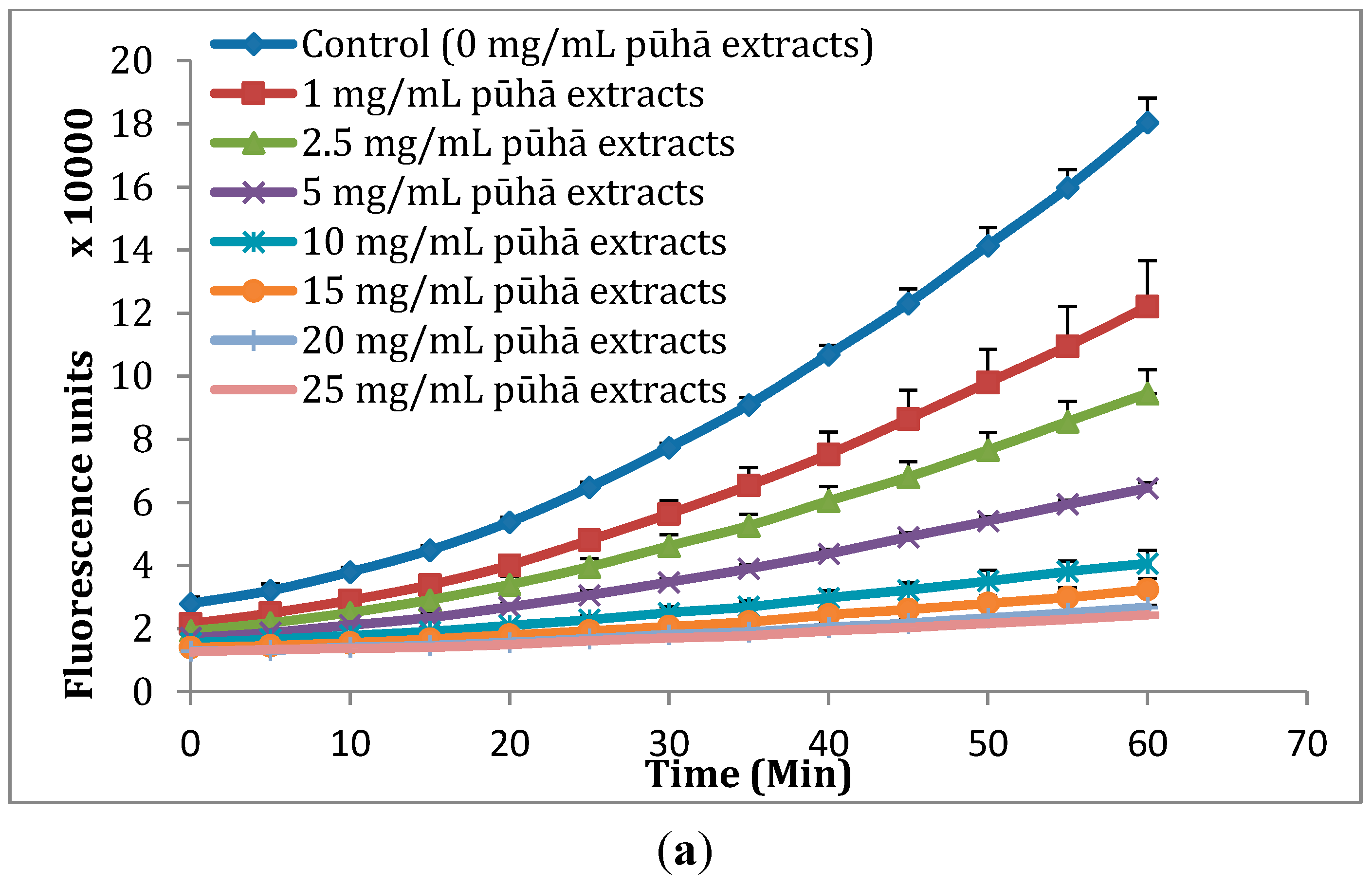
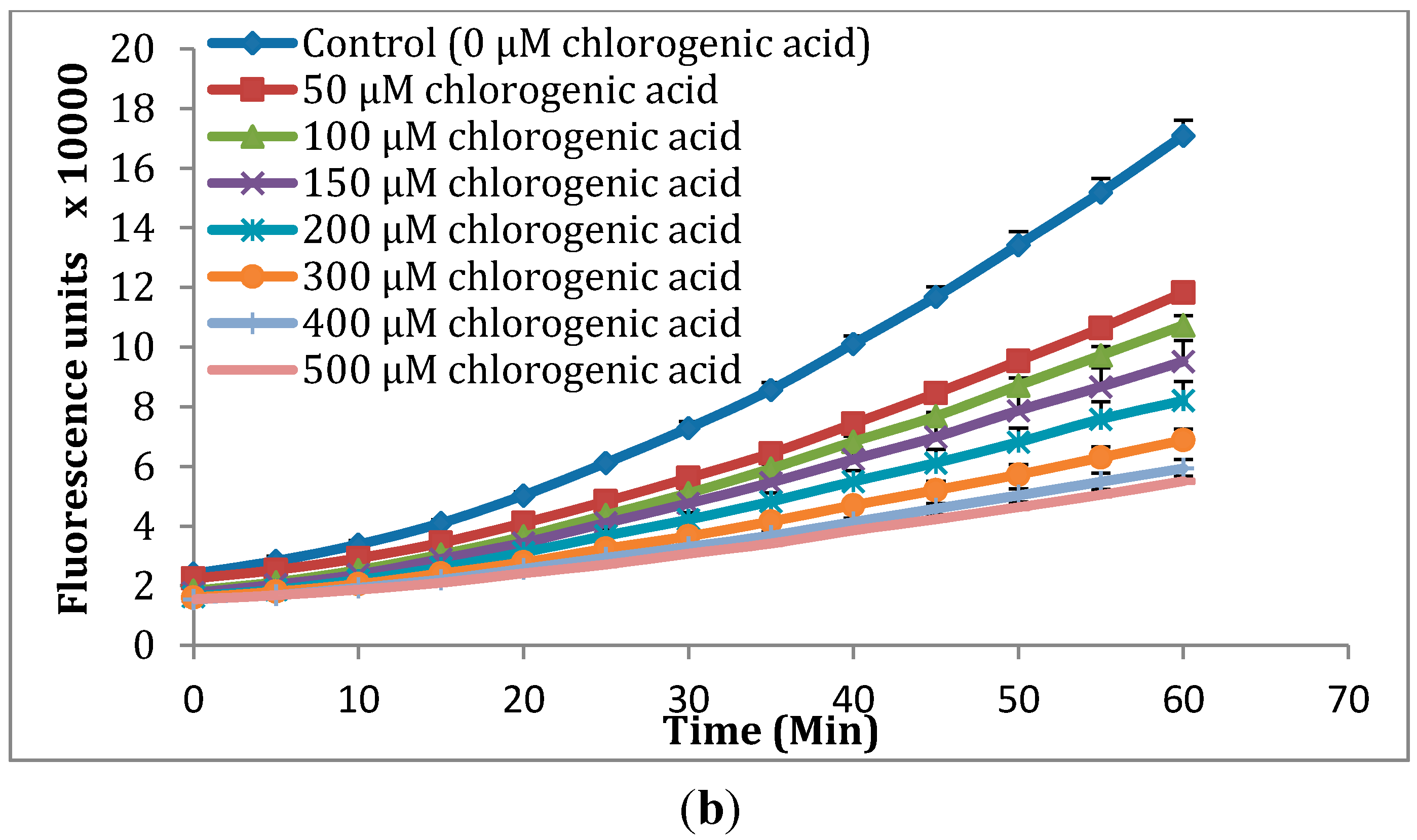
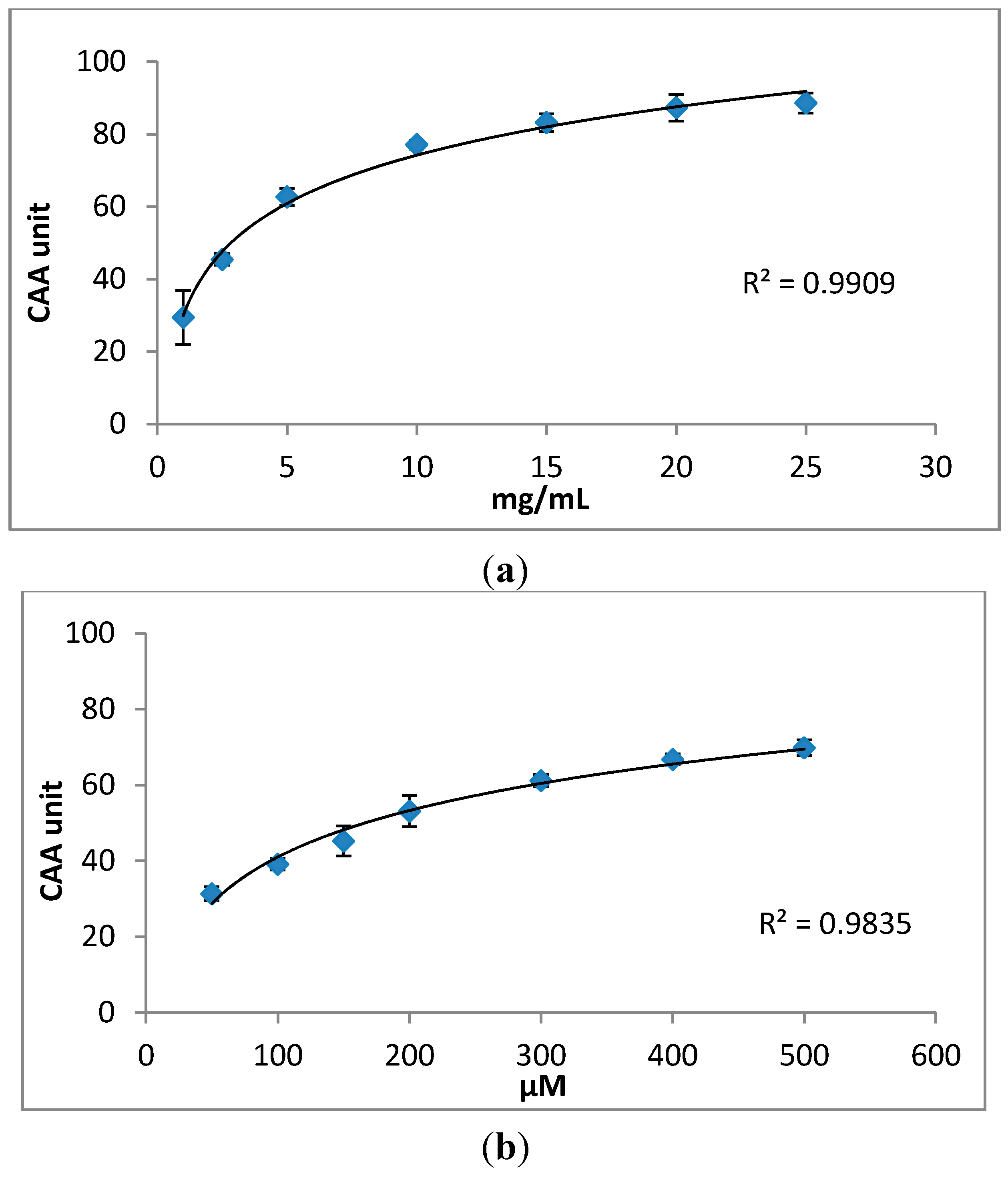
2.2. Cellular Antioxidant Activity (CAA) Assay
2.3. Effects of S. oleraceus Leaf Extracts on Stress-Induced Premature Senescence in WI-38 Cells
2.3.1. Protective Effect

2.3.2. Recovery Effect

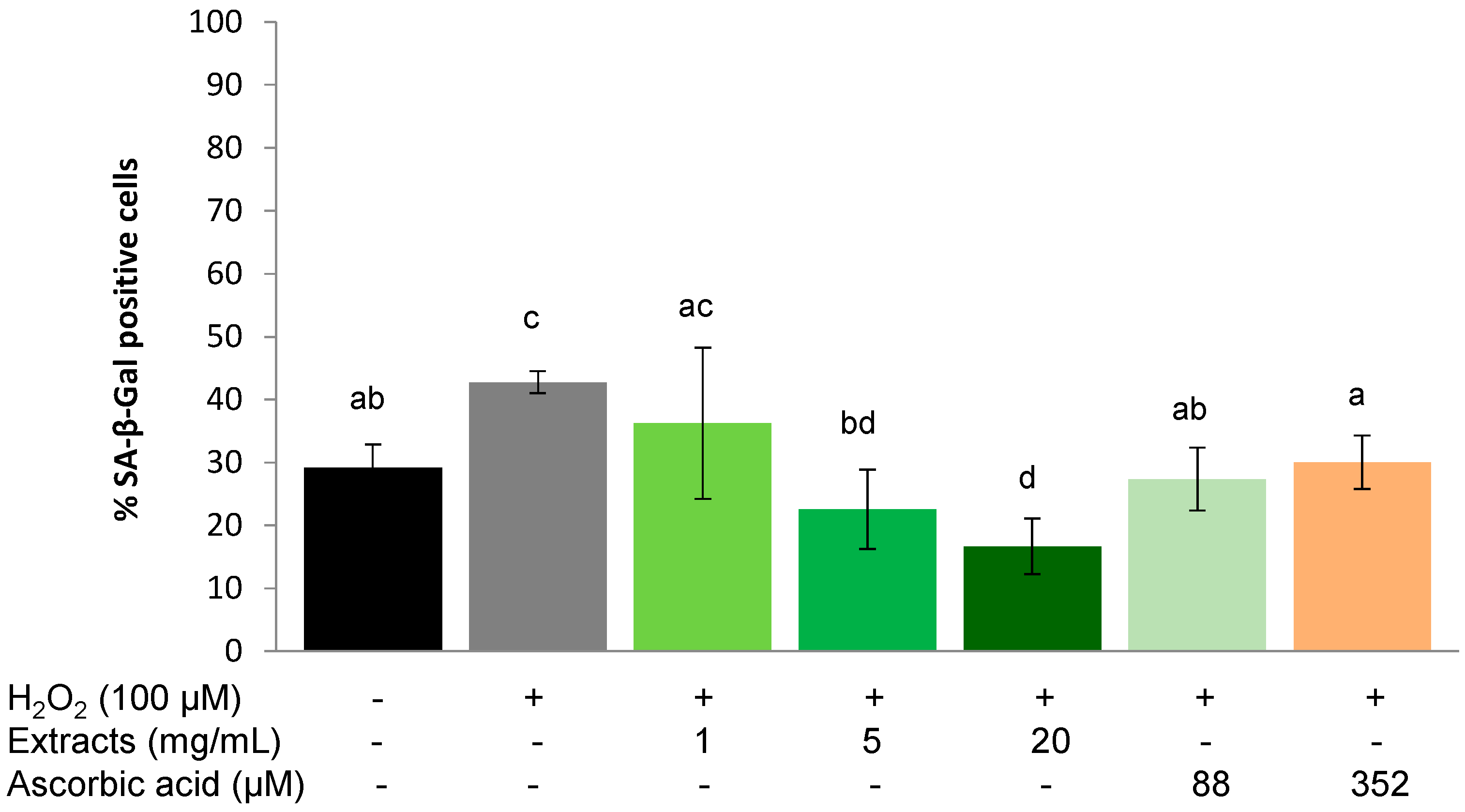
2.3.3. Protective Effect on Chronic Stress
2.4. Discussion
3. Experimental Section
3.1. Plant Material
3.2. Preparation of Leaf Extracts
3.3. Preparation of Extract Samples and Chemical Solutions
3.4. Cell Culture
3.5. Cell Viability
3.6. Cellular Antioxidant Activity (CAA) Assay
3.7. Quantification of CAA
3.8. Stress-Induced Premature Senescence in WI-38 Cells
3.8.1. Protective Effect of S. oleraceus Leaf Extracts
3.8.2. Recovery Ability of S. oleraceus Leaf Extracts
3.8.3. Protective Effect from Chronic Stress
3.9. Analysis of Senescence-Associated β-Galactosidase (SA-β-gal) Activity in WI-38 Cells
3.10. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Getoff, N. Anti-aging and aging factors in life. The role of free radicals. Radiat. Phys. Chem. 2007, 76, 1577–1586. [Google Scholar] [CrossRef]
- Toussaint, O.; Medrano, E.E.; von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (sips) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000, 35, 927–945. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-β-gal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Von Zglinicki, T. Role of oxidative stress in telomere length regulation and replicative senescence. Annu. N. Y. Acad. Sci. 2000, 908, 99–110. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.C.; Halliwell, B. Metals and oxygen: Respiration, oxidation and oxygen toxicity. In Antioxidants in Nutrition, Health, and Disease; Oxford University Press: Oxford, UK; New York, NY, USA, 1994; pp. 24–39. [Google Scholar]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Torsello, A.; Covacci, V.; Fasanella, S.; Montanari, M.; Boninsegna, A.; Cittadini, A. Oxidative DNA damage as a marker of aging in WI-38 human fibroblasts. Exp. Gerontol. 2002, 37, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Smith, J.R. Extension of the lifespan of cultured normal human diploid cells by vitamin E. Proc. Natl. Acad. Sci. USA 1974, 71, 4763–4767. [Google Scholar] [CrossRef] [PubMed]
- Fusco, D.; Colloca, G.; Lo Monaco, M.R.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377–387. [Google Scholar] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.-S.; Park, S.-H.; Kim, H.-S.; Kang, H.-J.; Kim, M.-J.; Oh, S.-J.; Park, J.-B.; Kim, J.; Kim, S.C.; Lee, J.-Y. Ascorbic acid extends replicative life span of human embryonic fibroblast by reducing DNA and mitochondrial damages. Nutr. Res. Pract. 2007, 1, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, G.F.; Liu, X.Z.; An, L.J.; Guan, S. Anti-ageing effects of protocatechuic acid from Alpinia on spleen and liver antioxidative system of senescent mice. Cell Biochem. Funct. 2011, 29, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Wiegant, F.A.C.; Surinova, S.; Ytsma, E.; Langelaar-Makkinje, M.; Wikman, G.; Post, J.A. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 2009, 10, 27–42. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, P.; Wu, J.; Xiong, S.; Jin, N.; Huang, Z. The neuroprotective and lifespan-extension activities of Damnacanthus officinarum extracts in Caenorhabditis elegans. J. Ethnopharmacol. 2012, 141, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar]
- Safer, A.M.; Al-Nughamish, A.J. Hepatotoxicity induced by the anti-oxidant food additive, butylated hydroxytoluene (BHT), in rats: An electron microscopical study. Histol. Histopathol. 1999, 14, 391–406. [Google Scholar] [PubMed]
- Choi, M.J.; Kim, B.K.; Park, K.Y.; Yokozawa, T.; Song, Y.O.; Cho, E.J. Anti-aging effects of cyanidin under a stress-induced premature senescence cellular system. Biol. Pharm. Bull. 2010, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Noppe, G.; Dekker, P.; de Koning-Treurniet, C.; Blom, J.; van Heemst, D.; Dirks, R.W.; Tanke, H.J.; Westendorp, R.G.; Maier, A.B. Rapid flow cytometric method for measuring senescence associated β-galactosidase activity in human fibroblasts. Cytom. A 2009, 75, 910–916. [Google Scholar] [CrossRef]
- Hwang, E.; Yoon, G.; Kang, H. A comparative analysis of the cell biology of senescence and aging. Cell. Mol. Life Sci. 2009, 66, 2503–2524. [Google Scholar] [CrossRef] [PubMed]
- Von Zglinicki, T.; Saretzki, G.; Ladhoff, J.; D’Adda di Fagagna, F.; Jackson, S.P. Human cell senescence as a DNA damage response. Mech. Ageing Dev. 2005, 126, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Pascal, T.; Boilan, E.; Bastin, C.; Bauwens, E.; Toussaint, O. Screening of senescence-associated genes with specific DNA array reveals the role of IGFBP-3 in premature senescence of human diploid fibroblasts. Free Radic. Bio. Med. 2008, 44, 1817–1832. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Itahana, K.; Campisi, J.; Dimri, G. Methods to detect biomarkers of cellular senescence. In Biological Aging; Tollefsbol, T., Ed.; Humana Press: Totowa, NJ, USA, 2007; Volume 371, pp. 21–31. [Google Scholar]
- Guil-Guerrero, J.L.; Gimenez-Gimenez, A.; Rodriguez-Garcia, I.; Torija-Isasa, M.E. Nutritional composition of Sonchus species (S. asper L., S. oleraceus L. and S. tenerrimus L.). J. Sci. Food Agric. 1998, 76, 628–632. [Google Scholar]
- Yin, J.; Si, C.-L.; Wang, M.-H. Antioxidant activity of flavonoids and their glucosides from Sonchus oleraceus L. J. Appl. Biol. Chem. 2008, 51, 57–60. [Google Scholar] [CrossRef]
- Gould, K.S.; Thodey, K.; Philpott, M.; Ferguson, L.R. Antioxidant activities of extracts from traditional māori food plants. N. Z. J. Bot. 2006, 44, 1–4. [Google Scholar] [CrossRef]
- Xia, D.-Z.; Yu, X.-F.; Zhu, Z.-Y.; Zou, Z.-D. Antioxidant and antibacterial activity of six edible wild plants (Sonchus spp.) in china. Nat. Prod. Res. 2011, 25, 1893–1901. [Google Scholar] [CrossRef]
- McDowell, A.; Thompson, S.; Stark, M.; Ou, Z.-Q.; Gould, K.S. Antioxidant activity of puha (Sonchus oleraceus L.) as assessed by the cellular antioxidant activity (CAA) assay. Phytother. Res. 2011, 25, 1876–1882. [Google Scholar]
- Ou, Z.-Q.; Schmierer, D.M.; Rades, T.; Larsen, L.; McDowell, A. Application of an online post-column derivatization HPLC-DPPH assay to detect compounds responsible for antioxidant activity in Sonchus oleraceus L. leaf extracts. J. Pharm. Pharmacol. 2013, 65, 271–279. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Kwon, G.-J.; Wang, M.-H. The antioxidant and cytotoxic activities of Sonchus oleraceus L. extracts. Nutr. Res. Pract. 2007, 1, 189–194. [Google Scholar] [CrossRef]
- Argyropoulou, A.; Aligiannis, N.; Trougakos, I.P.; Skaltsounis, A.-L. Natural compounds with anti-ageing activity. Nat. Prod. Rep. 2013, 30, 1412–1437. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.-Q.; Schmierer, D.M.; Strachan, C.J.; Rades, T.; McDowell, A. Influence of postharvest processing and storage conditions on key antioxidants in pūhā (Sonchus oleraceus L.). J. Pharm. Pharmacol. 2014, 66, 998–1008. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Kang, X.M.; He, X.J.; Dong, M.; Zhang, Q.Y.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food. Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.V.; Kobira, K.; Keck, A.-S.; Juvik, J.A.; Jeffery, E.H. Correlation analyses of phytochemical composition, chemical, and cellular measures of antioxidant activity of broccoli (Brassica oleracea Var.Italica). J. Agric. Food Chem. 2005, 53, 7421–7431. [Google Scholar] [CrossRef]
- Roy, M.K.; Juneja, L.R.; Isobe, S.; Tsushida, T. Steam processed broccoli (Brassica oleracea) has higher antioxidant activity in chemical and cellular assay systems. Food Chem. 2009, 114, 263–269. [Google Scholar] [CrossRef]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular antioxidant activity of common vegetables. J. Agric. Food Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Selman, C.; McLaren, J.S.; Meyer, C.; Duncan, J.S.; Redman, P.; Collins, A.R.; Duthie, G.G.; Speakman, J.R. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech. Ageing Dev. 2006, 127, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Borlon, C.; Pascal, T.; Royer, V.; Eliaers, F.; Ninane, N.; Carrard, G.; Friguet, B.; de Longueville, F.; Boffe, S.; et al. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J. Cell Sci. 2005, 118, 743–758. [Google Scholar]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Zhang, R.; Piao, M.J.; Chae, S.; Kim, K.N.; Jeon, Y.J.; Park, D.B.; You, H.J.; Kim, J.S.; et al. Caffeic acid protects hydrogen peroxide induced cell damage in WI-38 human lung fibroblast cells. Biol. Pharm. Bull. 2006, 29, 1820–1824. [Google Scholar]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar] [PubMed]
- Zhou, Y.; Dong, Y.; Xu, Q.G.; Zhu, S.Y.; Tian, S.L.; Huo, J.J.; Hao, T.T.; Zhu, B.W. Mussel oligopeptides protect human fibroblasts from hydrogen peroxide (H2O2)-induced premature senescence. Arch. Gerontol. Geriatr. 2014, 58, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.T.; Arroteia, K.F.; Lago, J.C.; de Sa-Rocha, V.M.; Gesztesi, J.; Moreira, P.L. A new potent natural antioxidant mixture provides global protection against oxidative skin cell damage. Int. J. Cosmet. Sci. 2011, 33, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Bartholomew, J.C.; Campisi, J.; Acosta, M.; Reagan, J.D.; Ames, B.N. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: P53 and Rb control G1 arrest but not cell replication. Biochem. J. 1998, 332, 43–50. [Google Scholar] [PubMed]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K.H. Bioactive polyphenol antioxidants protect oral fibroblasts from ROS-inducing agents. Arch. Oral. Biol. 2012, 57, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivao, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Z.-Q.; Rades, T.; McDowell, A. Anti-Ageing Effects of Sonchus oleraceus L. (pūhā) Leaf Extracts on H2O2-Induced Cell Senescence. Molecules 2015, 20, 4548-4564. https://doi.org/10.3390/molecules20034548
Ou Z-Q, Rades T, McDowell A. Anti-Ageing Effects of Sonchus oleraceus L. (pūhā) Leaf Extracts on H2O2-Induced Cell Senescence. Molecules. 2015; 20(3):4548-4564. https://doi.org/10.3390/molecules20034548
Chicago/Turabian StyleOu, Zong-Quan, Thomas Rades, and Arlene McDowell. 2015. "Anti-Ageing Effects of Sonchus oleraceus L. (pūhā) Leaf Extracts on H2O2-Induced Cell Senescence" Molecules 20, no. 3: 4548-4564. https://doi.org/10.3390/molecules20034548







