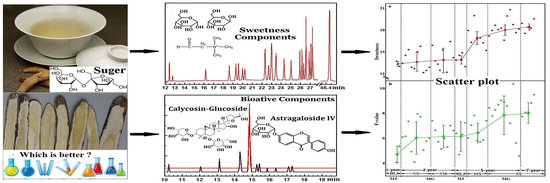
Potential Quality Evaluation Method for Radix Astragali Based on Sweetness Indicators
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Sweetness Components of RA
2.1.1. Qualitative and Quantitative Analysis of the Main Chemical Components of “RA Sweetness”

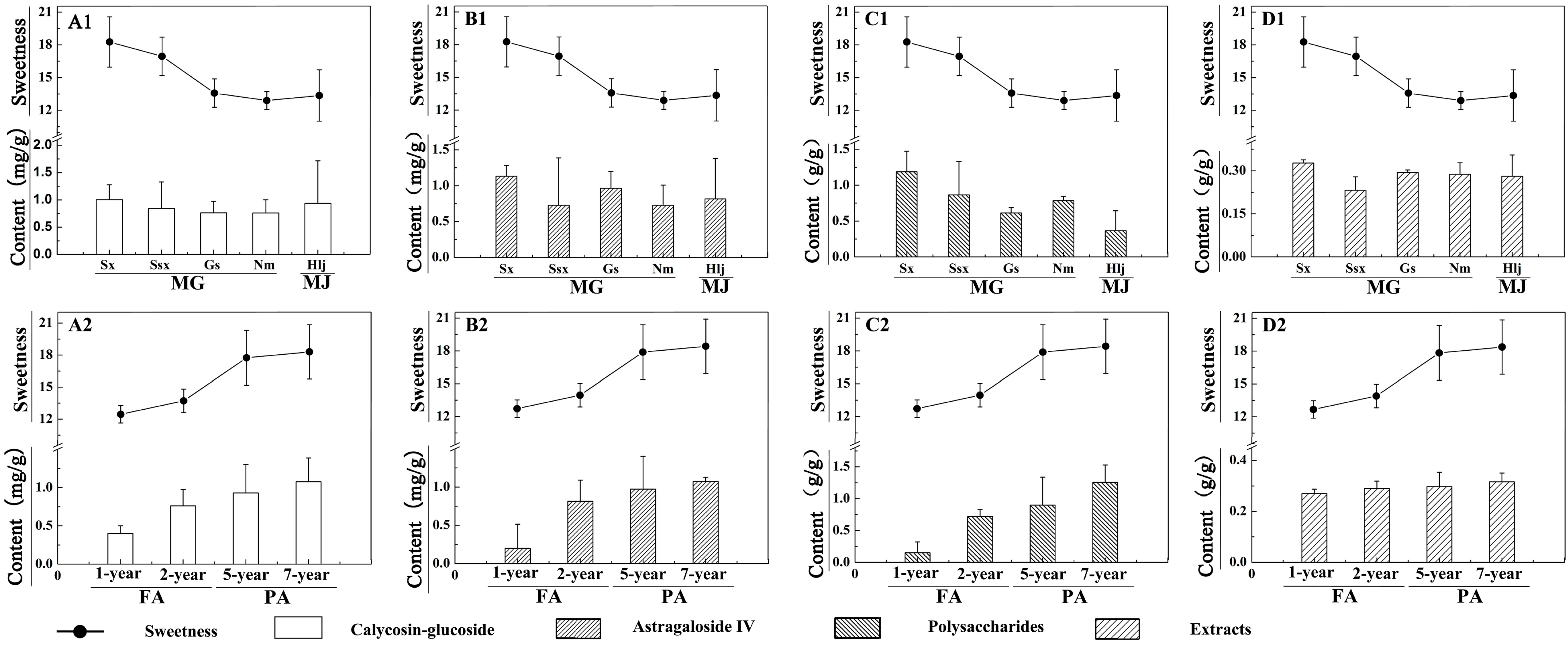
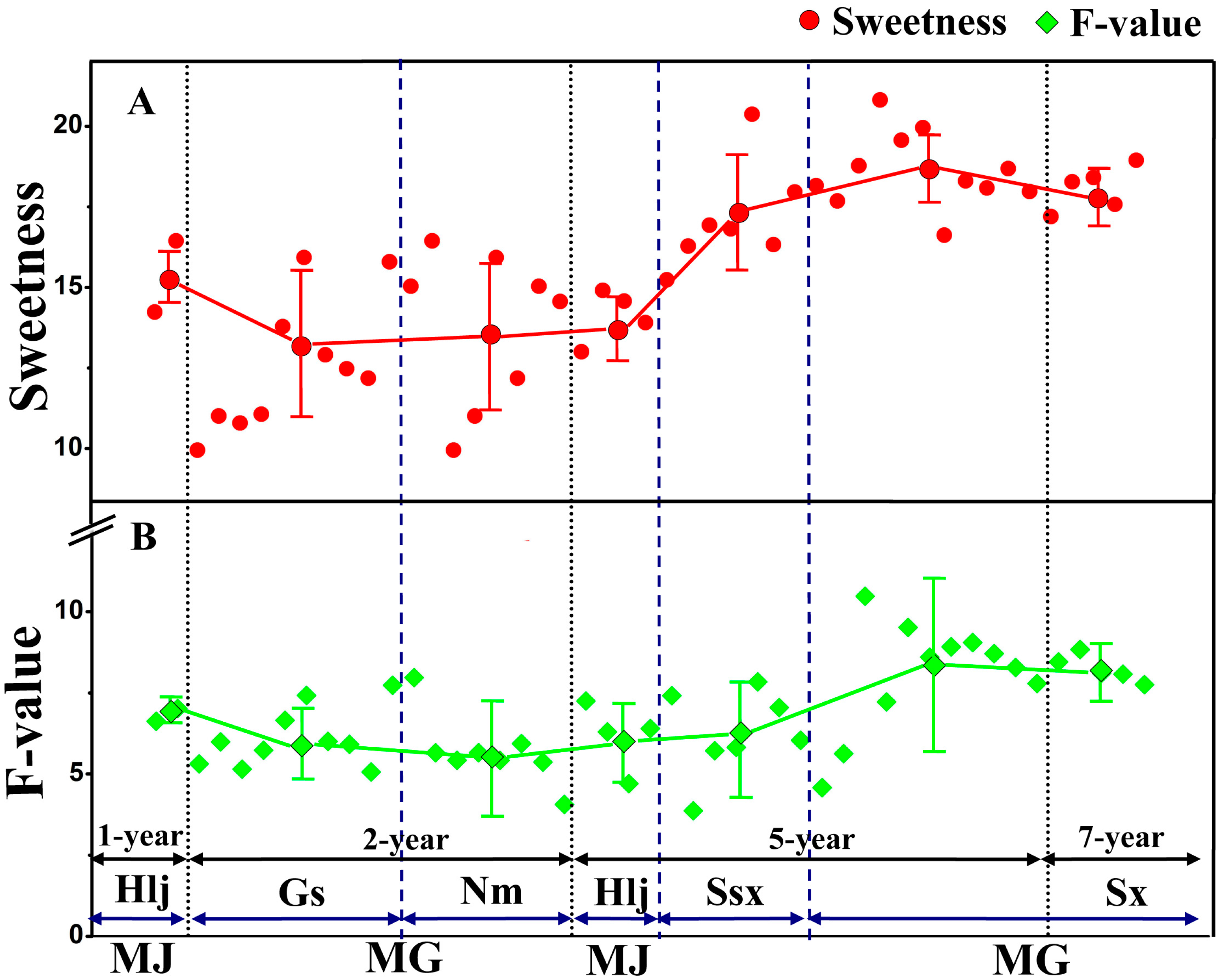
2.1.2. Differences in Sweetness of Samples from Different Species, Regions, and Growth Years
2.2. Analysis of Content Variation Trends between the Sweetness and Four Quality Control Indicators of RA
2.3. Correlation Analysis between the Sweetness and Four Quality Control Indicators of RA
| Contents | Varieties | Glc (μg/g) | Fru (μg/g) | Ino (μg/g) | Sor (μg/g) | Dul (μg/g) | Suc (mg/g) | Betaine (mg/g) | Sweetness | |
|---|---|---|---|---|---|---|---|---|---|---|
| Batches | R.S. = 0.75 | R.S. = 1.7 | R.S. = 0.5 | R.S. = 0.55 | R.S. = 0.3 | R.S. = 1.0 | R.S. = 0.5 | |||
| A. membranaceus var. Mongholicus (Bge.) | Sx (17) | 3.4 ± 1.4 | 1.3 ± 0.9 | 75.5 ± 23.6 | 1.5 ± 0.7 | 5.1 ± 1.2 | 17.5 ± 2.3 | 1.4 ± 0.7 | 18.3 ± 2.2 | |
| Ssx (7) | 1.9 ± 0.8 | 0.7 ± 0.5 | 79.0 ± 18.9 | 1.0 ± 0.4 | 4.3 ± 0.8 | 16.4 ± 1.8 | 1.4 ± 0.8 | 17.1 ± 1.6 | ||
| Gs (10) | 1.0 ± 0.3 | 0.2 ± 0.1 | 79.2 ± 13.6 | 0.8 ± 0.2 | 2.9 ± 0.8 | 12.2 ± 1.3 | 2.8 ± 0.1 | 13.7 ± 1.3 | ||
| Nm (8) | 0.6 ± 0.3 | 0.1 ± 0.1 | 88.4 ± 14.1 | 1.0 ± 0.6 | 2.0 ± 0.9 | 11.8 ± 0.8 | 2.2 ± 0. 9 | 13.7 ± 2.3 | ||
| A. membranaceus (Fisch.) Bge. | Hlj (6) | 0.9 ± 0.5 | 0.3 ± 0.2 | 49.0 ± 18.1 | 0.6 ± 0.3 | 2.6 ± 1.1 | 12.3 ± 2.4 | 2.2 ± 1.3 | 13.9 ± 0.8 | |
| Fast-growing RA | 1Y (2) | 0.7 ± 0.4 | 0.2 ± 0.2 | 38.1 ± 7.4 | 0.4 ± 0.2 | 1.9 ± 1.4 | 13.1 ± 0.2 | 0.7 ± 0.1 | 13.4 ± 0.5 | |
| 2Y (18) | 0.8 ± 0.3 | 0.2 ± 0.1 | 83.3 ± 14.3 | 0.9 ± 0.4 | 2.5 ± 0.9 | 12.0 ± 1.1 | 1.4 ± 0.7 | 13.7 ± 1.7 | ||
| Perennial RA | 5Y (22) | 2.4 ± 1.4 | 0.7 ± 0.4 | 69.3 ± 21.7 | 1.2 ± 0.6 | 4.3 ± 1.3 | 16.0 ± 2.8 | 2.5 ± 1.4 | 17.3 ± 2.1 | |
| 7Y (6) | 3.8 ± 1.2 | 2.4 ± 0.6 | 91.0 ± 19.9 | 1.6 ± 0.7 | 5.4 ± 1.1 | 17.1 ± 0.3 | 2.2 ± 0.3 | 18.2 ± 0.5 | ||
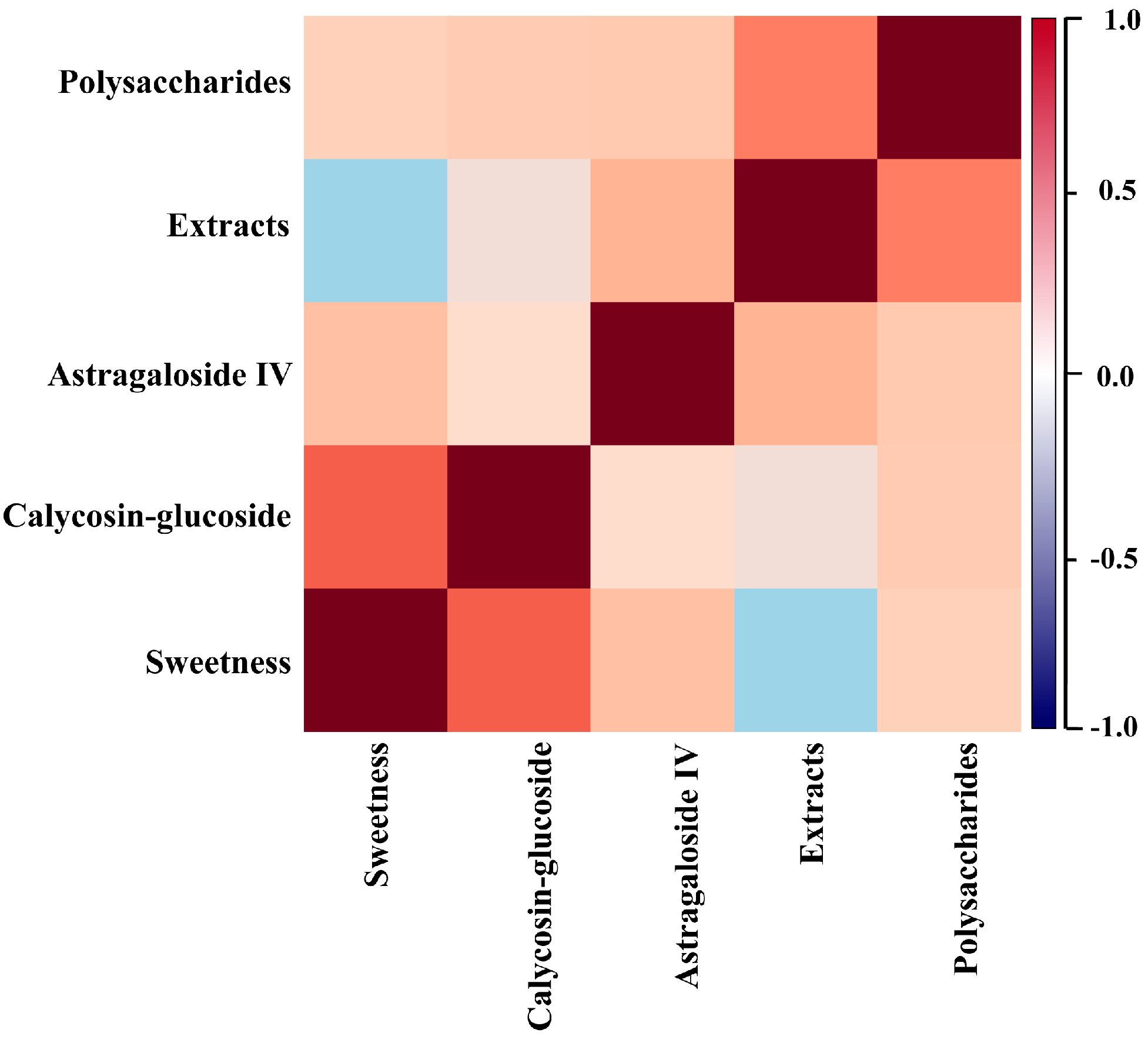
| Chemical Index | No. | Regression Equations | Related Coefficient | p Value |
|---|---|---|---|---|
| Polysaccharides | 48 | Y = 0.007X + 0.090 | 0.486 | 0.147 |
| Astragaloside IV | 48 | Y = 0.004X + 0.926 | 0.412 | 0.068 |
| calycosin-glucoside | 48 | Y = 0.079X − 0.344 | 0.659 | 0.001 |
| Extracts | 48 | Y = −0.003X + 0.243 | 0.183 | 0.315 |
3. Experimental Section
3.1. Plant Materials
3.2. Solvents and Chemicals
| Species | Cultivation Pattern | Regions | No. | Harvesting Time | Growth Year |
|---|---|---|---|---|---|
| A. membranaceus var. Mongholicus (Bge.) | FA | Gs, Longxi County | 1–3 | 2010.12 | 2 |
| Gs, Dangchang County | 4–5 | 2010.12 | 2 | ||
| Gs, Minxian County | 6–7 | 2011.1 | 2 | ||
| Gs, Weiyuan County | 8–10 | 2011.1 | 2 | ||
| Nm, Guyang County | 11–13 | 2011.1 | 2 | ||
| Nm, Chifeng | 14–15 | 2011.11 | 2 | ||
| Nm, Ulanqab | 16 | 2009.5 | 2 | ||
| Nm, Shangdu County | 17 | 2011.11 | 2 | ||
| Nm, Xinghe County | 18 | 2011 | 2 | ||
| PA | Sx, Hunyuan County | 19–22 | 2011.11 | ≥5 | |
| 22–26 | 2011.1 | 5 | |||
| Sx, Daixian County | 27–28 | 2011.1 | 5 | ||
| Sx, Yinxian County | 29–31 | 2011.9 | 5 | ||
| 32 | 2011.1 | ≥5 | |||
| Sx, Tianzhen County | 33 | 2011.1 | 5 | ||
| Sx, Yanggao County | 34 | 2011.1 | 5 | ||
| Sx, Xinzhou | 35 | 2011.9 | 5 | ||
| Ssx, Zizhou County | 36 | 2011.9 | 6 | ||
| Ssx | 37 | 2009.6 | 5 | ||
| Ssx, Yulin | 38–42 | 2011 | 5 | ||
| A. membranaceus (Fisch.) Bge. | FA | Hlj, Hulan County | 43 | 2011.1 | 1 |
| Hlj, Hulan County | 44 | 2011 | 1 | ||
| PA | Hlj, Hulan County | 45–47 | 2011.11 | 5 | |
| Hlj, Jiagedaqi | 48 | 2011 | 5 |
3.3. Apparatus
3.4. Determination of Monosaccharide, Disaccharide, and Alditol Contents in RA by GC-MS
3.5. Determination of Betaine and Total Polysaccharide Contents in RA by UV-Vis
3.5.1. Betaine
3.5.2. Polysaccharides
3.6. Determination of Astragaloside IV and Calycosin-Glucoside in RA by HPLC
3.6.1. Astragaloside IV
3.6.2. Calycosin-Glucoside
3.7. Determination of the Content of Aqueous Extracts in RA
3.8. Calculation of Sweetness
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Abbreviation
| RA | Radix Astragali |
| MG | A. membranaceus var. Mongholicus (Bge.), and MJ, A. membranaceus (Fisch.) Bge., represent the two species of RA |
| FA | fast-growing RA, and PA, perennial RA, represent the two different growth years of RA |
| Sx | Shanxi Province |
| Ssx | Shaanxi Province |
| Gs | Gansu Province |
| Nm | Inner Mongolia |
| Hlj | Heilongjiang Province |
| GC-MS | gas chromatography-mass spectrometry |
| UV-Vis | ultraviolet visible |
| DMSO | dimethyl sulfoxide |
| HPLC | high-performance liquid chromatography |
| ELSD | evaporative light scattering detector |
Conflicts of Interest
References
- Liu, Y.; Chen, H.B.; Zhao, Y.Y.; Wang, B.; Zhang, Q.Y.; Zhang, L.; Tu, P.F. Quantification and Stability Studies on the Flavonoids of Radix hedysari. J. Agric. Food Chem. 2006, 54, 6634–6639. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S. Chinese herbs: A clinical review of Astragalus, Ligusticum, and Schizandrae. Altern. Med. Rev. 1998, 3, 338–344. [Google Scholar] [PubMed]
- Mao, X.J.; Wan, Y.Q.; Yan, A.P.; Shen, M.Y.; Wei, Y.L. Simultaneous determination of organophosphorus, organochlorine, pyrethriod and carbamate pesticides in Radix astragali by microwave-assisted extraction/dispersive-solid phase extraction coupled with GC-MS. Talanta 2012, 97, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Yang Kuo, L.M.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New Isoflavonoid Glycosides and Related Constituents from Astragali Radix (Astragalus membranaceus) and Their Inhibitory Activity on Nitric Oxide Production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.L.; Susan, M.N.; Keduo, Q.; Dong, Y.Z.; Yang, X.M.; Zhou, T.; Eileen, B.; Wu, S.F.; Koji, W.; Toshiyuki, A. Recent Progress of Research on Herbal Products Used in Traditional Chinese Medicine: the Herbs belonging to The Divine Husbandman’s Herbal Foundation Canon. J. Tradit. Complement. 2012, 2, 6–26. [Google Scholar]
- Li, S.Z. (Ming Dynasty); Ben Cao Gang Mu; National Research Institute of Chinese Medicine: Taipei, Taiwan, 1994; Volume 3, pp. 403–405. [Google Scholar]
- Yang, D.J.; Zhong, Z.C.; Xiao, Z.Y. Advances in Research on the Natural Sweetness Composition. West China J. Pharm. 1989, 4, 155–160. [Google Scholar]
- Huang, H.L.; Zhang, Z.L. The primary investigation of the medicinal property of the chemical mechanism for Radix Astragali. Today Anim. Husb. Vet. Med. 1996, S1, 52–54. [Google Scholar]
- Chu, C.; Qi, L.W.; Liu, E.H.; Li, B.; Gao, W.; Li, P. Radix Astragali (Astragalus): Latest Advancements and Trends in Chemistry, Analysis, Pharmacology and Pharmacokinetics. Curr. Org. Chem. 2010, 14, 792–1807. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.M.; Zhao, J.C.; Zhang, Y.L. The literatures of astragalus. Chin. Pharm. 1999, 2, 211–213. [Google Scholar]
- Shao, B.M.; Xu, W.; Dai, H.; Tu, P.F.; Li, Z.J.; Gao, X.M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004, 320, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Zhuang, Y.; Tian, Y.P.; Thomas, G.N.; Ying, M.; TomLinson, B. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid. Med. Cell Longrev. 2012, 1115, 282–383. [Google Scholar]
- Iliana, I.O.; Aleksandar, S.; Ilina, K.; Todor, I. Recent progress in phytochemistry, pharmacology and biotechnology of Astragalus saponins. Phytochem. Rev. 2014, 13, 343–374. [Google Scholar] [CrossRef]
- Song, X.W.; Li, Q.; Luo, H.J.; Ye, J.; Chen, X.H.; Bi, K.S. Synthetical quality evaluation of Astragali Radix. J. Shenyang Pharm. Univ. 2011, 28, 599–606. [Google Scholar]
- Yang, M. Comprehensive Principal Components Analysis on Quality Evaluation of Radix Astragali. J. Math. Med. 2008, 21, 474–476. [Google Scholar]
- Pharmacopoeia of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2010; Volume 1, pp. 283–284. [Google Scholar]
- David, W.; Jeff, X. MetaboAnalyst 3.0—A comprehensive server for metabolomic data analysis. Available online: http://www.metaboanalyst.ca/MetaboAnalyst/ (accessed on 5 February 2015).
- Dennis, D.T.; Blakely, S.D. Carbohydrate Metabolism. In Plant Biochemistry and Molecular Biology; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; Wiley: Oxford, UK, 2000; pp. 400–460. [Google Scholar]
- Bray, E.A.; Serres, J.B.; Weretilnyk, E. Responses to Abiotic Stresses. In Plant Biochemistry and Molecular Biology; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; Wiley: Oxford, UK, 2000; pp. 550–600. [Google Scholar]
- Sala, O.E.; Lauenroth, W.K.; Golluscio, R.A. Plant functional types in temperate semi-arid regions. In Plant Functional Types: Their Relevance to Ecosystem Properties and Global Change; Smith, T.M., Shugart, H.H., Woodward, F.I., Eds.; Cambridge University Press: New York, NY, USA, 1997; pp. 217–234. [Google Scholar]
- Tony, H.C.; Norio, M. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 9, 499–505. [Google Scholar]
- Zhao, J.T.; Li, X.F.; Li, H.; Xu, R.M. Research on the Role of the Soluble Sugar in the Regulation of Physiological Metabolism in Higher Plant. J. Anhui Agric. Sci. 2006, 34, 6423–6427. [Google Scholar]
- Yin, H.; Wang, W.X.; Zhao, X.M.; Du, Y.G. Research Progress in Plant Glycobiology. Chin. Bull. Bot. 2010, 45, 521–529. [Google Scholar]
- Yang, Q.Z.; Liu, D.W.; Wang, D.M.; Fu, J.; Huang, L.F. Correlation on quality and ecotype of Astragalus membranaceus var. mongholicus Chinese. Tradit. Herb. Drugs. 2014, 45, 2395–2399. [Google Scholar]
- Su, W.H.; Zhang, G.F.; Li, X.H.; Ou, X.K. Relationship between accumulation of secondary metabolism in medicinal plant and environmental condition. Tradit. Herb. Drugs. 2005, 9, 1415–1418. [Google Scholar]
- Li, K.; Liu, S.; Tan, Y.; Chao, N.; Tian, X.; Qi, L.; Powell, W.A.; Jiang, X.; Gai, Y. An Optimized GC-MS Method to Simultaneously Quantify Acetylated Aldose, Ketose and Alditol for Plant Tissues Based on Derivatization in Methyl Sulfoxide/1 Methylimidazole System. J. Agric. Food Chem. 2013, 61, 4011–4018. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, B.J. Diffusion Profiles of Health Beneficial Components from Goji Berry (Lyceum barbarum) Marinated in Alcohol and Their Antioxidant Capacities as Affected by Alcohol Concentration and Steeping Time. J. Foods 2013, 2, 32–42. [Google Scholar] [CrossRef]
- Michel, D.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Fred, S. Colorimetric Method for Determination of Sugars and Related Substances. J. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Du, G.J.; Qin, X.M.; Li, Z.Y.; He, P.; Gao, F.R.; Li, K.; Zhou, R. Comparative study on quality of Astragali Radix by two different planting patterns in major producing areas. J. Chin. Tradit. Herb. Drugs 2013, 44, 3386–3393. [Google Scholar]
- Sweetener values, including Calories and Glycemic Index. Available online: http://www.sugar-and-sweetener-guide.com (accessed on 5 February 2015).
- Rebecca, L. Johnson. The World of Medicinal Herbs. In National Geographic Shorts 36 Healing Herbs: The World’s Best Medicinal Plants; National Geographic Society: Washington, DC, USA, 2012; Volume 3.1, pp. 10–11. [Google Scholar]
- Kojo, A.; Han, L.F.; Liu, E.W.; Zhang, Y.; Wang, T.; Gao, X.M. Recent Advances in Astragalus membranaceus Anti-Diabetic Research: Pharmacological Effects of Its Phytochemical Constituents. Evid.-Based Complement. Altern. Med. 2013, 11. [Google Scholar] [CrossRef]
- Jin, M.L.; Zhao, K.; Huang, Q.S.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Bus. Manag. 2014, 64, 257–266. [Google Scholar]
- Deng, S.H.; Song, L.; Duan, X.J.; Zhang, D.L.; Nie, L. Correlation analysis between HPLC fingerprint of astragali radix extracts and anti-fatigue effect. J. Chin. Med. Mater. 2013, 36, 260–264. [Google Scholar]
- Sun, Z.Q. The Principal Component Analysis & Factor Analysis. In Medical Statistics, 3rd ed.; People’s Medical Publishing House: Beijing, China, 2010; Volume 8, pp. 354–373. [Google Scholar]
- Lu, S.; Chen, K.J.; Yang, Q.Y.; Sun, H.R. Progress in the Research of Radix Astragali in Treating Chronic Heart Failure: Effective Ingredients, Dose-Effect Relationship and Adverse Reaction. Chin. J. Integr. Med. 2011, 17, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.F.; Xie, D.S.; Guo, X.Q.; Zhang, L.Z.; Li, Z.Y.; Wu, B.; Qin, X.M. Study on the relevance between beany flavor and main bioactive components in Radix Astragali. J. Agric. Food Chem. 2010, 58, 5568–5573. [Google Scholar] [CrossRef] [PubMed]
- Gurib, F.A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Gao, F.; Li, Z.; Qin, X.; Sun, H.; Xing, J.; Zhang, L.; Du, G. Potential Quality Evaluation Method for Radix Astragali Based on Sweetness Indicators. Molecules 2015, 20, 3129-3145. https://doi.org/10.3390/molecules20023129
Li K, Gao F, Li Z, Qin X, Sun H, Xing J, Zhang L, Du G. Potential Quality Evaluation Method for Radix Astragali Based on Sweetness Indicators. Molecules. 2015; 20(2):3129-3145. https://doi.org/10.3390/molecules20023129
Chicago/Turabian StyleLi, Ke, Fanrong Gao, Zhenyu Li, Xuemei Qin, Haifeng Sun, Jie Xing, Lizeng Zhang, and Guanhua Du. 2015. "Potential Quality Evaluation Method for Radix Astragali Based on Sweetness Indicators" Molecules 20, no. 2: 3129-3145. https://doi.org/10.3390/molecules20023129