Effect of Diterpenes Isolated of the Marine Alga Canistrocarpus cervicornis against Some Toxic Effects of the Venom of the Bothrops jararaca Snake
Abstract
:1. Introduction
2. Results and Discussion
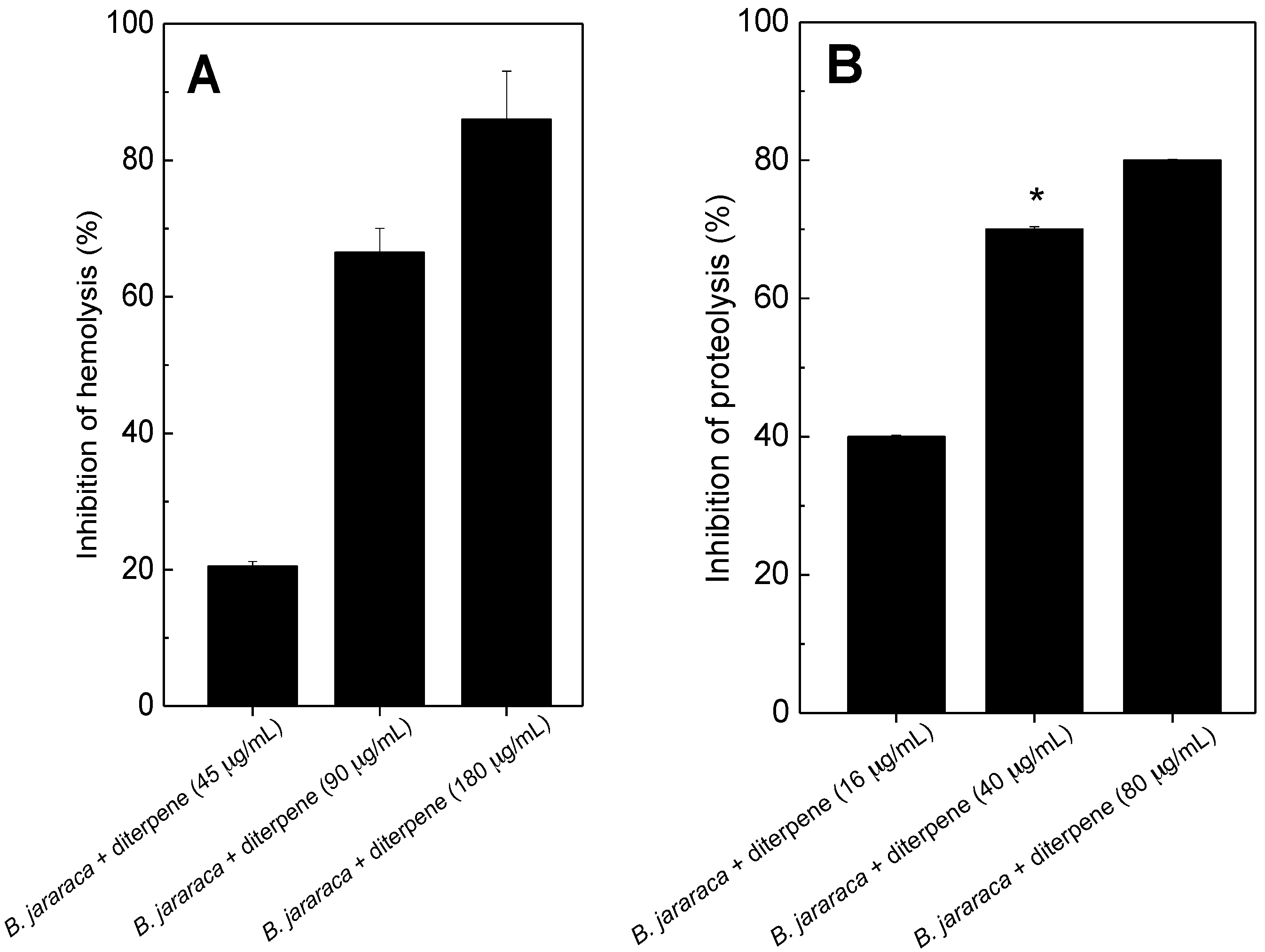
2.1. Antihemolytic and Antiproteolytic Effect
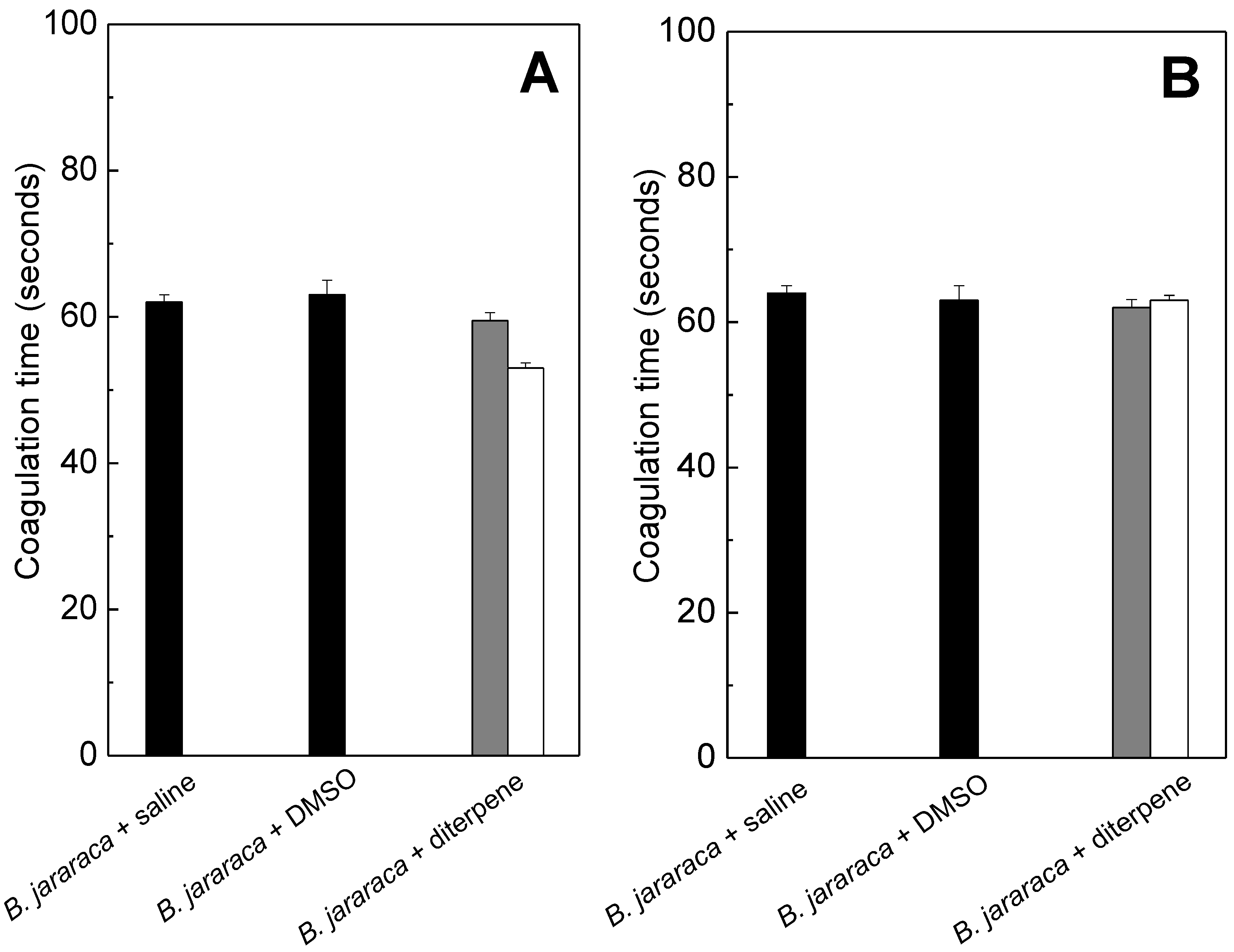
2.2. Anticoagulation
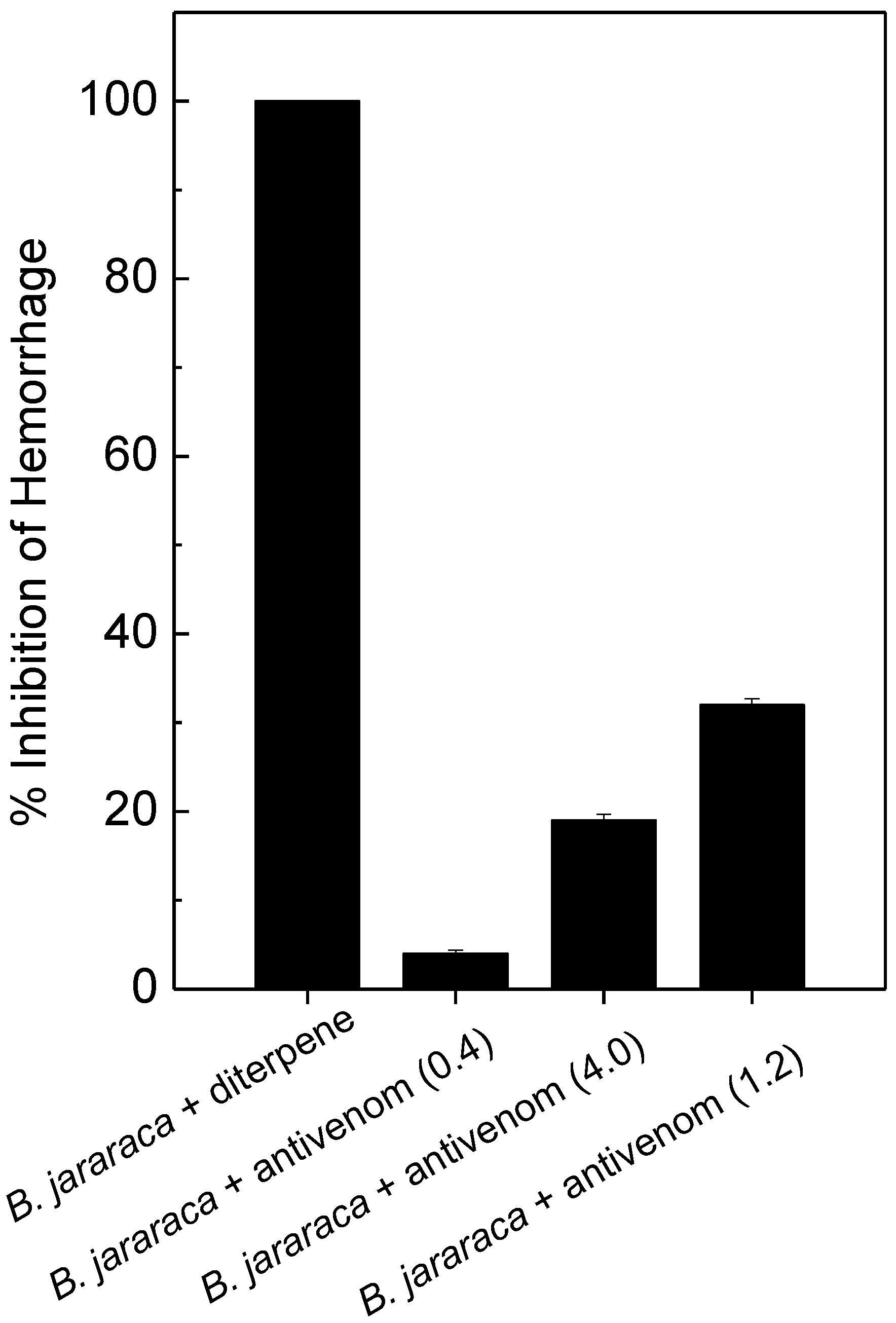
2.3. Antihemorrhagic and Antiedematogenic Activities
2.4. Antilethality Activity
| Groups | Death (min) |
|---|---|
| B. jararaca + saline | 320 ± 14 |
| B. jararaca + DMSO | 350 ± 21 |
| Saline + diterpenes | ND |
| B. jararaca + diterpenes | 310 ± 20 |
| B. jararaca + antibothropic serum (0.4 mL/mg) | ND |
| B. jararaca + antibothropic serum (1.2 mL/mg) | ND |
| B. jararaca + antibothropic serum (4.0 mL/mg) | ND |
3. Experimental Section
3.1. Algal Material
3.2. Isolation of Diterpenes
3.3. Snake Venom, Antivenom and Animals
3.4. Antihemolytic Activity
3.5. Anticoagulant Activity
3.6. Antiproteolytic Activity
3.7. Antihemorrhagic Activity
3.8. Antiedematogenic Activity
3.9. Antilethal Activity
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Production Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Gutiérrez, J.M. Comprendiendo los venenos de serpientes: 50 años de investigaciones en América Latina. Rev. Biol. Trop. 2002, 50, 377–394. [Google Scholar] [PubMed]
- Soares, A.M.; Fontes, M.R.M.; Giglio, J.R. Phospholipase A2 myotoxins from Bothrops snake venoms: Structure–function relationship. Curr. Org. Chem. 2004, 8, 1677–1690. [Google Scholar] [CrossRef]
- Bochner, R.; Struchiner, C.J. Snake bite epidemiology in the last 100 years in Brazil: A review. Cad. Saúde Pública 2003, 19, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.J.; Jorge, M.T.; Ribeiro, L.A. Epidemiology of snakebite in a central region of Brazil. Toxicon 2003, 41, 251–255. [Google Scholar]
- Morais, V.M.; Massaldi, H. Snake antivenoms: Adverse reactions and production technology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 2–18. [Google Scholar] [CrossRef]
- León, G.; Herrera, M.; Segura, Á.; Villalta, M.; Vargas, M.; Gutiérrez, J.M. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon 2013, 15, 63–76. [Google Scholar]
- Da Silva, N.M.; Arruda, E.Z.; Murakami, Y.L.; Moraes, R.A.; El-Kik, C.Z.; Tomaz, M.A.; Fernandes, F.F.; Oliveira, C.Z.; Soares, A.M.; Giglio, J.R.; et al. Evaluation of three Brazilian antivenom ability to antagonize myonecrosis and hemorrhage induced by Bothrops snake venoms in a mouse model. Toxicon 2007, 50, 196–205. [Google Scholar]
- Gutiérrez, J.M.; Lomonte, B.; León, G.; Alape-Girón, A.; Flores-Díaz, M.; Sanz, L.; Ângulo, Y.; Calvete, J.J. Snake venomics and antivenomics: Proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J. Proteom. 2009, 72, 165–182. [Google Scholar] [CrossRef]
- Clavico, E.E.; Muricy, G.G.; Gama, B.A.P.; Batista, D.; Ventura, C.R.R.; Pereira, R.C. Ecological roles of natural products from the marine sponge Geodia corticostylifera. Mar. Biol. 2006, 148, 479–488. [Google Scholar] [CrossRef]
- González del Val, A.; Platas, G.; Basílio, A.; Cabello, A.; Gorrochategui, J.; Suay, I.; Vicente, F.; Portillo, E.; Jiménez del Río, M.; Reina, G.G.; et al. Screening of antimicrobial activities in red, green and brown macroalgae from Gram Canária (Canary Islands, Spain). Int. Microb. 2001, 4, 35–40. [Google Scholar]
- Vallim, M.A.; Barbosa, J.E.; Cavalcanti, D.N.; De-Paula, J.C.; Giongo, V.A.; da Silva, G.; Teixeira, V.L.; Paixão, I.C.N.P. In vitro antiviral activity of diterpenes isolated from the Brazilian brown alga Canistrocarpus cervicornis. J. Med. Plant Res. 2010, 4, 2379–2382. [Google Scholar]
- Namvar, F.; Tahir, P.M.; Mohamad, R.; Mahdavi, M.; Abedi, P.; Najafi, T.F.; Rahmanand, H.S.; Jawaid, M. Biomedical properties of edible seaweed in cancer therapy and chemoprevention trials: A review. Nat. Prod. Commun. 2013, 8, 1811–1820. [Google Scholar] [PubMed]
- Domingos, T.F.; Carvalho, C.; Moura, L.A.; Teixeira, V.L.; Pereira, R.C.; Bianco, E.M.; Ferreira, W.J.; Ramos, C.J.; de Miranda, A.L.; Melo, P.A.; et al. Antilonomic effects of different Brazilian brown seaweeds crude extracts. Nat. Prod. Commun. 2009, 4, 1075–1078. [Google Scholar]
- Moura, L.A.; Sanchez, E.F.; Bianco, E.M.; Pereira, R.C.; Teixeira, V.L.; Fuly, A.L. Antiophidian properties of a dolastane diterpene isolated from the marine brown alga Canistrocarpus cervicornis. Biomed. Prev. Nutr. 2011, 1, 61–66. [Google Scholar] [CrossRef]
- Moura, L.A.; Bianco, E.M.; Pereira, R.C.; Teixeira, V.L.; Fuly, A.L. Anticoagulation and antiplatelet effects of a dolastane diterpene isolated from the marine brown alga Canistrocarpus cervicornis. J. Thromb. Thrombol. 2011, 31, 235–240. [Google Scholar] [CrossRef]
- Jongaramruong, J.; Kongkam, N. Novel diterpenes with cytotoxic, anti-malarial and anti-tuberculosis activities from a brown alga Dictyota sp. J. Asian Nat. Prod. Res. 2007, 9, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Vallim, M.A.; de Paula, J.C.R.; Pereira, C.; Teixeira, V.L. The diterpenes from Dictyotacean marine brown algae in the Tropical Atlantic American region. Biochem. System. Ecol. 2005, 33, 1–16. [Google Scholar] [CrossRef]
- Bianco, E.M.; Rogers, R.; Teixeira, V.L.; Pereira, R.C. Antifoulant diterpenes produced by the brown seaweed Canistrocarpus cervicornis. J. Appl. Phycol. 2009, 21, 341–346. [Google Scholar] [CrossRef]
- Domingos, T.F.S.; Vallim, M.A.; Carvalho, C.; Sanchez, E.F.; Teixeira, V.L.; Fuly, A.L. Anti-snake venom effect of secodolastane diterpenes isolated from Brazilian marine brown alga Canistrocarpus cervicornis against Lachesis muta venom. Rev. Bras. Farmacogn. 2011, 21, 234–238. [Google Scholar] [CrossRef]
- Bhatnagar, I.; Kim, Se-Kwon. Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural products. Nat. Prod. Rep. 2004, 21, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.R.; Harle, U.N.; Chaugule, B.B. Therapeutic potential and health benefits of Sargassum species. Pharmacogn. Rev. 2014, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Vargas, A.; de Barcelos, O.I.; Stephens, P.R.; Cirne-Santos, C.C.; de Palmer, I.C.; Ramos, F.A.; Jiménez, C.; Rodríguez, J.; Resende, J.A.; Teixeira, V.L.; et al. Dolabelladienols A-C, new diterpenes isolated from Brazilian brown alga Dictyota pfaffii. Mar. Drugs 2014, 23, 4247–4259. [Google Scholar]
- Bianco, E.M.; Teixeira, V.L.; Pereira, R.C. Chemical defenses of the tropical marine seaweed Canistrocarpus cervicornis against herbivory by sea urchin. Braz. J. Oceanog. 2010, 58, 213–218. [Google Scholar] [CrossRef]
- Teixeira, V.L.; Tomassini, T.; Fleury, B.G.; Kelecom, A. Dolastane and secodolastane diterpenes from the marine brown alga Dictyota cervicornis. J. Nat. Prod. 1986, 49, 570–575. [Google Scholar] [CrossRef]
- De Paula, R.C.; Castro, H.C.; Rodrigues, C.R.; Melo, P.A.; Fuly, A.L. Structural and pharmacological features of phospholipases A2 from snake venoms. Prot. Pep. Lett. 2009, 16, 899–907. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef]
- Otero, R.; Núñez, V.; Jiménez, S.L.; Fonnegra, R.; Osorio, R.G.; García, M.E.; Díaz, A. Snakebites and ethnobotany in the northwest region of Colombia part II: Neutralization of lethal and enzymatic effects of Bothrops atrox venom. J. Ethnopharmacol. 2000, 71, 505–511. [Google Scholar] [CrossRef]
- Potts, B.C.M.; Faulkner, D.J. Phospholipase A2 inhibitors from marine organisms. J. Nat. Prod. 1992, 55, 1701–1717. [Google Scholar] [CrossRef]
- Serrano, M.T. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S. Snake venoms. Drugs 1997, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.H.; Alves, D.L.; Raslan, D.S.; Piló-Veloso, D.; Rodrigues, V.M.; Homsi-Brandeburgo, M.I.; de Lima, M.E. Neutralizing properties of Musa paradisiaca L. (Musaceae) juice on phospholipase A2, myotoxic, hemorrhagic and lethal activities of crotalidae venoms. J. Ethnopharmacol. 2005, 8, 21–29. [Google Scholar]
- Oliveira, A.S.; Cavalcanti, D.N.; Bianco, E.M.; de Paula, J.C.; Pereira, R.C.; Yoneshigue-Valentin, Y.; Teixeira, V.L. Chemical composition of diterpenes from the brown alga Canistrocarpus cervicornis (Dictyotaceae, Phaeophyceae). Nat. Prod. Commun. 2008, 9, 1469–1472. [Google Scholar]
- Fuly, A.L.; de Miranda, A.L.; Zingali, R.B.; Guimarães, J.A. Purification and characterization of a phospholipase A2 isoenzyme isolated from Lachesis muta snake venom. Biochem. Pharmacol. 2002, 63, 1589–1597. [Google Scholar]
- Garcia, E.S.; Guimarães, J.A.; Prado, J.L. Purification and characterization of a sulfhydryl-dependent protease from Rhodnius prolixus midgut. Arch. Biochem. Biophys. 1978, 188, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Kondo, S.; Ikezawa, H.; Murata, R. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 1960, 13, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Nikai, T.; Mori, N.; Kishida, M.; Sugihara, H.; Tu, A.T. Isolation and biochemical characterization of hemorrhagic toxin from the venom of Crotalus atrox. Arch. Biochem. Biophys. 1984, 231, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Nozani, M.; Hokama, Z. Toxins: Animal, Plant and Microbial; Plenum Press: New York, NY, USA, 1976; pp. 97–120. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingos, T.F.S.; Vallim, M.A.; Cavalcanti, D.N.; Sanchez, E.F.; Teixeira, V.L.; Fuly, A.L. Effect of Diterpenes Isolated of the Marine Alga Canistrocarpus cervicornis against Some Toxic Effects of the Venom of the Bothrops jararaca Snake. Molecules 2015, 20, 3515-3526. https://doi.org/10.3390/molecules20033515
Domingos TFS, Vallim MA, Cavalcanti DN, Sanchez EF, Teixeira VL, Fuly AL. Effect of Diterpenes Isolated of the Marine Alga Canistrocarpus cervicornis against Some Toxic Effects of the Venom of the Bothrops jararaca Snake. Molecules. 2015; 20(3):3515-3526. https://doi.org/10.3390/molecules20033515
Chicago/Turabian StyleDomingos, Thaisa Francielle Souza, Magui Aparecida Vallim, Diana Negrão Cavalcanti, Eládio Flores Sanchez, Valéria Laneuville Teixeira, and André Lopes Fuly. 2015. "Effect of Diterpenes Isolated of the Marine Alga Canistrocarpus cervicornis against Some Toxic Effects of the Venom of the Bothrops jararaca Snake" Molecules 20, no. 3: 3515-3526. https://doi.org/10.3390/molecules20033515





