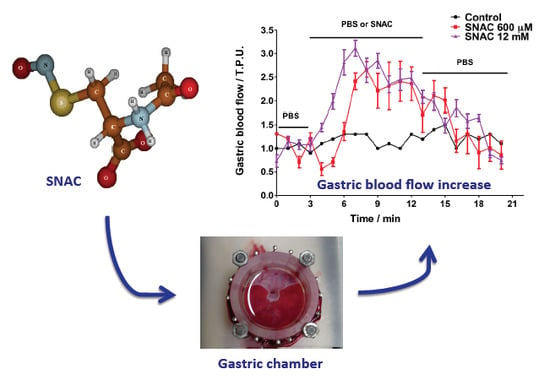
Nitric Oxide Released from Luminal S-Nitroso-N-Acetylcysteine Increases Gastric Mucosal Blood Flow
Abstract
:1. Introduction
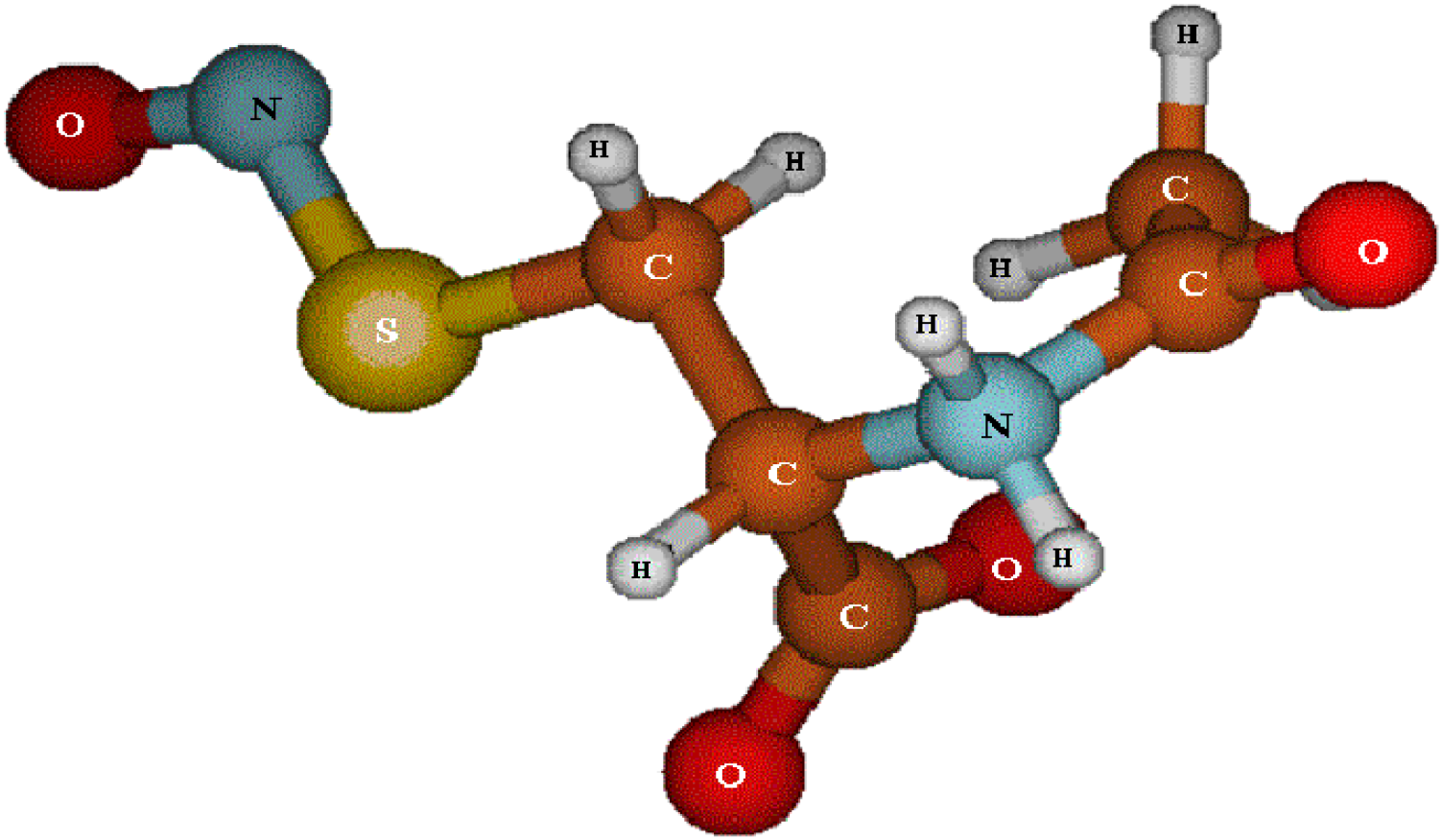
2. Results and Discussion
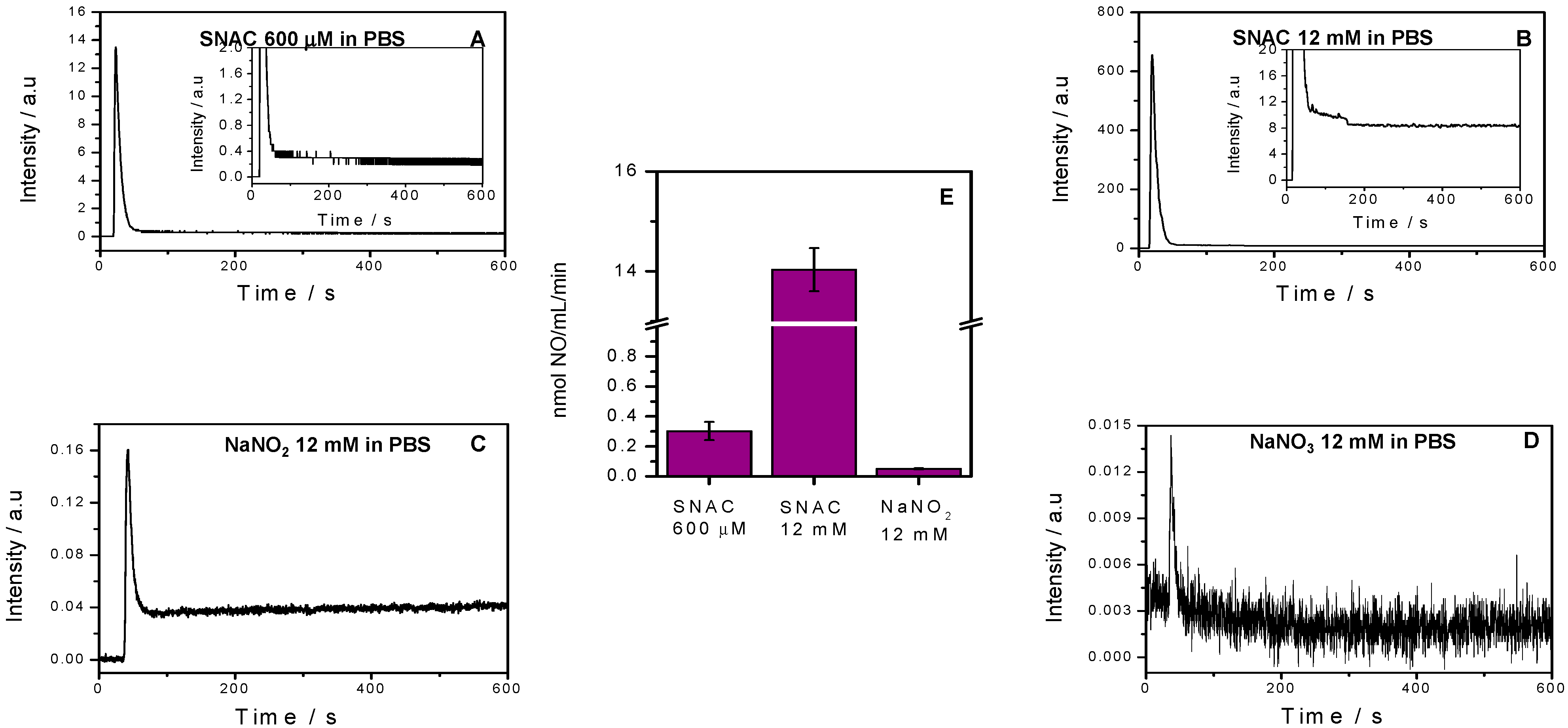
2.1. NO Release Profiles
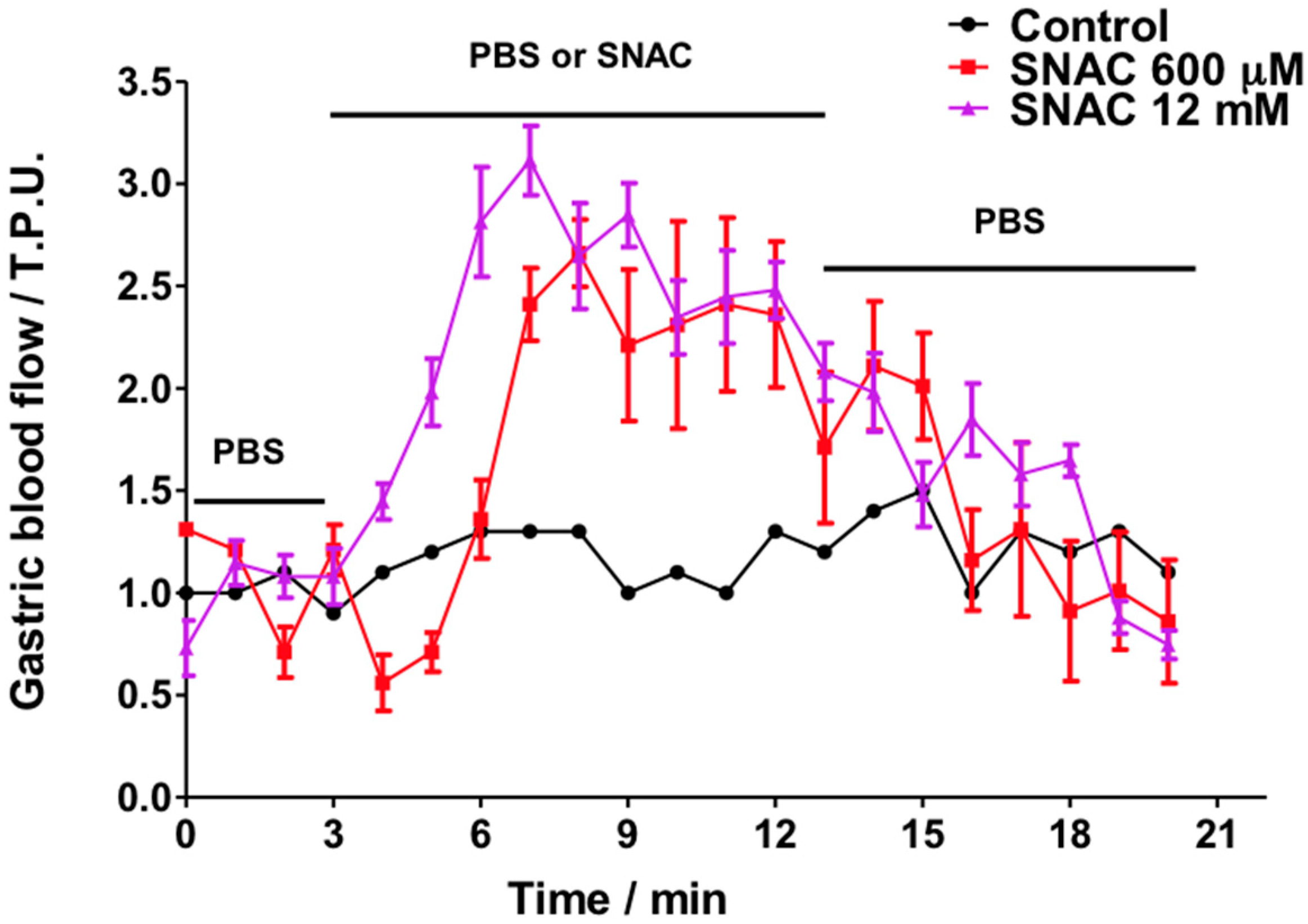
2.2. Gastric Blood Flow Measurements by Laser Doppler Flowmetry
2.3. Quantification of Plasma NOx Levels
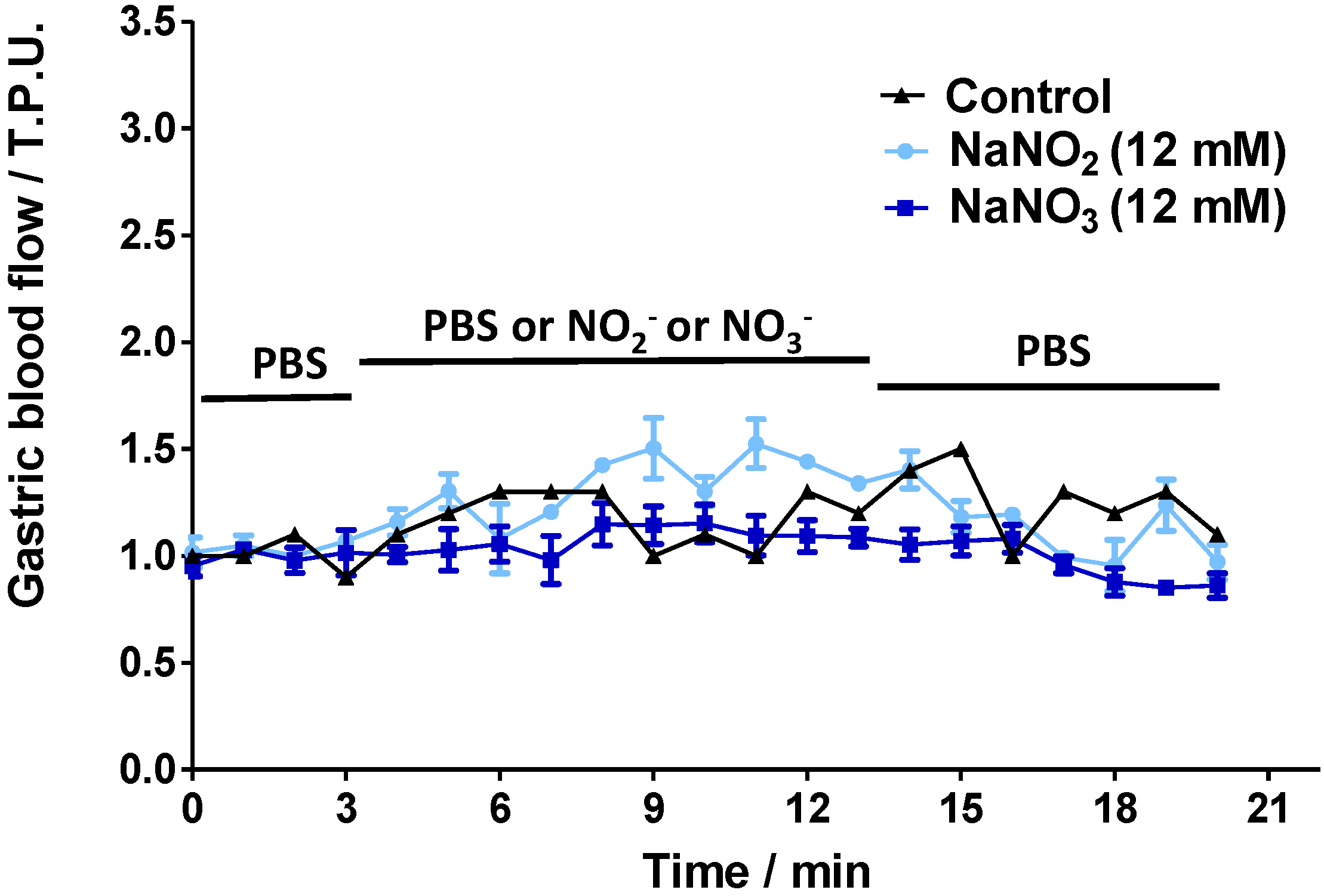
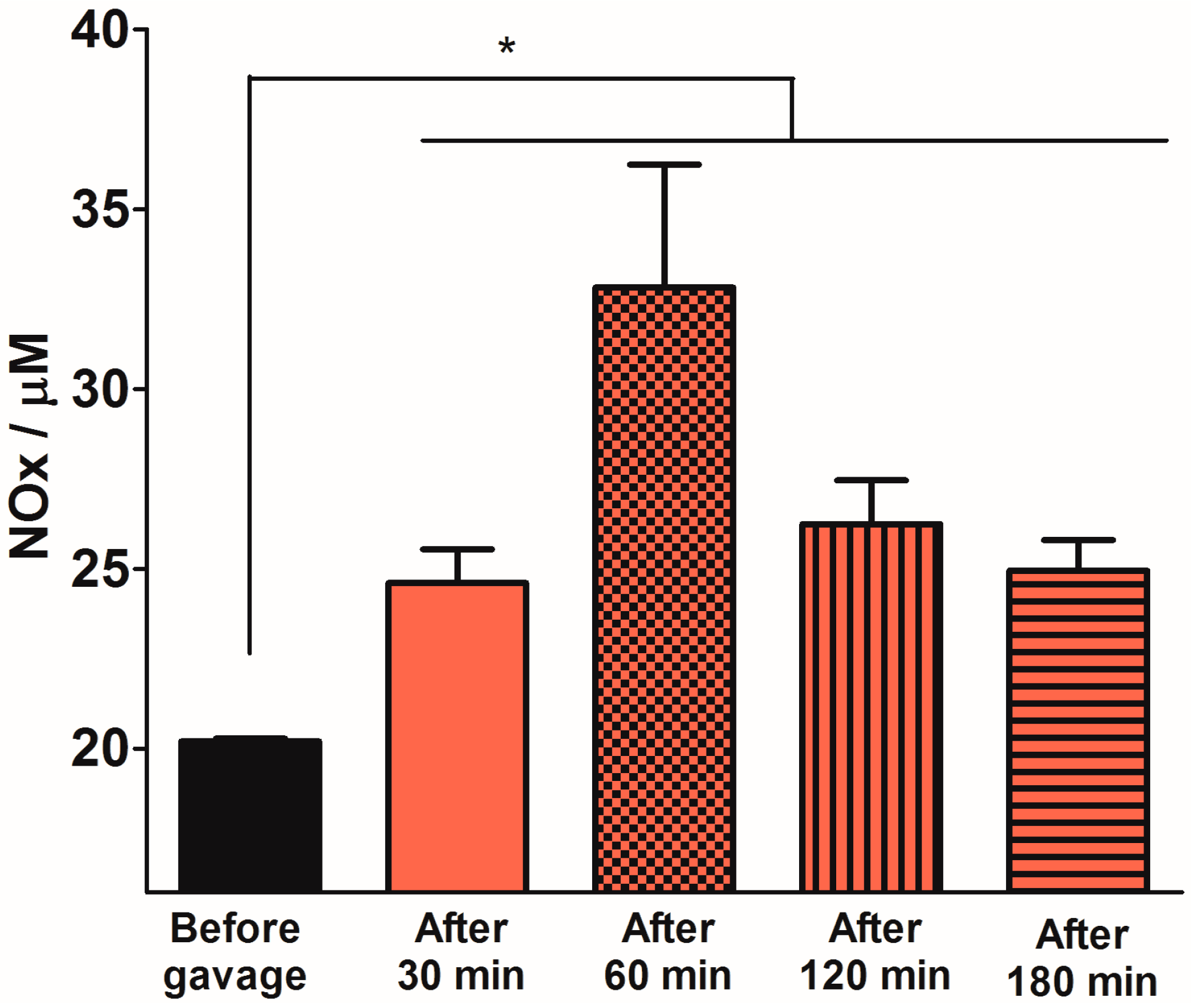
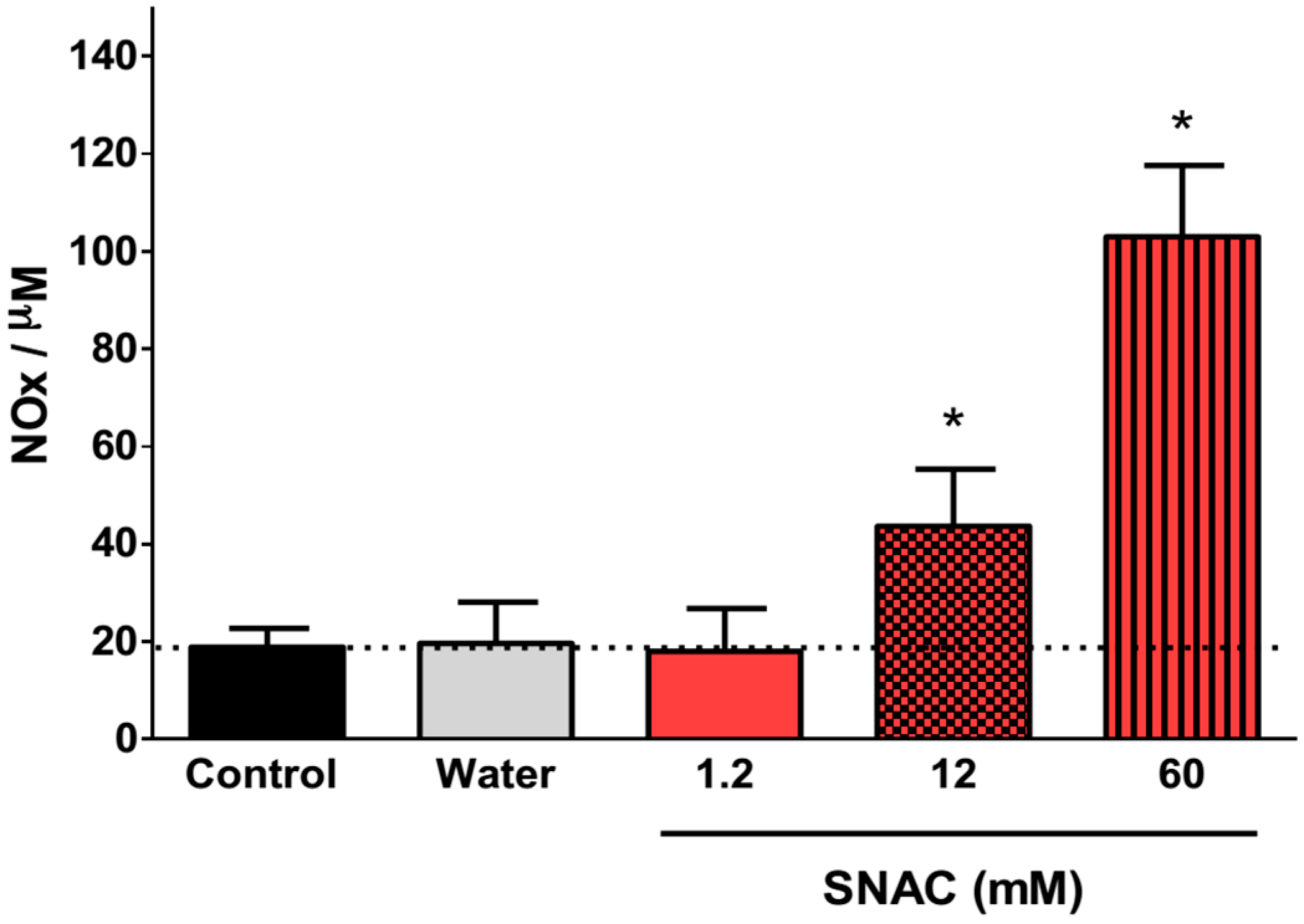
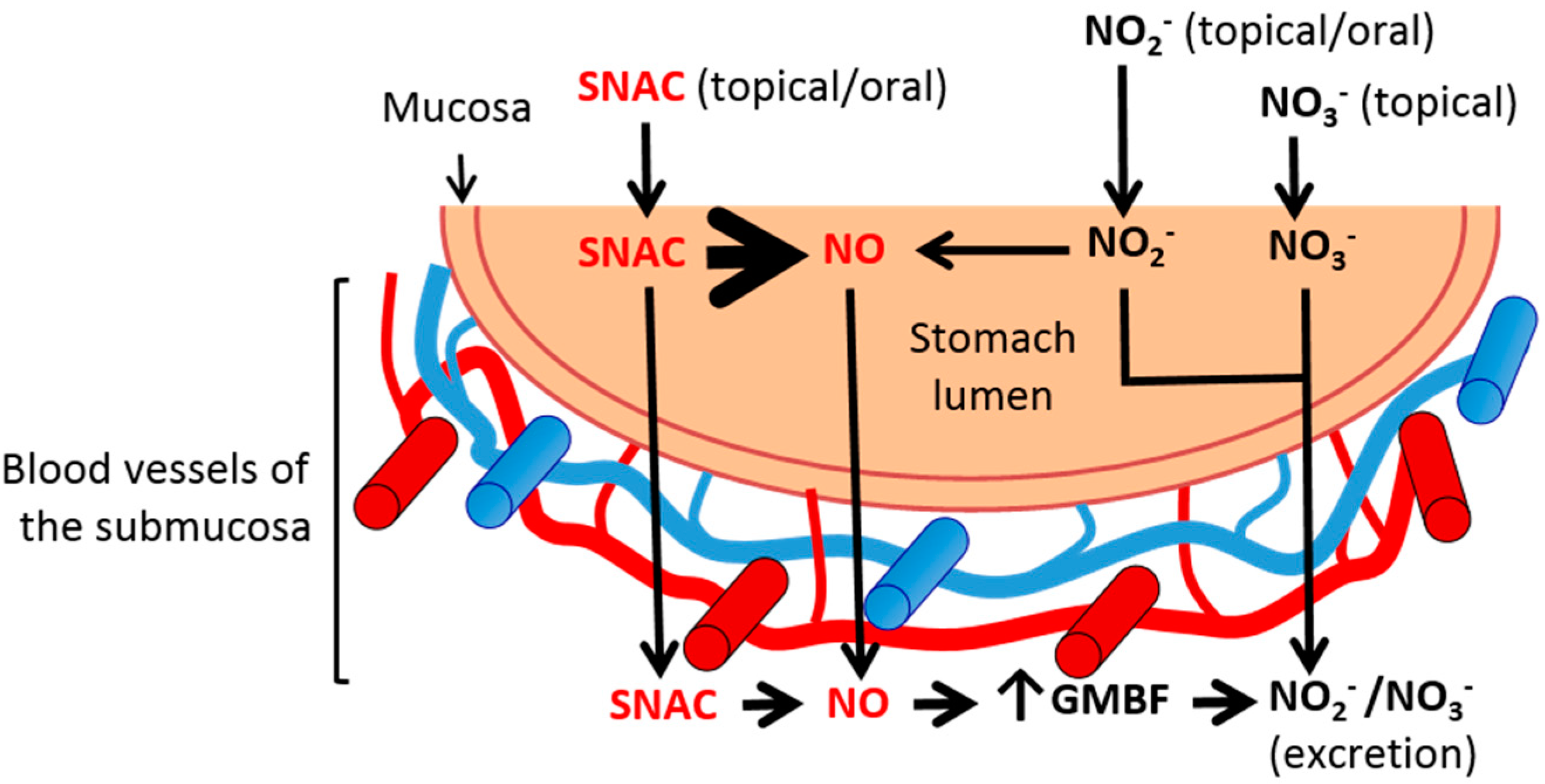
2.4. Discussion
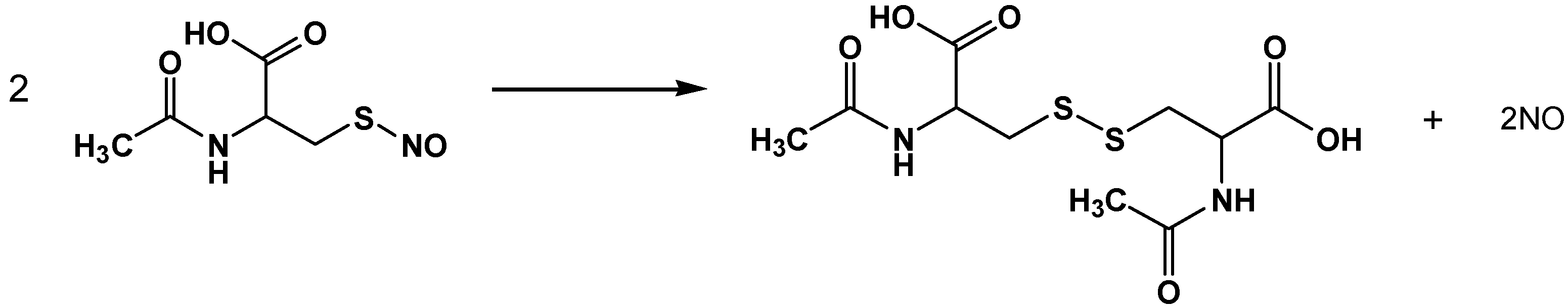

3. Experimental Section
3.1. SNAC Synthesis
3.2. Characterization of NO Release from SNAC, NaNO2 and NaNO3 Solutions
3.3. Animal Care
3.4. Experimental Design
3.5. Materials
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez-Ruiz, A.; Cadenas, S.; Lamas, S. Nitric oxide signaling: Classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 2011, 51, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kochar, N.I.; Chandewal, A.V.; Bakal, R.L.; Kochar, P.N. Nitric oxide and the gastrointestinal tract. Int. J. Pharmacol. 2011, 7, 31–39. [Google Scholar] [CrossRef]
- Brown, J.F.; Hanson, P.J.; Whittle, B.J. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur. J. Pharmacol. 1992, 223, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Oh, S.Y.; Ahn, B.; Kim, Y.B.; Jang, D.D.; Yang, K.H.; Hahm, K.B.; Kim, D.Y. Decreased helicobacter pylori associated gastric carcinogenesis in mice lacking inducible nitric oxide synthase. Gut 2004, 53, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Sahah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Miller, M.J. Nitric oxide in mucosal defense: A little goes a long way. Gastroenterology 2000, 119, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A. Role of nitric oxide in the gastrointestinal tract. Arthritis Res. Ther. 2008, 10, 1–6. [Google Scholar] [CrossRef]
- Pawlik, M.W.; Kwiecien, S.; Pajdo, R.; Ptak-Belowska, A.; Brzozowski, B.; Krzysiek-Maczka, G.; Strzalka, M.; Konturek, S.J.; Brzozowski, T. Esophagoprotective activity of angiotensin-(1–7) in experimental model of acute reflux esophagitis. Evidence for the role of nitric oxide, sensory nerves, hypoxia-inducible factor-1alpha and proinflammatory cytokines. J. Physiol. Pharmacol. 2014, 65, 809–822. [Google Scholar] [PubMed]
- Brzozowski, T.; Konturek, P.C.; Pajdo, R.; Ptak-Belowska, A.; Kwiecien, S.; Pawlik, M.; Drozdowicz, D.; Sliwowski, Z.; Brzozowski, B.; Konturek, S.J.; et al. Physiological mediators in nonsteroidal anti-inflammatory drugs (NSAIDs)-induced impairment of gastric mucosal defense and adaptation. Focus on nitric oxide and lipoxins. J. Physiol. Pharmacol. 2008, 59, 89–102. [Google Scholar] [PubMed]
- Zwolinska-Wcislo, M.; Brzozowski, T.; Ptak-Belowska, A.; Targosz, A.; Urbanczyk, K.; Kwiecien, S.; Sliwowski, Z. Nitric oxide-releasing aspirin but not conventional aspirin improves healing of experimental colitis. World J. Gastroenterol. 2011, 17, 4076–4089. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.W.; Saha, J.K.; Garvey, D.S.; Schroeder, J.D.; Shelekhin, T.E.; Janero, D.R.; Chen, L.; Glavin, A.; Letts, L.G. Nitrosothiol-based no-donors inhibit the gastrointestinal mucosal damaging actions of nsaids. Inflammopharmacology 2000, 8, 81–88. [Google Scholar] [CrossRef]
- Ricardo, K.F.S.; Shishido, S.M.; de Oliveira, M.G.; Krieger, M.H. Characterization of the hypotensive effect of s-nitroso-n-acetylcysteine in normotensive and hypertensive conscious rats. Nitric Oxide Biol. Chem. 2002, 7, 57–66. [Google Scholar] [CrossRef]
- Seabra, A.B.; Fitzpatrick, A.; Paul, J.; de Oliveira, M.G.; Weller, R. Topically applied s-nitrosothiol-containing hydrogels as experimental and pharmacological nitric oxide donors in human skin. Br. J. Dermatol. 2004, 5, 977–983. [Google Scholar] [CrossRef]
- Andraus, W.; Souza, G.F.; Oliveira, M.G.; Haddad, L.B.; Coelho, A.M.; Galvao, F.H.; Leitao, R.M.; D’Albuquerque, L.A.; Machado, M.C. S-nitroso-n-acetylcysteine ameliorates ischemia-reperfusion injury in the steatotic liver. Clinics 2010, 65, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazo, D.F.; de Oliveira, M.G.; Pereira, I.V.; Cogliati, B.; Stefano, J.T.; de Souza, G.F.; Rabelo, F.; Lima, F.R.; Ferreira Alves, V.A.; Carrilho, F.J.; et al. S-nitroso-n-acetylcysteine attenuates liver fibrosis in experimental nonalcoholic steatohepatitis. Drug Des. Dev. Ther. 2013, 7, 553–563. [Google Scholar]
- Vercelino, R.; Crespo, I.; de Souza, G.F.; Cuevas, M.J.; de Oliveira, M.G.; Marroni, N.P.; Gonzalez-Gallego, J.; Tunon, M.J. S-nitroso-N-acetylcysteine attenuates liver fibrosis in cirrhotic rats. J. Mol. Med. 2010, 88, 401–411. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.G.; Shishido, S.M.; Seabra, A.B.; Morgon, N.H. Thermal stability of primary S-nitrosothiols: Roles of autocatalysis and structural effects on the rate of nitric oxide release. J. Phys. Chem. A 2002, 106, 8963–8970. [Google Scholar] [CrossRef]
- Jia, L.; Bonaventura, C.; Bonaventura, J.; Stamler, J.S. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature 1996, 380, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, T.; Chono, K.; Hirata, T.; Keto, Y.; Kimoto, A.; Sasamata, M. Mucosal acid causes gastric mucosal microcirculatory disturbance in nonsteroidal anti-inflammatory drug-treated rats. Eur. J. Pharmacol. 2007, 554, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Phillipson, M.; Jansson, E.A.; Patzak, A.; Lundberg, J.O.; Holm, L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G718–G724. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Gladwin, M.T.; Ahluwalia, A.; Benjamin, N.; Bryan, N.S.; Butler, A.; Pedro, C.; Fago, A.; Feelisch, M.; Ford, P.C.; et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009, 5, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.M. The use of proton pump inhibitors in treating and preventing NSAID-induced mucosal damage. Arthritis Res. Ther. 2013, 15, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Shah, A.; Bemanian, S. Intragastric pH with oral vc intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology 2008, 134, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Heikal, L.; Martin, G.P.; Dailey, L.A. Characterisation of the decomposition behaviour of S-nitrosoglutathione and a new class of analogues: S-Nitrosophytochelatins. Nitric Oxide 2009, 20, 157–165. [Google Scholar]
- Pannala, A.S.; Mani, A.R.; Spencer, J.P.E.; Skinner, V.; Bruckdorfer, K.R.; Moore, K.P.; Rice-Evans, C.A. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic. Biol. Med. 2003, 34, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.S.; Widmer, W.R.; Luell, S.; Christen, A.; Orevillo, C.; Shah, S.; Visco, D. Therapeutic administration of a selective inhibitor of nitric oxide synthase does not ameliorate the chronic inflammation and tissue damage associated with adjuvant-induced arthritis in rats. J. Pharmacol. Exp. Ther. 1998, 284, 714–721. [Google Scholar] [PubMed]
- Van Amsterdam, J.G.; van den Berg, C.; Zuidema, J.; te Biesebeek, J.D.; Rokos, H. Effect of septicaemia on the plasma levels of biopterin and nitric oxide metabolites in rats and rabbits. Biochem. Pharmacol. 1996, 52, 1447–1451. [Google Scholar]
- Kino, M.; Yamato, T.; Aomine, M. Simultaneous measurement of nitric oxide, blood glucose, and monoamines in the hippocampus of diabetic rat: An in vivo microdialysis study. Neurochem. Int. 2004, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Scherer de Fraga, R.; Camacho, V.R.; Souza, G.F.; Cerski, C.T.; de Oliveira, J.R.; de Oliveira, M.G.; Alvares-da-Silva, M.R. S-nitroso-n-acetylcysteine: A promising drug for early ischemia/reperfusion injury in rat liver. Transpl. Proc. 2010, 42, 4491–4495. [Google Scholar] [CrossRef]
- Taladriz-Blanco, P.; de Oliveira, M.G. Enhanced photochemical nitric oxide release from a flutamidederivative incorporated in pluronic f127 micelles. J. Photochem. Photobiol. A Chem. 2014, 293, 65–71. [Google Scholar] [CrossRef]
- Samouilov, A.; Zweier, J.L. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal. Biochem. 1998, 258, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Camara, P.R.; Ferraz, G.J.; Velloso, L.A.; Zeitune, J.M.; Suassuna, F.A.; Ferraz, J.G. Endothelin and neonatal capsaicin regulate gastric resistance to injury in bdl rats. World J. Gastrointest. Pathophysiol. 2012, 3, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Troncy, E.; Francoeur, M.; Vinet, B.; Vinay, P.; Czaika, G.; Blaise, G. Effects of reducing reagents and temperature on conversion of nitrite and nitrate to nitric oxide and detection of no by chemiluminescence. Clin. Chem. 1997, 43, 657–662. [Google Scholar] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza, G.F.P.; Taladriz-Blanco, P.; Velloso, L.A.; De Oliveira, M.G. Nitric Oxide Released from Luminal S-Nitroso-N-Acetylcysteine Increases Gastric Mucosal Blood Flow. Molecules 2015, 20, 4109-4123. https://doi.org/10.3390/molecules20034109
De Souza GFP, Taladriz-Blanco P, Velloso LA, De Oliveira MG. Nitric Oxide Released from Luminal S-Nitroso-N-Acetylcysteine Increases Gastric Mucosal Blood Flow. Molecules. 2015; 20(3):4109-4123. https://doi.org/10.3390/molecules20034109
Chicago/Turabian StyleDe Souza, Gabriela F. P., Patricia Taladriz-Blanco, Lício A. Velloso, and Marcelo G. De Oliveira. 2015. "Nitric Oxide Released from Luminal S-Nitroso-N-Acetylcysteine Increases Gastric Mucosal Blood Flow" Molecules 20, no. 3: 4109-4123. https://doi.org/10.3390/molecules20034109