Inhibitory Effect of Triterpenoids from Dillenia serrata (Dilleniaceae) on Prostaglandin E2 Production and Quantitative HPLC Analysis of Its Koetjapic Acid and Betulinic Acid Contents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of Compounds
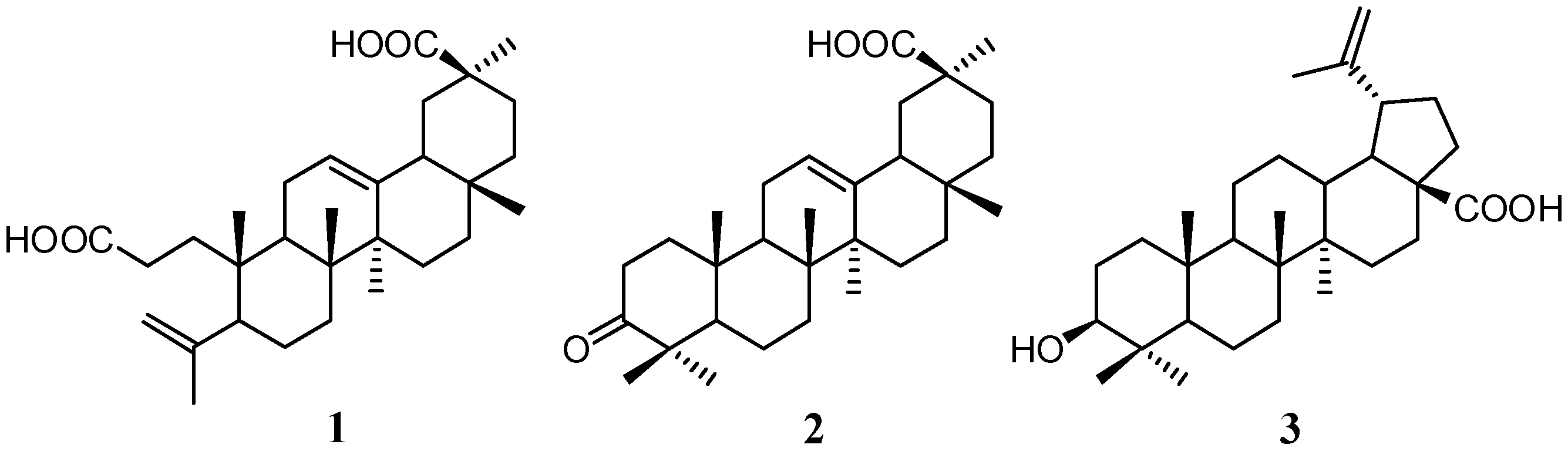
2.2. Quantitative Analysis of Koetjapic Acid (1) and Betulinic Acid (3)
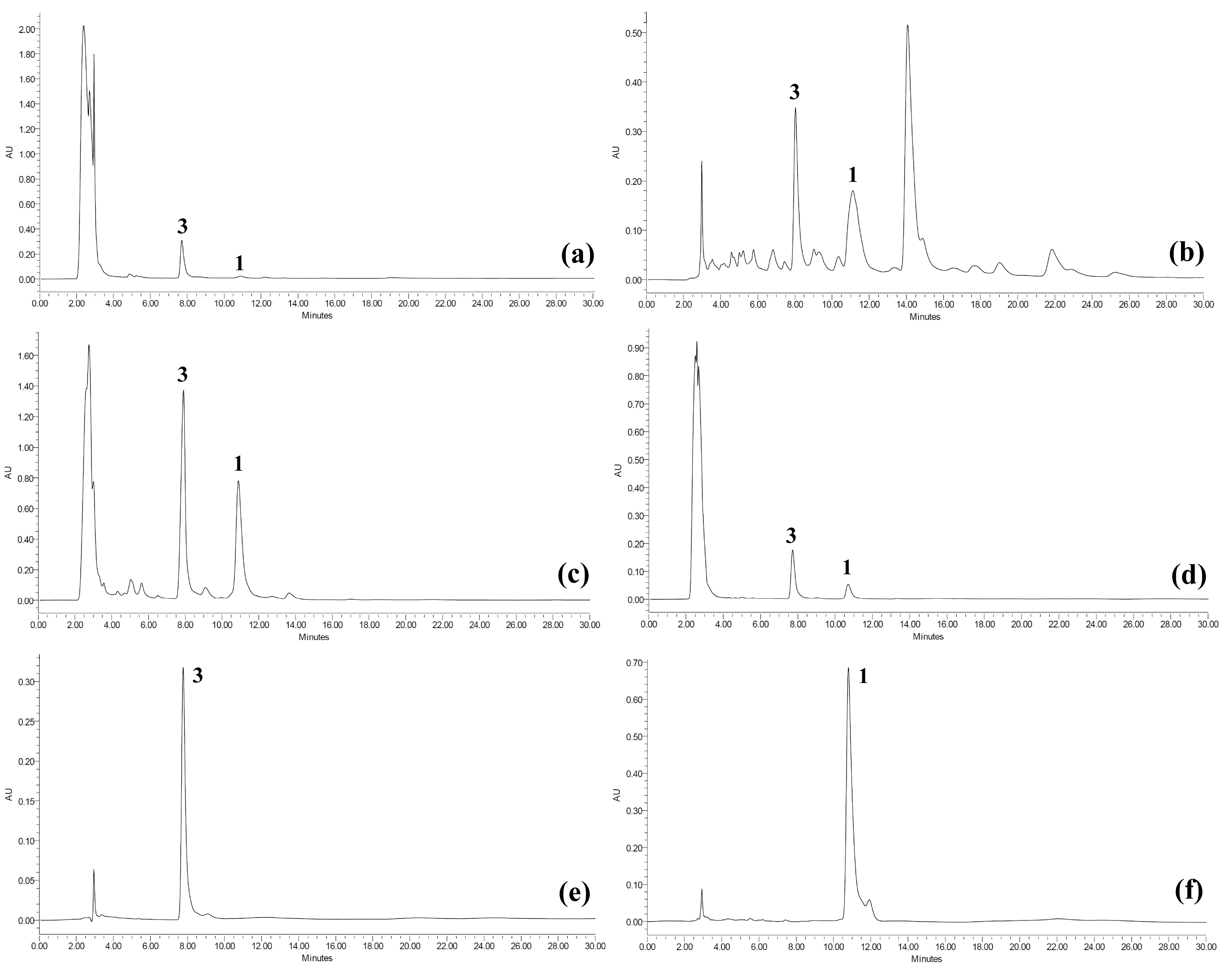
| Sample | Code | Concentration of 1 (mg/g) * | Concentration of 3 (mg/g) * |
|---|---|---|---|
| Root bark | |||
| Crude MeOH | CRB | 21.31 ± 1.99 | 81.76 ± 1.40 |
| Petroleum ether fraction | RB-PEF | 46.32 ± 0.61 | 128.20 ± 3.91 |
| Ethyl acetate fraction | RB-EAF | 117.62 ± 1.54 | 424.26 ± 2.97 |
| MeOH fraction | RB-MF | 19.78 ± 1.41 | 90.93 ± 2.53 |
| Stem bark | |||
| Crude MeOH | CSB | 4.27 ± 1.09 | 102.59 ± 2.99 |
| Petroleum ether fraction | SB-PEF | 45.37 ± 1.68 | 137.58 ± 3.09 |
| Ethyl acetate fraction | SB-EAF | 151.29 ± 2.40 | 528.08 ± 1.11 |
| MeOH fraction | SB-MF | 8.49 ± 0.50 | 58.81 ± 1.21 |
2.3. Inhibition of Prostaglandin E2 (PGE2)
| Sample | Code | % Inhibition (10 µg/mL) *,† | IC50 (µg/mL) * |
|---|---|---|---|
| Root bark | |||
| Crude MeOH | CRB | 73.03 ± 0.77 | 1.80 ± 0.09 |
| Petroleum ether fraction | RB-PEF | 72.36 ± 1.18 | 0.23 ± 0.15 |
| Ethyl acetate fraction | RB-EAF | 73.86 ± 2.90 | 2.00 ± 0.13 |
| MeOH fraction | RB-MF | 65.01 ± 0.21 | 3.33 ± 0.53 |
| Stem bark | |||
| Crude MeOH | CSB | 71.88 ± 1.80 | 0.96 ± 0.32 |
| Petroleum ether fraction | SB-PEF | 64.26 ± 0.98 | 4.61 ± 0.09 |
| Ethyl acetate fraction | SB-EAF | 64.06 ± 1.62 | 1.31 ± 0.33 |
| MeOH fraction | SB-MF | 69.02 ± 0.90 | 1.24 ± 0.25 |
| Indomethacin (positive control) | 83.90 ± 0.27 | 0.16 ± 0.02 |
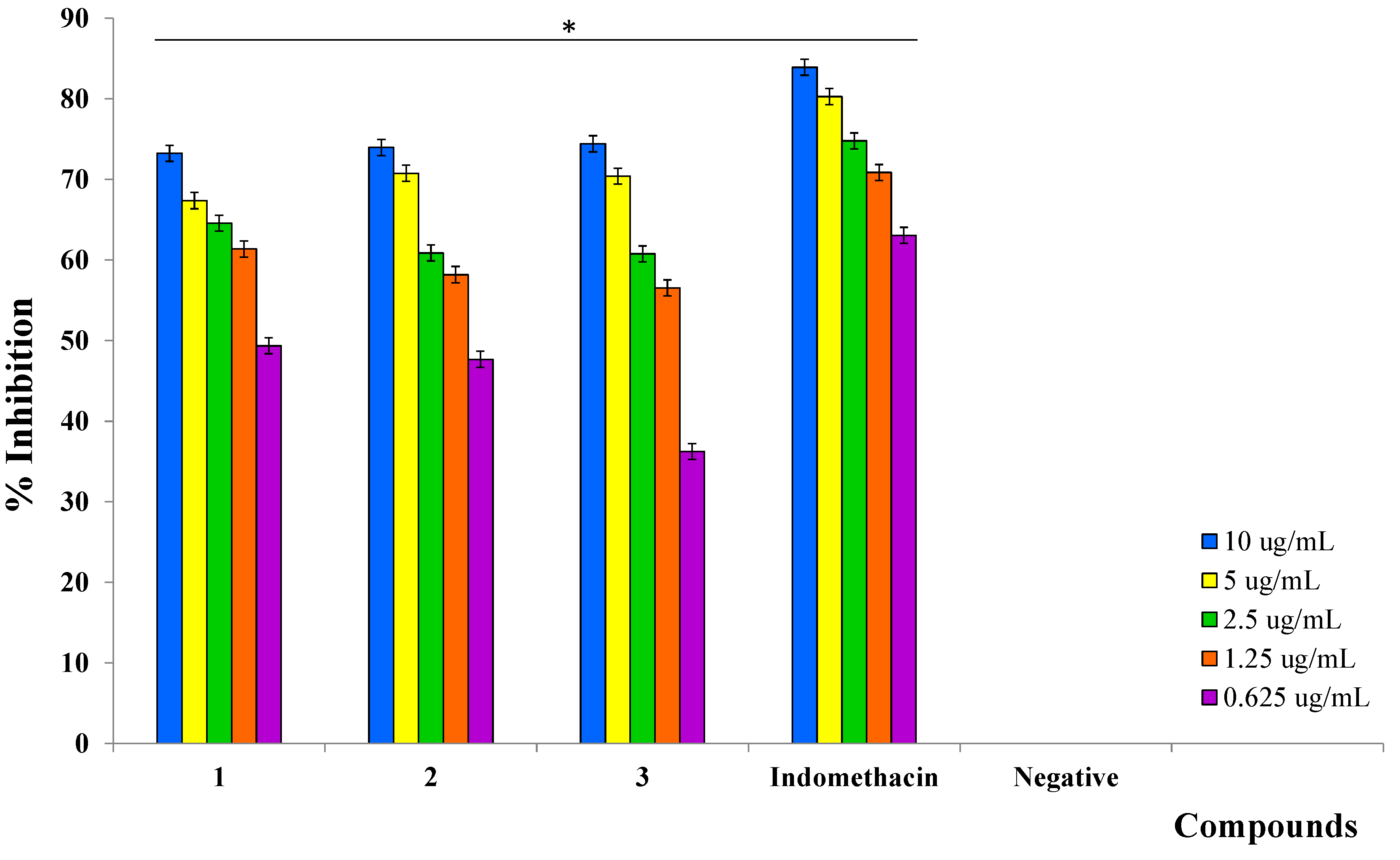
| Compounds | IC50 (µM) * |
|---|---|
| 1 | 1.05 ± 0.02 |
| 2 | 1.54 ± 0.05 |
| 3 | 2.59 ± 0.07 |
| Indomethacin (positive control) | 0.45 ± 0.02 |
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation of Compounds
3.4. Quantification of Koetjapic Acid (1) and Betulinic Acid (3) Using HPLC
| Compound | Conc. 1 | Intra-day precision 2 | Inter-day precision 4 | Equation (r2) | LOD 5 | LOQ 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rt 3 | Area | |||||||||||
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Rt 3 | Area | |||||
| Koetjapic acid (1) | 62.5 | 1.51 | 2.16 | 0.06 | 4.51 | 3.62 | 3.68 | 1.20 | 1.48 | y = 13523x − 44326 (0.9994) | 1.89 | 5.75 |
| 125 | 2.58 | 2.52 | 1.48 | 3.09 | 1.15 | 2.08 | 3.57 | 1.89 | ||||
| 250 | 2.50 | 0.56 | 1.57 | 1.33 | 0.95 | 4.04 | 2.26 | 3.41 | ||||
| 500 | 0.68 | 1.98 | 0.56 | 1.58 | 2.04 | 2.19 | 3.82 | 2.11 | ||||
| 1000 | 0.94 | 0.83 | 1.74 | 1.62 | 1.27 | 1.59 | 2.49 | 2.66 | ||||
| Betulinic acid (1) | 62.5 | 0.48 | 0.44 | 1.31 | 4.92 | 3.82 | 3.45 | 2.29 | 2.46 | y = 4851x − 42307 (0.9999) | 9.23 | 27.97 |
| 125 | 1.07 | 1.50 | 1.11 | 4.53 | 3.98 | 2.28 | 2.18 | 3.53 | ||||
| 250 | 0.50 | 0.88 | 0.66 | 1.03 | 2.83 | 2.82 | 2.40 | 3.32 | ||||
| 500 | 1.69 | 1.07 | 1.57 | 1.15 | 2.08 | 4.96 | 2.55 | 2.58 | ||||
| 1000 | 0.83 | 0.89 | 0.83 | 2.86 | 2.57 | 2.86 | 1.55 | 4.03 | ||||
3.5. Radioimmunoassay for Prostaglandin E2 (PGE2)
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Milatovic, D.; Montine, T.J.; Aschner, M. Prostanoid signaling: dual role for prostaglandin E2 in neurotoxicity. NeuroToxicology 2011, 32, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T. Roles of prostaglandin E2 in the cochlea. Hear. Res. 2011, 276, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Bruckner, M.; Uetz-von Allmen, E.; Krause, P. Prostaglandin E2 at new glance: Novel insights in functional diversity offer therapeutic chances. Int. J. Biochem. Cell Biol. 2010, 42, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.G.; Padilla, J.; Koumas, L.; Ray, D.; Phipps, R.P. Prostaglandins as modulators of immunity. TRENDS Immunol. 2002, 23, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [PubMed]
- Jabbour, H.N.; Sales, K.J. Prostaglandin receptor signaling and function in human endometrial pathology. TRENDS Endocrin. Met. 2004, 15, 398–404. [Google Scholar] [CrossRef]
- Offermanns, S.; Rosenthal, W. Encyclopedia of Molecular Pharmacology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Lim, T.K. Dillenia Serrata. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 2. [Google Scholar]
- Hedberg, I.; Hedberg, O.; Madati, P.J.; Mshigeni, K.E.; Mshiu, E.N.; Samuelsson, G. Inventory of plants used in traditional medicine in Tanzania. II. Plants of the families Dilleniaceae-Opiliaceae. J. Ethnopharmacol. 1983, 9, 105–128. [Google Scholar]
- Grosvenor, P.W.; Gothard, P.K.; McWilliam, N.C.; Supriono, A.; Gray, D.O. Medicinal plants from Riau Province, Sumatra, Indonesia. Part 1: Uses. J. Ethnopharmacol. 1995, 45, 75–95. [Google Scholar] [CrossRef]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula; Ministry of Agriculture and Cooperatives: Kuala Lumpur, Malaysia, 1966. [Google Scholar]
- Kerrigan, R.A.; Craven, L.A.; Dunlop, C.R. Dilleniaeae. In Flora of the Darwin Region; Short, P.S., Cowie, I.D., Eds.; Northern Territory Herbarium, Department of Natural Resources, Environment, the Arts and Sport: Palmerston, Australia, 2011. [Google Scholar]
- Jansen, P.C.M.; Jukema, J.; Oyen, L.P.A.; van Lingen, T.G. Dillenia serrata Thunb. In Plant Resources of South-East Asia No. 2: Edible Fruit and Nuts; Verheij, E.W.M., Coronol, R.E., Eds.; Prosea Foundation: Bogor, Indonesia, 1992. [Google Scholar]
- Windadri, F.I.; Rahayu, M.; Uji, T.; Rustiami, H. Pemanfaatan tumbuhan sebagai bahan obat oleh masyarakat lokal suku Muna di kecamatan Wakarumba, kabupaten Muna, Sulawesi Utara. Biodiversitas 2006, 7, 333–339. [Google Scholar] [CrossRef]
- Bhattacharjee, S.R.; Chatterjee, A. Betulinic acid and betulin, the triterpenoid constituents of Dillenia indica. J. Indian Chem. Soc. 1962, 39, 276–284. [Google Scholar]
- Pavanasasivam, G.; Sultanbawa, M.U.S. Chemical investigation of Ceylonese plants. IX. Betulinic acid in the Dilleniaceae and a review of its natural distribution. Phytochemistry 1974, 13, 2002–2006. [Google Scholar]
- Dan, S.; Dan, S.S. Triterpenoids of Indian Dilleniaceae. J. Indian Chem. Soc. 1980, 57, 760. [Google Scholar]
- Nick, A.; Wright, A.D.; Rali, T.; Sticher, O. Antibacterial triterpenoids from Dillenia papuana and their structure-activity relationships. Phytochemistry 1995, 40, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Macahig, R.A.S.; Matsunami, K.; Otsuka, H. Chemical studies on an endemic Philippine plant: Sulfated glucoside and seco-A-ring triterpenoids from Dillenia philippinensis. Chem. Pharm. Bull. 2011, 59, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Bate-Smith, E.C.; Harborne, J.B. Differences in flavonoid content between fresh and herbarium leaf tissue in Dillenia. Phytochemistry 1981, 10, 1055–1058. [Google Scholar] [CrossRef]
- Pavanasasivam, G.; Sultanbawa, M.U.S. Flavonoids of some Dilleniaceae species. Phytochemistry 1975, 14, 1127–1128. [Google Scholar] [CrossRef]
- Gurni, A.A.; Kubitzki, K. Flavonoid chemistry and systematics of the Dilleniaceae. Biochem. Syst. Ecol. 1981, 9, 109–114. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Cui, C.; Li, M.; Fan, M. Chemical constituents of Dillenii kerrii and their activities on antitumor and anti-hypoxia in vitro. Chin. J. Med. Chem. 2009, 19, 130–134. [Google Scholar] [CrossRef]
- Nick, A.; Wright, A.D.; Sticher, O.; Rali, T. Antibacterial triterpenoid acids from Dillenia papuana. J. Nat. Prod. 1994, 57, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Muliawan, S.Y. Effect of Dillenia suffruticosa extract on dengue virus type 2 replication. Univ. Med. 2008, 27, 1–5. [Google Scholar]
- Parvin, M.N.; Rahman, M.S.; Islam, M.S.; Rashid, M.A. Chemical and biological investigations of Dillenia indica Linn. Bangladesh J. Pharmacol. 2009, 4, 122–125. [Google Scholar]
- Yeshwante, S.B.; Juvekar, A.R.; Nagmoti, D.M.; Wankhede, S.S.; Shah, A.S.; Pimprikar, R.B.; Saindane, D.S. Anti-inflammatory activity of methanolic extracts of Dillenia indica L. leaves. Pharmacology 2009, 1, 63–66. [Google Scholar]
- Abdille, M.H.; Sigh, R.P.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 2005, 90, 891–896. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 347–352. [Google Scholar] [CrossRef]
- Kumar, D.; Mallick, S.; Vedasiromoni, J.R.; Pal, B.C. Anti-leukemic activity of Dillenia indica L. Fruit and quantification of betulinic acid by HPLC. Phytomedicine 2010, 17, 431–435. [Google Scholar]
- Rosangkima, G.; Rongpi, T.; Prasad, S.B. Role of glutathione and glutathione-related enzymes in the antitumor activity of Dillenia pentagyna in Dalton’s lymphoma-bearing mice. Int. J. Cancer Res. 2008, 4, 92–102. [Google Scholar] [CrossRef]
- Nyman, U.; Joshi, P.; Madsen, L.B.; Pedersen, T.B.; Pinstrup, M.; Rajasekharan, S.; George, V.; Pushpangadan, P. Ethnomedical information and in vitro screening for angiotensin-converting enzyme inhibition of plants utilized as traditional medicines in Gujarat, Rajasthan and Kerala (India). J. Ethnophamacol. 1998, 60, 247–263. [Google Scholar] [CrossRef]
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; El Sayed, K.; Mossa, J.S.; Al-Said, M.S.; El-Feraly, F.S.; Clark, A.M.; Hufford, C.D.; Oh, S.; Mayer, A.M.S. Bioactive 12-oleanene triterpene and secotriterpene acids from Maytenus undata. J. Nat. Prod. 2000, 63, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Wachter, G.A.; Valcic, S.; Flagg, M.L.; Franzblau, S.G.; Montenegro, G.; Suarez, E.; Timmermann, B.N. Antitubercular activity of pentacyclic triterpenoids from plants of Argentina and Chile. Phytomedicine 1999, 6, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.S.; Ito, H.; Mukainaka, T.; Higashihara, H.; Enjo, F.; Tokuda, H.; Nishino, H.; Yoshida, T. Ichthyotoxic and anticarcinogenic effects of triterpenoids from Sandoricum koetjape bark. Biol. Pharm. Bull. 2003, 26, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-H.; Starck, S.R.; Locke, E.P.; Hecht, S.M. DNA polymerase beta inhibitors from Sandoricum koetjape. J. Nat. Prod. 1999, 62, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, A.C.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar]
- Schmidt, M.L.; Kuzmanoff, K.L.; Ling-Indeck, L.; Pezzuto, J.M. Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur. J. Cancer 1997, 33, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Safayhi, H.; Sailer, E.-R. Anti-inflammatory actions of pentacyclic triterpenes. Planta Med. 1997, 63, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Huguet, A.-I.; Recio, M.C.; Manez, S.; Giner, R.M.; Rios, J.-L. Effect of triterpenoids on the inflammation induced by protein kinase C activators, neuronally acting irritants and other agents. Eur. J. Pharmacol. 2000, 410, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, R.E.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.-S. Anti-HIV principles from Syzygium claviflorum and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, F.; Kashiwada, Y.; Cosentino, L.M.; Chen, C.-H.; Garret, P.E.; Lee, K.-H. Anti-AIDS agents-XXVII. Synthesis and anti-HIV activity of betulinic acid and dihydrobetulinic acid derivatives. Bioorganic Med. Chem. 1997, 5, 2133–2143. [Google Scholar]
- Kaneda, N.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R.; Santisuk, T.; Tuchinda, P.; Udchachon, J.; Reutrakul, V. Plant anticancer agents, L.1 Cytotoxic triterpenes from Sandoricum koetjape stems. J. Nat. Prod. 1992, 55, 654–659. [Google Scholar]
- Peng, C.; Bodenhausen, G.; Qiu, S.; Fong, H.H.S.; Farnsworth, N.R.; Yuanm, S.; Zheng, C. Computer-assisted structure elucidation: Application of CISOC-SES to the resonance assignment and structure generation of betulinic acid. Magn. Reson. Chem. 1998, 36, 267–278. [Google Scholar] [CrossRef]
- Bae, K.H.; Lee, S.M.; Lee, E.S.; Lee, J.S.; Kang, J.S. Isolation and quantitative analysis of betulinic acid and alphitolic acid from Zyziphi fructus. Yakhak Hoeji 1996, 40, 558–562. [Google Scholar]
- Oliveira, B.H.; Santos, C.A.M.; Espíndola, A.P.D.M. Determination of the triterpenoid, betulinic acid, in Doliocarpus schottianus by HPLC. Phytochem. Anal. 2002, 13, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yan, W.; Cao, D. Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J. Pharm. Biomed. Anal. 2007, 43, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Bretnall, A.E.; Clarke, G.S. Validation of analytical test methods. In Separation Scince and Technology; Ahuja, S., Ed.; Academic Press: Amsterdam, The Netherlands, 2011; Volume 10. [Google Scholar]
- Crowther, J.B. Validation of pharmaceutical methods. In Handbook of Modern Pharmaceutical Analysis; Ahuja, S., Scypinski, S., Eds.; Academic Press: San Diego, CA, USA, 2001; Volume 3. [Google Scholar]
- Patrignani, P.; Panara, M.R.; Greco, A.; Fusco, O.; Natoli, C.; Iacobelli, S.; Cipollone, F.; Ganci, A.; Créminon, C.; Maclouf, J.; et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J. Pharmacol. Exp. Ther. 1994, 271, 1705–1712. [Google Scholar]
- Rasadah, M.A.; Khozirah, S.; Aznie, A.A.; Nik, M.M. Anti-inflammatory agents from Sandoricum koetjape Merr. Phytomedicine 2004, 11, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Viji, V.; Helen, A.; Luxmi, V.R. Betulinic acid inhibits endotoxin-stimulated phosphorylation cascade and pro-inflammatory prostaglandin E2 production in human peripheral blood mononuclear cells. Br. J. Pharmacol. 2011, 162, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Wenzig, E.M.; Widowitz, U.; Kunert, O.; Chrubasik, S.; Bucar, F.; Knauder, E.; Bauer, R. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology; ICH Harmonized Tripartite Guidelines, 2005. Available online: http://www.ich.org (accessed on 1 December 2013).
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalil, J.; Sabandar, C.W.; Ahmat, N.; Jamal, J.A.; Jantan, I.; Aladdin, N.-A.; Muhammad, K.; Buang, F.; Mohamad, H.F.; Sahidin, I. Inhibitory Effect of Triterpenoids from Dillenia serrata (Dilleniaceae) on Prostaglandin E2 Production and Quantitative HPLC Analysis of Its Koetjapic Acid and Betulinic Acid Contents. Molecules 2015, 20, 3206-3220. https://doi.org/10.3390/molecules20023206
Jalil J, Sabandar CW, Ahmat N, Jamal JA, Jantan I, Aladdin N-A, Muhammad K, Buang F, Mohamad HF, Sahidin I. Inhibitory Effect of Triterpenoids from Dillenia serrata (Dilleniaceae) on Prostaglandin E2 Production and Quantitative HPLC Analysis of Its Koetjapic Acid and Betulinic Acid Contents. Molecules. 2015; 20(2):3206-3220. https://doi.org/10.3390/molecules20023206
Chicago/Turabian StyleJalil, Juriyati, Carla W. Sabandar, Norizan Ahmat, Jamia A. Jamal, Ibrahim Jantan, Nor-Ashila Aladdin, Kartiniwati Muhammad, Fhataheya Buang, Hazni Falina Mohamad, and Idin Sahidin. 2015. "Inhibitory Effect of Triterpenoids from Dillenia serrata (Dilleniaceae) on Prostaglandin E2 Production and Quantitative HPLC Analysis of Its Koetjapic Acid and Betulinic Acid Contents" Molecules 20, no. 2: 3206-3220. https://doi.org/10.3390/molecules20023206





