2.1. 1H-NMR Characterization of Saponins and Isoflavonoids of Astragalus Roots
Metabolomics has been applied recently to discriminate
Astragalus roots collected from diverse places in Korea and three provinces of China: Shandong, Hebei, and Liaoning [
24]. In that study, some discriminating primary metabolites were identified, including organic acids (acetic acid, fromic acid, fumaric acid, malic acid) and amino acids (alanine, arginine, proline, valine) as well as sucrose and a few inorganic substances. However there was scarce information on the variation of secondary metabolites between samples from different locations.
The two major activity-related secondary metabolite groups in Astragalus are saponins and isoflavonoids, though their content is very variable. Thus, it was reasonable to begin the search for chemomarkers of this drug with these groups. 1H-NMR has many advantages as a tool for metabolic profiling but when used directly—without any hyphenation to a chromatographic system—the signals in the obtained spectrum will mostly likely show a great deal of overlapping. Therefore, the first step of 1H-NMR-based metabolomics is to pick out the characteristic signals of possible metabolites present in the samples among the many other signals.
Saponins contain two kinds of moieties, a hydrophobic steroidal or triterpenic aglycone and a hydrophilic part made up of sugars. In the 1H-NMR structural elucidation of saponins, the most characteristic 1H resonances are from anomeric and methyl signals. Depending on the sugars and their bonding, each anomeric proton has its particular chemical shift and coupling constants. However, these anomeric protons are detected in the crowded crown region, δ 4.5–δ 5.5, where signals of many anomeric protons of free sugars and residual HDO are also detected and thus overlap.
Other important characteristic signals of saponins correspond to methyls detected in the range of δ 0.6–δ 1.5. The methyl signals are mostly singlets with a few highly intense doublets corresponding to 3 protons. In order to investigate the feasibility of the methyl signals as marker
1H resonances, H-18, H-21, H-26, H-27, H-28, H-29, and H-30 of astragaloside I, II, III, and IV (
Figure 1) were assigned using the 2D-NMR experiments, HSQC and HMBC. Particularly in the HMBC spectrum, each methyl signal can be well distinguished by characteristic correlations: H-18/C-13 and C-17, H-21/C-20 and C-17, H-26 and H-27/C-25 and C-24, H-28 and H-29/C-3, and H-30/C-13. The assignment of the seven methyl group protons and cyclopropane moiety of four astragalosides is listed in
Table 1, both in CD
3OD and CD
3OD -KH
2PO
4 buffer in D
2O (1:1, v/v).
Table 1.
1H-NMR chemical shifts (ppm) and coupling constants (Hz) of methyls and H-19 of astragaloside I, II, III, and IV in CD3OD a (Solvent 1) and CD3OD-KH2PO4 buffer in D2O (1:1, v/v) b (Solvent 2).
Table 1.
1H-NMR chemical shifts (ppm) and coupling constants (Hz) of methyls and H-19 of astragaloside I, II, III, and IV in CD3OD a (Solvent 1) and CD3OD-KH2PO4 buffer in D2O (1:1, v/v) b (Solvent 2).
| No. of H | Solvent | Astragaloside I | Astragaloside II | Astragaloside III | Astragaloside IV |
|---|
| H-18 | 1 | 1.24 (s) | 1.24 (s) | 1.25 (s) | 1.25 (s) |
| 2 | 1.24 (s) | 1.24 (s) | 1.25 (s) | 1.24 (s) |
| H-19a | 1 | 0.26 (d, 4.4 Hz) | 0.26 (d, 4.5 Hz) | 0.38 (d, 4.3 Hz) | 0.26 (d, 4.4 Hz) |
| 2 | 0.35 (d, 4.4 Hz) | 0.35 (d, 4.5 Hz) | 0.40 (d, 4.3 Hz) | 0.37 (d, 4.4 Hz) |
| H-19b | 1 | 0.58 (d, 4.4 Hz) | 0.58(d, 4.5 Hz) | 0.55 (d, 4.3 Hz) | 0.59 (d, 4.4 Hz) |
| 2 | 0.57(d, 4.4 Hz) | 0.57(d, 4.5 Hz) | 0.54 (d, 4.3 Hz) | 0.59 (d, 4.4 Hz) |
| H-21 | 1 | 1.19 (s) | 1.18 (s) | 1.23 (s) | 1.22 (s) |
| 2 | 1.20 (s) | 1.22 (s) | 1.23 (s) | 1.22 (s) |
| H-26 | 1 | 1.12 (s) | 1.12 (s) | 1.12 (s) | 1.13 (s) |
| 2 | 1.26 (s) | 1.26 (s) | 1.14 (s) | 1.27 (s) |
| H-27 | 1 | 1.22 (s) | 1.22 (s) | 1.25 (s) | 1.25 (s) |
| 2 | 1.26 (s) | 1.26 (s) | 1.29 (s) | 1.29 (s) |
| H-28 | 1 | 1.29 (s) | 1.29 (s) | 1.32 (s) | 1.28 (s) |
| 2 | 1.27 (s) | 1.27 (s) | 1.28 (s) | 1.28 (s) |
| H-29 | 1 | 0.90 (s) | 0.90 (s) | 1.02 (s) | 1.01 (s) |
| 2 | 0.90 (s) | 0.90 (s) | 1.01 (s) | 0.99 (s) |
| H-30 | 1 | 1.01 (s) | 1.00 (s) | 0.99 (s) | 1.02 (s) |
| 2 | 0.99 (s) | 0.99 (s) | 0.99 (s) | 1.01 (s) |
All the major
Astragalus saponins share a common aglycone, cycloastragenol, which is substituted with different sugar moieties at the C-3 or C-6 positions. This chemical resemblance makes it very difficult to distinguish the astragalosides. Furthermore, these methyl signals from saponins might also overlap with other methyl signals from amino acids such as isoleucine, leucine, and valine, which are commonly detected as free amino acids in plant extracts. However, cycloastragenol has a characteristic cyclopropane moiety in the ring of C-9/C-10/C-19 that is absent in most common triterpenoids such as oleanane- or ursane-type triterpenoids. The two protons of H-19 are detected as doublets upfield-shifted to a non-crowded region, one of them to δ 0.2–δ 0.4 and the other to δ 0.5–δ 0.6 (
Table 1). The absence of glycosylation at C-6 produces a downfield shift of the H-19 protons of δ 0.38 (d,
J = 4.3 Hz) in the case of astragaloside III as compared with d 0.26 (d,
J = 4.4 Hz) of other astragalosides in the solvent of CD
3OD-KH
2PO
4 buffer in D
2O (1:1, v/v). These signals that correspond to the cyclopropane moiety can be used as target signals for the saponins in the
1H-NMR spectrum of
Astragalus roots
The intensity of signals and consequently the sensitivity of the
1H-NMR method is generally affected by the solvent. To choose the best solvent, the spectra of CD
3OD or CD
3OD-KH
2PO
4 buffer in D
2O (1:1, v/v) extracts of
Astragalus were compared to determine which showed the highest resolution or intensity of the methyl and H-19 signals (
Figure 2). Most methyl signals such as H-18, H-21, H-26, H-27, H-28, H-29, and H-30 overlapped with other signals, but the H-19 signals were clearly detected in a non-crowded region in both cases. However, the highest sensitivity for these signals was observed in extracts of the non-aqueous solvent, CD
3OD. This is probably due to the fact that saponins generate micelles in an aqueous solvent resulting in signal broadening. Thus, in this case, CD
3OD proved to be a better solvent than CD
3OD-KH
2PO
4 buffer in D
2O (1:1, v/v), which is commonly used for NMR-based plant metabolomics.
Figure 2.
1H-NMR spectra of Astragalus membranaceus roots collected in Shanxi province in China (No. of voucher specimen: 20131206-2). (a) In CD3OD; (b) CD3OD-KH2PO4 buffer in D2O (1:1, v/v), 1: methyl signals of astragaloside I, II, III, IV, isoleucine, leucine, and valine, 2: H-24 of astragaloside VIII and soyasaponin I, 3: H-19a of astragalosides, 4: H-19b of astragalosides.
Figure 2.
1H-NMR spectra of Astragalus membranaceus roots collected in Shanxi province in China (No. of voucher specimen: 20131206-2). (a) In CD3OD; (b) CD3OD-KH2PO4 buffer in D2O (1:1, v/v), 1: methyl signals of astragaloside I, II, III, IV, isoleucine, leucine, and valine, 2: H-24 of astragaloside VIII and soyasaponin I, 3: H-19a of astragalosides, 4: H-19b of astragalosides.
The advantage of using CD
3OD rather than aqueous solvents was not limited to the detection of the H-19 of cycloastragenol saponins. The H-23 signal of another type of saponin—soyasapgenol B, an oleanane-type triterpenoid (
Figure 1)—was also clearly detected with higher intensity in the non-aqueous solvent.
Apart from saponins,
Astragalus roots also contain a high amount of isoflavonoids. To investigate characteristic isoflavonoid
1H-NMR resonances, the spectra of four reference compounds—calycosin, formononetin and their 7-
O-glucosides—in CD
3OD and CD
3OD-KH
2PO
4 buffer in D
2O (1:1, v/v) were recorded. In the
Figure 2, singlets corresponding to H-2 resonances in the C-ring can be observed to be well distinguished from other signals in the downfield region around δ 8.1–δ 8.4; this is due to the α-effect of oxygen and the effect of the β-position from the ketone group. If a B-ring is attached to H-2 as in the case of common flavones such as apigenin or luteolin, the H-3 of the C-ring proton is detected around δ 6.9 as a singlet. Thus, the greatly downfield-shifted H-resonance of H-2 can be used as an isoflavonoid signal in
1H-NMR-based metabolomics.
Figure 3.
1H-NMR spectra of calycosin (1), calycosin-7-O-glucoside (2), formononetin (3), and formononetin-O-glucoside (ononin, 4). (a) In CD3OD; (b) CD3OD-KH2PO4 buffer in D2O (1:1, v/v).
Figure 3.
1H-NMR spectra of calycosin (1), calycosin-7-O-glucoside (2), formononetin (3), and formononetin-O-glucoside (ononin, 4). (a) In CD3OD; (b) CD3OD-KH2PO4 buffer in D2O (1:1, v/v).
In addition to H-2, calycosin and formononetin have a hydroxyl group at the C-7 position, which causes a downfield shift of the H-5 proton signal to δ 8.2 (d,
J = 8.8 Hz) (
Figure 3). Calycosin and formononetin can be differentiated by the pattern of their B-ring. Formononetin has a typical 1,4-disubstituted benzene ring pattern, two multiplets at
ca. δ 7.0 (H-3' and H-5') and
ca. δ 7.5 (H-2' and H-6').
Additionally, the presence of glucose can be detected by the shift of H-6 and H-8 protons. The O-glycosidic linkage at the C-7 position causes a downfield shift of the signals of both protons of ca. 0.3 ppm as compared with that of their corresponding aglycones.
The presence of a low amount of isoflavonoids was confirmed in the CD
3OD extract of
Astragalus roots. Two kinds of H-2 and H-5 were clearly detected in the
1H-NMR spectrum and the coupling constants were confirmed by
1H-
1H-J-resolved spectrum (
Figure 4). Based on the information of the chemical shifts of the reference compounds, the two major isoflavonoids were assigned to be calycosin-7-
O-glucoside and formononetin-7-
O-glucoside (ononin).
Figure 4.
1H-1H-J-resolved spectrum of Astragalus membranaceus roots collected in in the Shanxi province in China (No. of voucher specimen: 20131206-5) in CD3OD. 1: H-2 of formononetin-7-O-glucoside, 2: H-2 of calycosin-7-O-glucoside, 3: H-5 of formononetin-7-O-glucoside and calycosin-7-O-glucoside.
Figure 4.
1H-1H-J-resolved spectrum of Astragalus membranaceus roots collected in in the Shanxi province in China (No. of voucher specimen: 20131206-5) in CD3OD. 1: H-2 of formononetin-7-O-glucoside, 2: H-2 of calycosin-7-O-glucoside, 3: H-5 of formononetin-7-O-glucoside and calycosin-7-O-glucoside.
In addition to saponins and flavonoids, the 1H-NMR spectrum of Astragalus roots revealed the presence of a number of primary metabolites, including amino acids (alanine, arginine, asparagine, leucine, isoleucine, proline, and valine), organic acids (citric-, formic and malic acid), and sugars (sucrose and glucose) as well as γ-aminobutyric acid (GABA) and choline. Among the metabolites detected, sucrose was found to be the most abundant, accounting for over 15% of the dry weight of Astragalus roots.
2.2. Multivariate Data Analysis of 1H-NMR Spectra to Investigate the Geographical Variation of Astragalus Roots
The
1H-NMR data were further reduced by principal component analysis to investigate the grouping between the samples. Apart from obvious factors such as species and variety, there are at least two other factors that can potentially influence the metabolite profile of
Astragalus root samples: age and geographical origin. The samples investigated in this study (
Table 2) were identified as
Astragalus membranaceus var. mongholicus,
Asgragalus membranaceus, and
Hedysarum polybotrys.
Astragalus membranaceus var. mongholicus and
A. membranaceus are defined as Radix Astragali but
Hedysarum polybotrys is used as a red-coloured substitute [
9].
Table 2.
Sample information of Astragalus roots evaluated in this study.
Table 2.
Sample information of Astragalus roots evaluated in this study.
| Sample Code * | Voucher Specimen | Collection | Wild/Cultivated | Age (Years) | Source of Materials |
|---|
| 1 | 304032-1302004 | a | Cultivated | 2–3 | g |
| 2 | 20131018 | a | Cultivated | 2–3 | h |
| 3 | 201210 | a | Cultivated | 2–3 | i |
| 4 | 304032-1306009 | a | Cultivated | 2–3 | i |
| 5 | 20131222-2 | a | Cultivated | 2–3 | j |
| 6 | 121103 | b | Cultivated | 2–3 | k |
| 7 | 20131120-1 | b | Cultivated | 2–3 | l |
| 8 | 20131120-2 | b | Cultivated | 2–3 | l |
| 9 | 20131122-3 | b | Cultivated | 2–3 | j |
| 10 | 20131119 | b | Cultivated | 2–3 | h |
| 11 | 21031020 | b | Cultivated | 2–3 | h |
| 12 | 20131206-1 | c | Wild | 5 | m |
| 13 | 20131206-2 | c | Cultivated | 2 | m |
| 14 | 20131206-3 | c | Wild | 20 | m |
| 15 | 20131206-4 | c | Wild | 12 | m |
| 16 | 20131206-5 | c | Wild | 30 | m |
| 17 | 20131222-1 | c | Cultivated | 2–3 | j |
| 18 | 20131203 | d | Wild | 10 | n |
| 19 | 20131207-1 | d | Wild | 10 | n |
| 20 | 20131207-2 | d | Wild | 5 | n |
| 21 | 20131209-1 | d | Wild | 10 | n |
| 22 | 20131209-2 | d | Cultivated | 2–3 | n |
| 23 | 20131210 | d | Wild | 10 | n |
| 24 | DA13121001 | e | Cultivated | 2–3 | o |
| 25 | DA13121002 | e | Cultivated | 2–3 | o |
| 26 | DA13121003 | e | Cultivated | 2–3 | o |
| 27 | DB13121001 | e | Cultivated | 2–3 | p |
| 28 | DB13121002 | e | Cultivated | 2–3 | p |
| 29 | DB13121003 | e | Cultivated | 2–3 | p |
| 30 | 20131222-4 | f | Cultivated | 2–3 | j |
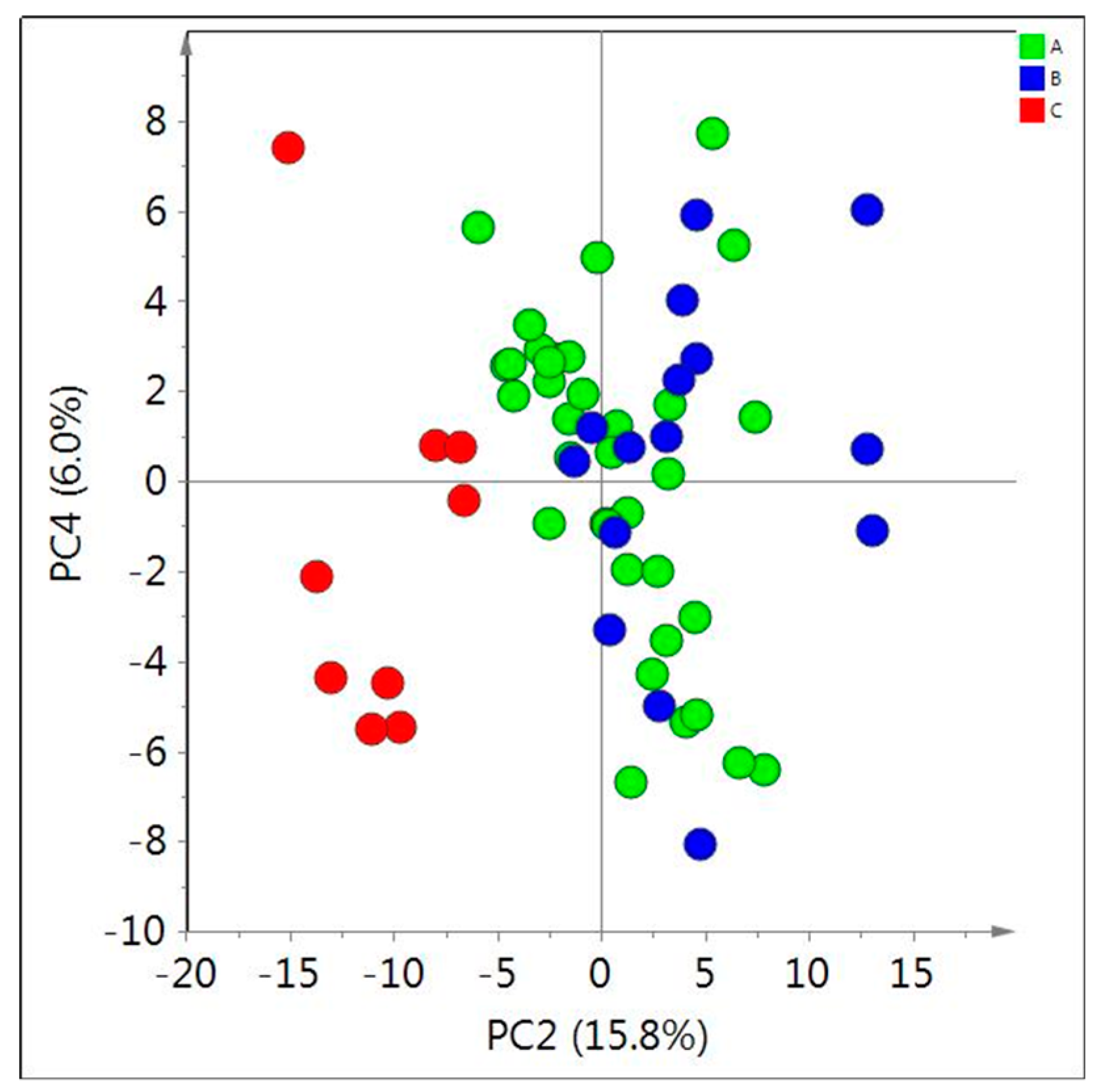
Prior to the investigation of the effect of age and location, the metabolic difference between the species and varieties were evaluated by PCA of their
1H-NMR data. For this, samples of a similar age (2–3 years old) were tested. As shown in
Figure 5, no clear separation was observed between
A. membranaceus var. mongholicus and
A. membranaceus. However,
H. polybotrys was distinguished from others by the low amount of isoflavonoids and saponins, which is consistent with the previous data [
9].
Figure 5.
Score plot of principal component analysis (PCA, PC2/PC4) obtained from 1H-NMR data of in CD3OD extracts of 2–3 year old Astragalus membranaceus var. mongholicus (A); Asgragalus membranaceus (B); and Hedysarum polybotrys (C).
Figure 5.
Score plot of principal component analysis (PCA, PC2/PC4) obtained from 1H-NMR data of in CD3OD extracts of 2–3 year old Astragalus membranaceus var. mongholicus (A); Asgragalus membranaceus (B); and Hedysarum polybotrys (C).
Geographical location can affect the metabolome of a plant due to a number of external factors comprising temperature, soil conditions, altitude rain and drought, whereas the effect of age is caused by internal factors related to gene expression during the plant development. Because of this wide range of variables, a large number of samples would be needed to reach significant conclusions. This problem can be circumvented by assuming that there is a specific marker for each developmental stage of the plant as well as specific markers for the sites of the collection. Markers which will be sufficiently selective as to stand out above the variability can be detected by means of supervised multivariate data analysis, in which specifically the differences between some classes are analysed, such as, for example, the difference between samples of different age. With this approach, all random variability such as that due to diurnal and seasonal changes for example, will be appear only as noise, so that, if present, specific markers will be revealed. Thus, after PCA, we applied partial least square (PLS) and orthogonal partial least square (OPLS) modeling, two supervised multivariate data analysis methods.
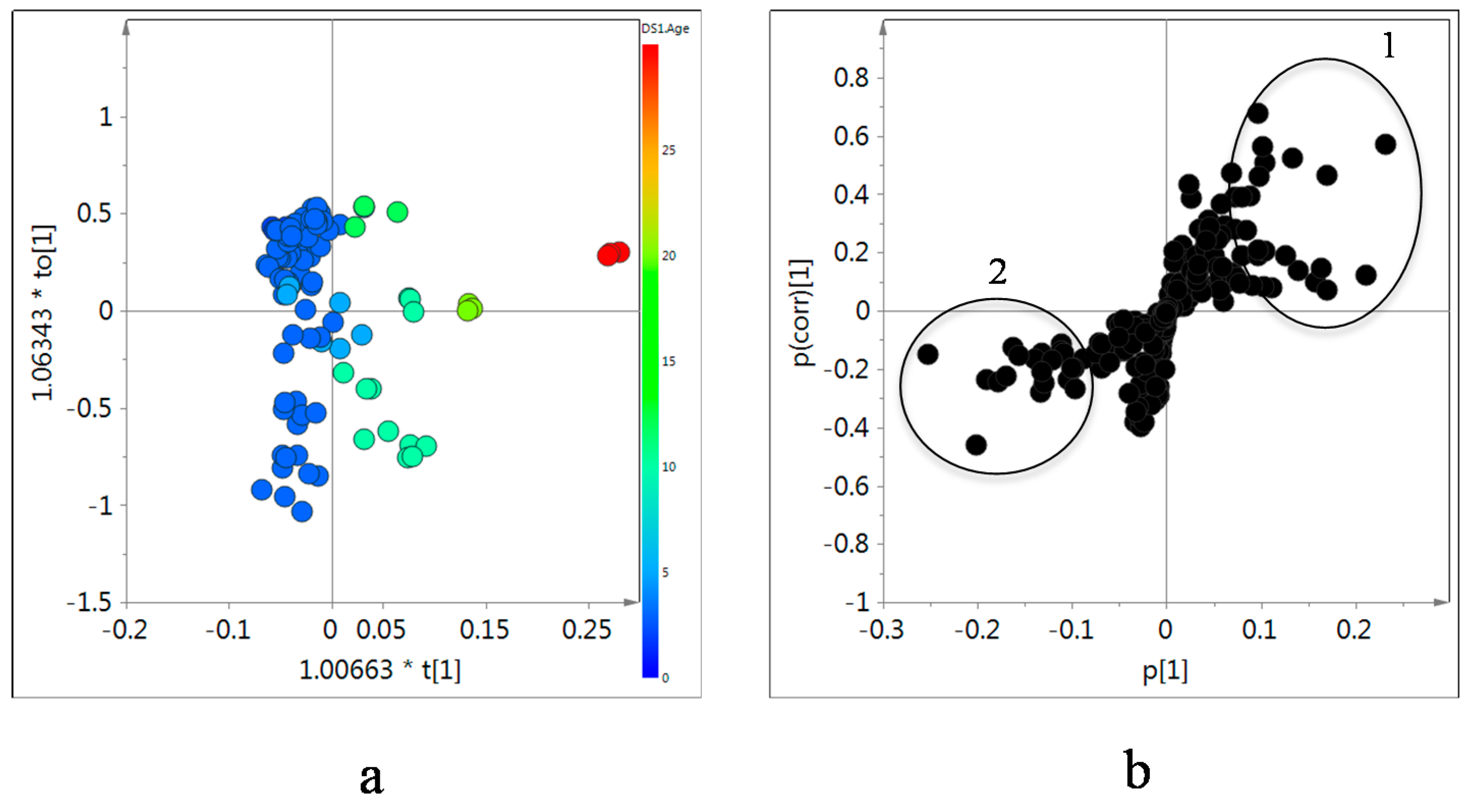
As expected, no grouping for age or geographical location of the samples was obtained by PCA since the random variability was too high. The samples were collected from diverse locations and with a wide range of ages. However, OPLS modeling using age as a Y data set showed a clear trend in the OPLS component 1, revealing which metabolites are influenced by ages (
Figure 6a). The samples were clearly separated into four groups depending on the age of the roots: 2–9, 9–16, 16–23, and 30 years. Obviously, the orthogonal component in y-axis reflects the large variability but this did not interfere with the age-dependant markers.
The metabolites contributing to the age separation were analyzed by an S-plot of the OPLS modeling (
Figure 6b). Older samples showed higher levels of isoflavonoids than younger ones. The signals of H-5, H-6, and H-8 of isoflavonoids including calycosin and formononetin, their 7-
O-glucosides were found be signals that most contributed differentiate the old
Astragalus samples. In addition, sucrose and alanine were found to be higher in old samples. In the case of saponins, we could find no difference in the intensity of H-19, the characteristic
1H resonances of astragalosides, nor for any other methyl signal throughout all samples.
Figure 6.
Score (a) and S-plot (b) of orthogonal partial least square (OPLS) modeling obtained from 1H-NMR data of in CD3OD extracts of Astragalus roots as X-data and age of each sample as Y-data. 1: H-2, H-5, H-6 and H-8 of isoflavonoids, H-1 and H-2' of sucrose, and H-3 of alanine, 2: H-2 and H-3 of asparagine and aspartic acid, CH3 of choline, H-4 of GABA, and H-4 of proline.
Figure 6.
Score (a) and S-plot (b) of orthogonal partial least square (OPLS) modeling obtained from 1H-NMR data of in CD3OD extracts of Astragalus roots as X-data and age of each sample as Y-data. 1: H-2, H-5, H-6 and H-8 of isoflavonoids, H-1 and H-2' of sucrose, and H-3 of alanine, 2: H-2 and H-3 of asparagine and aspartic acid, CH3 of choline, H-4 of GABA, and H-4 of proline.
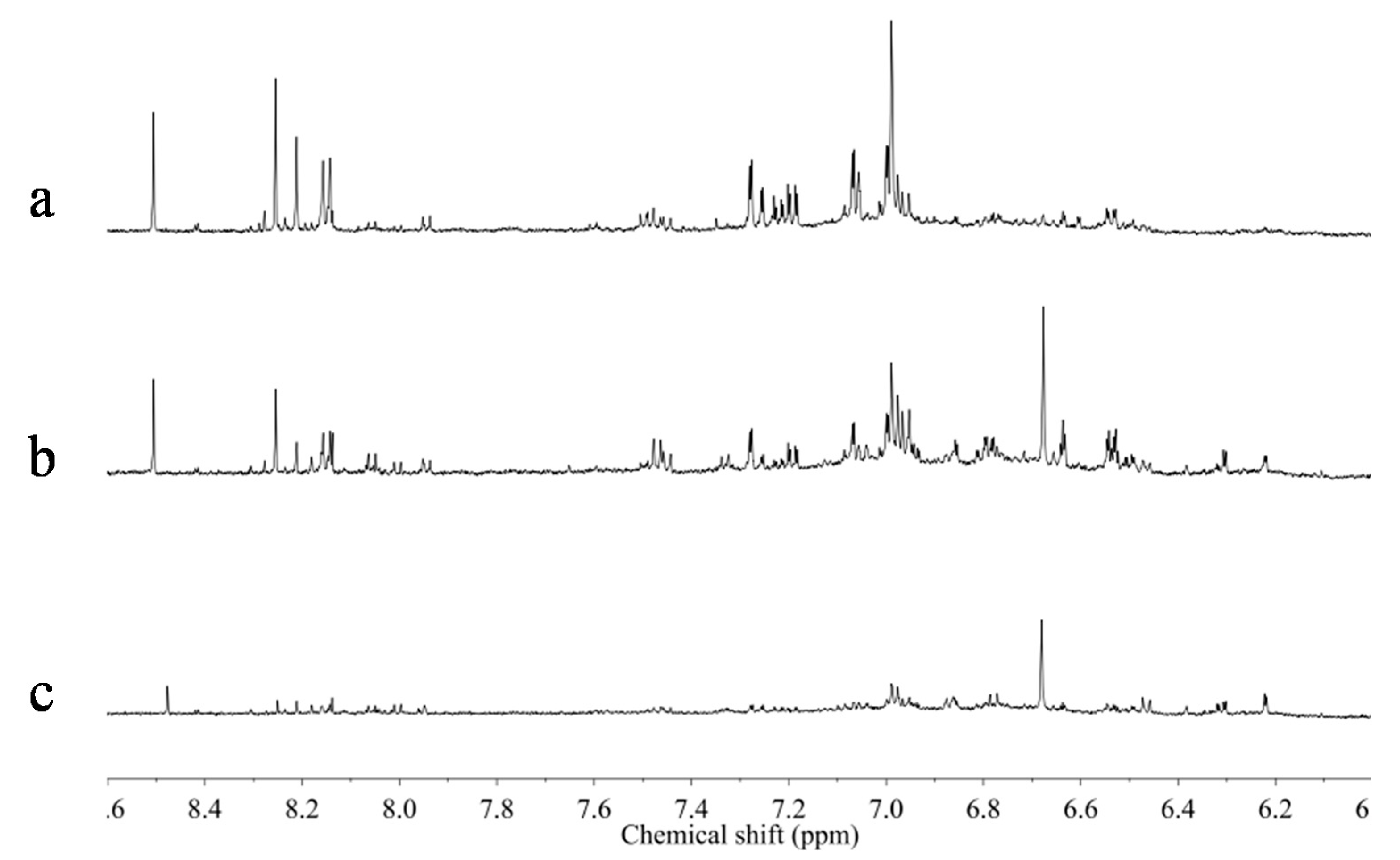
In the younger roots, higher levels of some primary metabolites including asparagine, aspartic acid, GABA and choline were observed. To confirm this result,
1H-NMR spectra of one set of materials, which was collected in the same location but were of different ages, were compared. In
Figure 7, the aromatic region of the
1H-NMR spectra of 5, 20 and 30, year-old
Astragalus roots collected from Shanxi province in China were compared. The resonances of isoflavonoids were clearly higher in the older samples than younger ones. However, the effect of age on the metabolome should be further studied on samples of diverse regions and ages because there were only a small number of old-aged samples among all those tested.
A previous study by Ma and colleagues [
9] had revealed metabolic differences in
Astragalus roots during their first 4 years. Total polysaccharides and GABA were reported to be significantly higher in 3-year-old plants, while 1-year-old plants contained the highest amount of total amino acids, which is in accordance with our current data. However, they did not find any significant change in the total isoflavonoid and saponin levels in plants that were up to 4 years old with the exception of astragaloside I that was slightly increased. The different time scale used in the studies could account for the difference in results. As can be seen in
Figure 5, the level of isoflavonoids increased only in roots that were more than 10 years old. This change would not thus be detected in the 1- to 4-year-old roots that were evaluated by Ma and colleagues [
9]
Figure 7.
1H-NMR spectra (CD3OD) of 30 (a), 20 (b), and 5 years (c) old Astragalus roots collected from Shanxi province in China.
Figure 7.
1H-NMR spectra (CD3OD) of 30 (a), 20 (b), and 5 years (c) old Astragalus roots collected from Shanxi province in China.
Neither PCA nor supervised multivariate data analysis (PLS or OPLS-modeling) revealed changes in the metabolome of the samples collected from different locations. This could be because these were much less significant than the variations due to age and/or other variables such as collection time or seasonal variation. Thus, to investigate differences according to their geographical origin, same-age samples from Neimenggu, Gansu, Shanxi, and Shanxi were analysed. These four locations were chosen because the number of similar age samples was sufficient; 2–3 year-old samples and other minor samples were removed for this analysis.
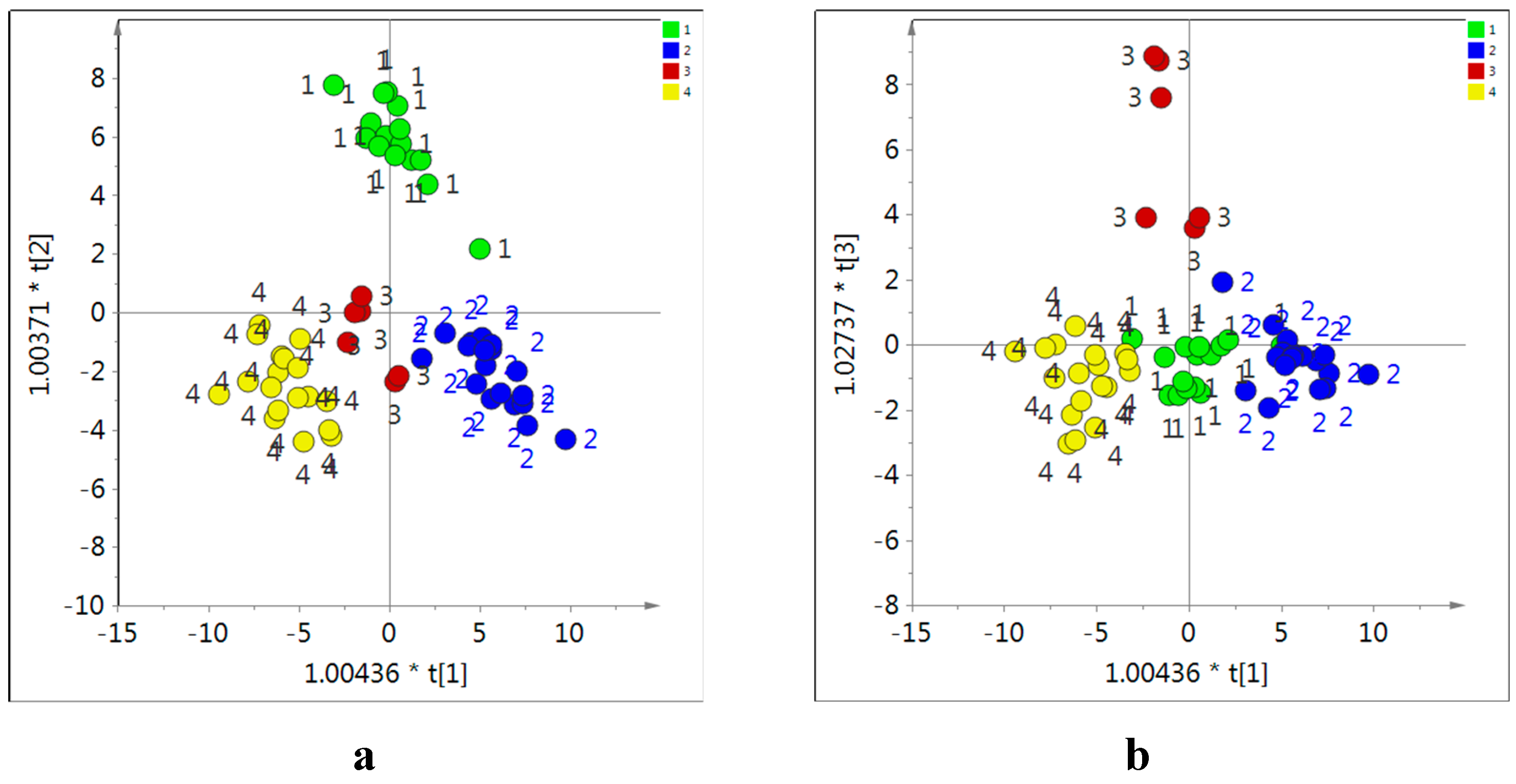
Figure 8.
Score of orthogonal partial least square (OPLS) modeling obtained from 1H-NMR data of in CD3OD extracts of Astragalus roots as X-data and locations of each sample as Y-data using the combination of OPLS component 1 and 2 (a) and 1 and 3 (b). 1: Neimengu, 2: Ganxu, 3: Shanxi, 4: Shaanxi in China.
Figure 8.
Score of orthogonal partial least square (OPLS) modeling obtained from 1H-NMR data of in CD3OD extracts of Astragalus roots as X-data and locations of each sample as Y-data using the combination of OPLS component 1 and 2 (a) and 1 and 3 (b). 1: Neimengu, 2: Ganxu, 3: Shanxi, 4: Shaanxi in China.
In these conditions, the four groups of Astragalus samples were well distinguished using OPLS component 1, 2 and 3 (
Figure 8). The samples from Neimunggu, Shanxi and Ganxu in China were separated by the component 3, 2, and 1, respectively. The variable importance for the projection (VIP) method was used to determine which metabolites contributed to the separation, This showed that saponins, trigonelline, isoflavonoids including calycosin, formononetin and their glycosides, glucose, sucrose, citric acid, malic acid, and some amino acids (asparagine, aspartic acid and proline) were the main discriminating metabolites.
The loading plot revealed that
Astragalus roots grown in Neimunggu had higher levels of isoflavonoids, while those from Shaanxi had more asparagine, aspartic acid, citric acid and malic acid and Gansu samples had more trigonelline and sucrose. Shanxi samples were distinguished from all others for their high content in saponins (astragaloside I, II, III, and IV) and proline. This is interesting because a recent study had concluded that the highest quality roots produced in China were from this province [
10] suggesting that it could be related to the saponin content.