Promoting Effect of Foliage Sprayed Zinc Sulfate on Accumulation of Sugar and Phenolics in Berries of Vitis vinifera cv. Merlot Growing on Zinc Deficient Soil
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Soil Constituents of Experimental Field Site and Initial Concentration of Zinc in Grape Petiole
| Soil Constituents | Depth | Critical Value | |
|---|---|---|---|
| 0–20 cm | 20–40 cm | ||
| Available N (mg/kg) | 34.29 | 17.50 | 10–100 |
| Available P (mg/kg) | 54.42 | 20.05 | 18–25 |
| Available K (mg/kg) | 75.19 | 67.78 | 60–100 |
| Organic matter (%) | 0.59 | 0.43 | 1–3.5 |
| pH | 8.63 | 8.74 | - |
| Available Cu (mg/kg) | 0.81 | 0.49 | 0.5–1.0 |
| Available Zn (mg/kg) | 0.81 | 0.33 | 1.1–2.0 |
| Available Fe (mg/kg) | 4.19 | 3.33 | 4.5–10.0 |
| Available Mn (mg/kg) | 6.52 | 5.17 | 9–15 |
2.2. The Photosynthesis at Three Developmental Stages
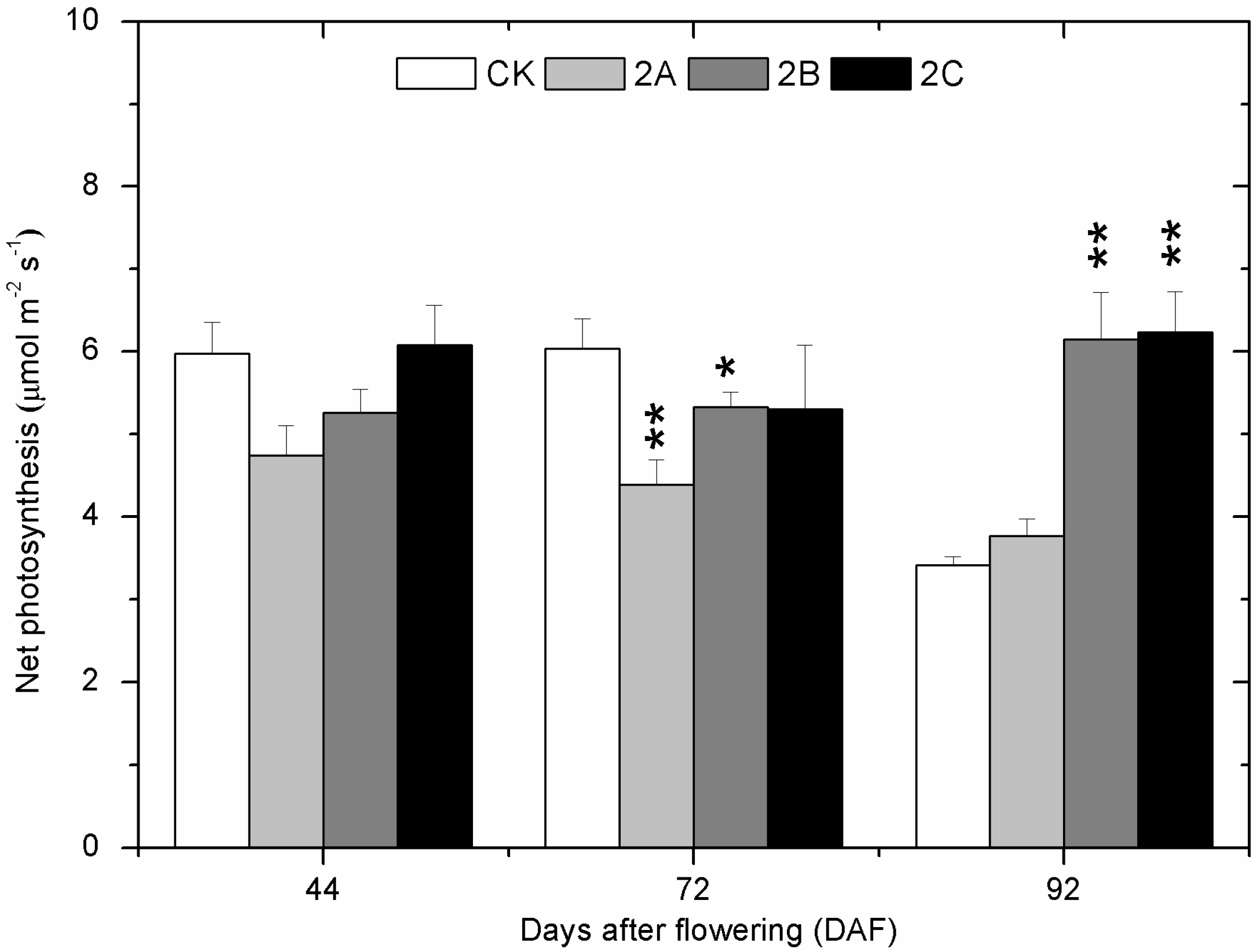
2.3. Berry Weight, Total Soluble Solids, pH and Titratable Acidity of Berry Juice throughout the Developmental Stages
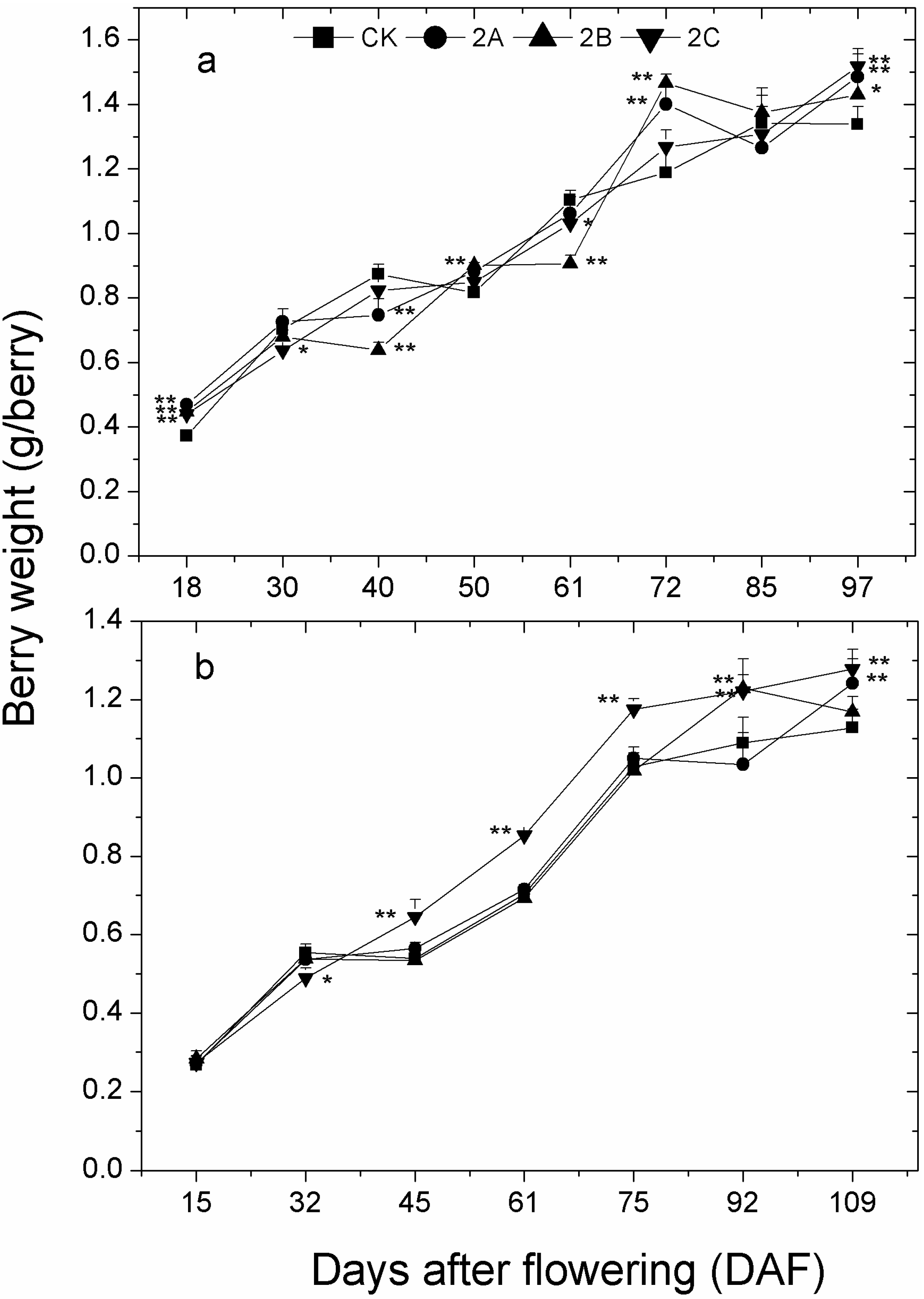
| DAF | 2013 | DAF | 2014 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CK | 2A | 2B | 2C | CK | 2A | 2B | 2C | ||
| 18 | 3.9 ± 0.07 | 3.4 ± 0.04 ** | 3.6 ± 0.06 * | 3.8 ± 0.07 | 15 | 3.4 ± 0.12 | 2.9 ± 0.23 * | 3.1 ± 0.11 | 3.2 ± 0.09 |
| 30 | 4.0 ± 0.05 | 3.6 ± 0.14 * | 3.6 ± 0.07 * | 3.7 ± 0.07 * | 32 | 4.1 ± 0.11 | 3.8 ± 0.20 | 4.0 ± 0.09 | 4.0 ± 0.13 |
| 40 | 3.9 ± 0.08 | 3.8 ± 0.10 | 3.7 ± 0.07 | 3.8 ± 0.12 | 45 | 4.2 ± 0.21 | 4.0 ± 0.08 | 4.0 ± 0.21 | 4.1 ± 0.03 |
| 50 | 5.5 ± 0.06 | 5.4 ± 0.06 | 5.7 ± 0.07 * | 6.1 ± 0.06 ** | 61 | 12.5 ± 0.14 | 11.3 ± 0.17 ** | 11.7 ± 0.07 ** | 11.1 ± 0.18 ** |
| 61 | 12.4 ± 0.13 | 12.7 ± 0.08 | 12.6 ± 0.06 | 13.0 ± 0.00 ** | 75 | 14.7 ± 0.08 | 14.4 ± 0.05 ** | 14.6 ± 0.09 | 15.0 ± 0.18 |
| 72 | 15.4 ± 0.15 | 16.5 ± 0.07 ** | 16.3 ± 0.22 ** | 16.1 ± 0.20 * | 92 | 18.9 ± 0.16 | 19.1 ± 0.20 | 19.9 ± 0.05 ** | 20.3 ± 0.22 ** |
| 85 | 17.9 ± 0.08 | 18.5 ± 0.19 | 19.0 ± 0.18 ** | 18.9 ± 0.09 ** | 109 | 20.1 ± 0.09 | 22.0 ± 0.16 ** | 21.8 ± 0.10 ** | 22.2 ± 0.12 ** |
| 97 | 19.6 ± 0.14 | 21.4 ± 0.34 ** | 21.6 ± 0.11 ** | 20.7 ± 0.21 * | - | - | - | - | - |
| DAF | pH | TA (g/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| CK | 2A | 2B | 2C | CK | 2A | 2B | 2C | |
| 32 | 2.63 ± 0.021 | 2.66 ± 0.014 | 2.68 ± 0.014 ** | 2.66 ± 0.006 | 28.08 ± 0.29 | 27.38 ± 0.17 | 27.66 ± 0.12 | 26.91 ± 0.23 * |
| 45 | 2.57 ± 0.057 | 2.55 ± 0.021 | 2.52 ± 0.005 | 2.56 ± 0.007 | 27.91 ± 0.01 | 27.78 ± 0.04 * | 28.41 ± 0.23 | 27.80 ± 0.04 |
| 61 | 2.89 ± 0.014 | 3.06 ± 0.028 ** | 3.08 ± 0.028 ** | 3.05 ± 0.014 ** | 11.75 ± 0.29 | 12.61 ± 0.10 | 12.23 ± 0.08 | 12.87 ± 0.04 ** |
| 75 | 3.24 ± 0.007 | 3.31 ± 0.021 ** | 3.27 ± 0.014 | 3.26 ± 0.012 | 7.38 ± 0.09 | 7.23 ± 0.12 ** | 6.71 ± 0.09 | 7.23 ± 0.09 |
| 92 | 3.62 ± 0.006 | 3.70 ± 0.014 ** | 3.55 ± 0.005 ** | 3.66 ± 0.007 * | 5.08 ± 0.11 | 4.44 ± 0.06 | 4.35 ± 0.19 | 4.33 ± 0.01 ** |
| 109 | 3.90 ± 0.007 | 3.86 ± 0.008 ** | 3.80 ± 0.007 ** | 3.77 ± 0.005 ** | 4.09 ± 0.02 | 3.75 ± 0.02 | 3.85 ± 0.04 | 3.63 ± 0.11 ** |
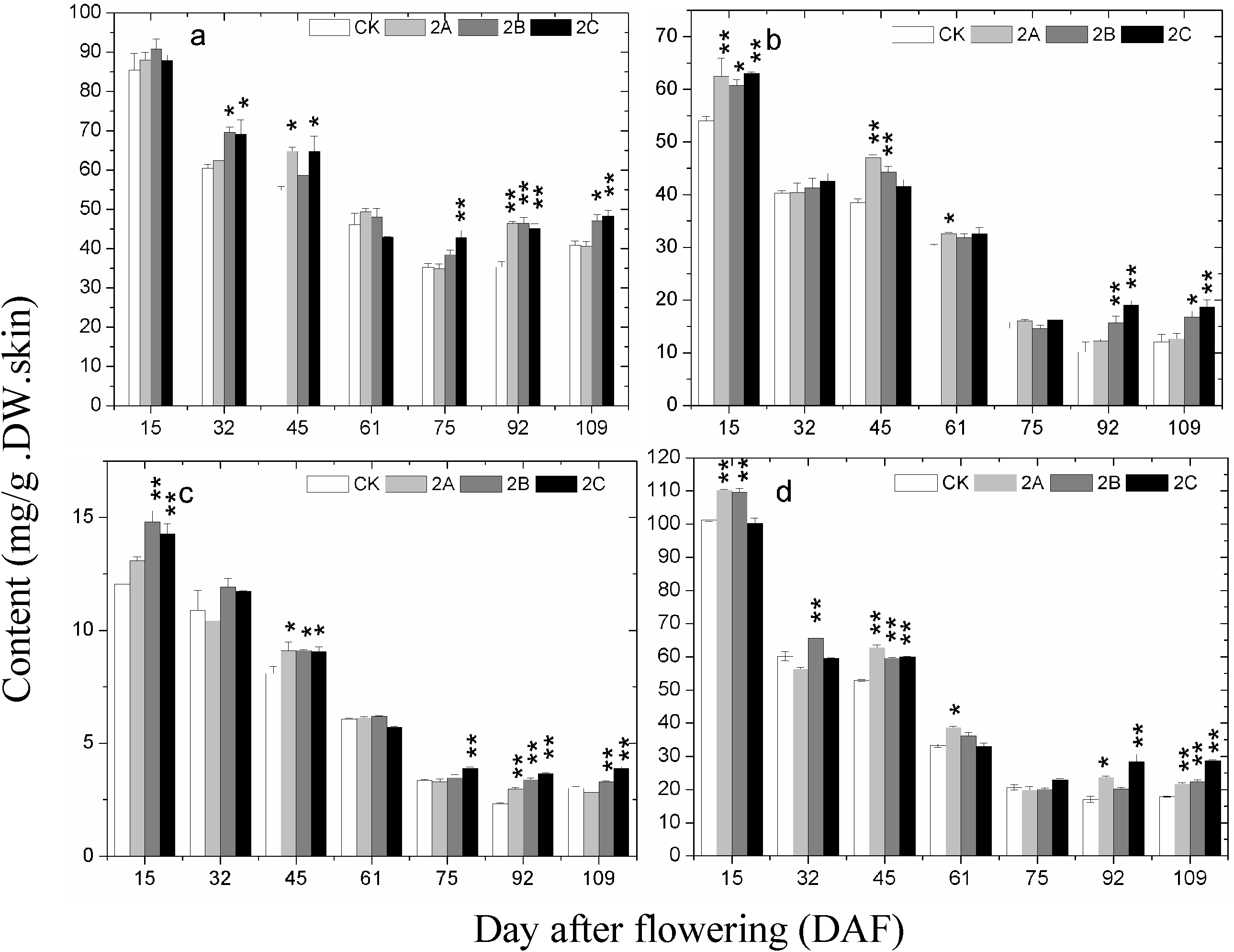
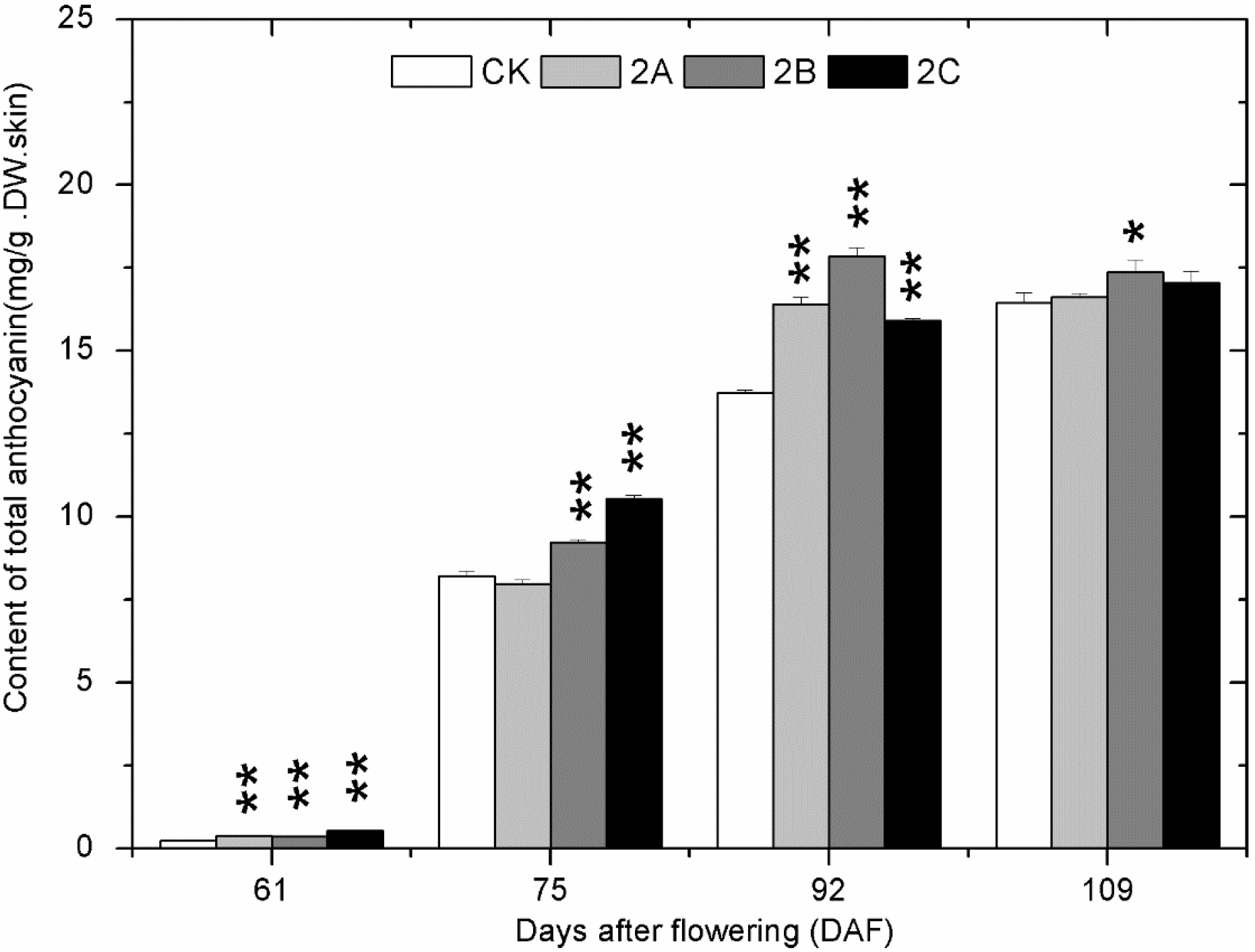
2.4. The Accumulation of Phenolics during Berry Development


2.5. The Expression of Phenolics Biosynthetic Pathway Genes during Berry Development

2.6. Discussion
3. Experimental Section
3.1. Experimental Field Site
3.2. Experimental Design
| Treatments | ZnSO4•7H2O Concentration (g/L) | Spraying Time | |
|---|---|---|---|
| 2 Weeks BF | 2 Weeks AF | ||
| CK | 0.0 | √ | √ |
| 2A | 1.0 | √ | √ |
| 2B | 4.0 | √ | √ |
| 2C | 8.0 | √ | √ |
3.3. Determination of Photosynthesis
3.4. Berry Sample Collection
3.5. Berry Weight, Total Soluble Solids, pH and Titratable Acidity of Berry Juice
3.6. Sample Preparation for Phenolics Analysis
3.7. Analysis of Phenolics in Skins of Grape Berries
3.8. Analysis of Transcriptional Level by Real-Time PCR
3.9. Presentation of Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U.; Clemens, S. Functions and homeostasis of zinc, copper, and nickel in plants. In Molecular Biology of Metal Homeostasis and Detoxification; Springer: Berlin, Germany, 2006; pp. 215–271. [Google Scholar]
- Alloway, B.J. Fundamental Aspects. In Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium, 2004; pp. 30–35. [Google Scholar]
- Srivastava, A.K.; Singh, S. Zinc Nutrition in “Nagpur” Mandarin on Haplustert. J. Plant Nutr. 2009, 32, 1065–1081. [Google Scholar] [CrossRef]
- Yang, X.-E.; Chen, W.-R.; Feng, Y. Improving human micronutrient nutrition through biofortification in the soil-plant system: China as a case study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Gatti, M.; Fregoni, M. Nutritional Deficiencies. In Methodologies and Results in Grapevine Research; Springer: Berlin, Germany, 2010; pp. 165–191. [Google Scholar]
- Li, H.; Wang, Y.J.; Meng, J.; Wang, H.; You, J.; Huo, X.S.; Wang, Y.Q. The Effect of Climate Change on the Climatic Zoning for Wine Grapes in China. Acta Hortic. Sin. 2009, 36, 313–320. [Google Scholar]
- Sun, Q.; Zhang, X.Y.; Wang, Z.P.; Wang, J.F.; Ma, K. Trace elements distribution in the soil profile of wine grape base at the eastern foot of Helan mountain of Ningxia. Sino Overseas Grapevine Wine 2008, 2, 4–8. [Google Scholar]
- Xiao, L.G.; Zhang, J.M. Assessment of Soil Comprehensive Nutrients Status in Irrigation District of Yellow River Based on GIS. Res. Soil Water Conserv. 2010, 17, 273–276. [Google Scholar]
- Fawzi, A.F.A.; Elfouly, M.M.; Moubarak, Z.M. The Need of Grain Legumes for Iron, Manganese, and Zinc Fertilization Under Egyptian Soil-Conditions—Effect and Uptake of Metalosates. J. Plant Nutr. 1993, 16, 813–823. [Google Scholar] [CrossRef]
- Pandey, N.; Gupta, B.; Pathak, G.C. Enhanced yield and nutritional enrichment of seeds of Pisum sativum L. through foliar application of zinc. Sci. Hortic. 2013, 164, 474–483. [Google Scholar] [CrossRef]
- Saadati, S.; Moallemi, N.; Mortazavi, S.M.H.; Seyyednejad, S.M. Effects of zinc and boron foliar application on soluble carbohydrate and oil contents of three olive cultivars during fruit ripening. Sci. Hortic. 2013, 164, 30–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.-X.; Tan, Q.-L.; Zheng, C.-S.; Gui, H.-P.; Zeng, W.-N.; Sun, X.-C.; Zhao, X.-H. Plant nutrition status, yield and quality of satsuma mandarin (Citrus unshiu Marc.) under soil application of Fe-EDDHA and combination with zinc and manganese in calcareous soil. Sci. Hortic. 2014, 174, 46–53. [Google Scholar] [CrossRef]
- Yamdagni, R.; Singh, D.; Jindal, P. Note on the effect of zinc sprays on yield and quality of Thompson Seedless grapes. Indian J. Agric. Res. 1979, 13, 117–118. [Google Scholar]
- Amiri, M.E.; Fallahi, E. Influence of mineral nutrients on growth, yield, berry quality, and petiole mineral nutrient concentrations of table grape. J. Plant Nutr. 2007, 30, 463–470. [Google Scholar] [CrossRef]
- Aydin, S.; Yagmur, B.; Hakerlerler, H.; Coban, H. Effects of different types and levels of zinc sulphate applications in vineyards (Vitis vinifera L.) in a semi-arid environment. Asian J. Chem. 2007, 19, 555–563. [Google Scholar]
- Bybordi, A.; Shabanov, J.A. Effects of the Foliar Application of Magnesium and Zinc on the Yield and Quality of Three Grape Cultivars Grown in the Calcareous Soils of Iran. Not. Sci. Biol. 2010, 2, 81–86. [Google Scholar]
- Er, F.; Gezgin, S.; Bayrakli, F. The effect of different zinc application methods and levels on yield and quality of hesapali (Vitis vinifera L.) grape. Bulg. J. Agric. Sci. 2009, 15, 410–416. [Google Scholar]
- Bao, S.-D. Soil Agricultural Chemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Gianquinto, G.; Abu-Rayyan, A.; Di Tola, L.; Piccotino, D.; Pezzarossa, B. Interaction effects of phosphorus and zinc on photosynthesis, growth and yield of dwarf bean grown in two environments. Plant Soil 2000, 220, 219–228. [Google Scholar] [CrossRef]
- Uygur, V.; Rimmer, D.L. Reactions of zinc with iron-oxide coated calcite surfaces at alkaline pH. Eur. J. Soil Sci. 2000, 51, 511–516. [Google Scholar] [CrossRef]
- Lopez-Millan, A.F.; Ellis, D.R.; Grusak, M.A. Effect of zinc and manganese supply on the activities of superoxide dismutase and carbonic anhydrase in Medicago truncatula wild type and raz mutant plants. Plant Sci. 2005, 168, 1015–1022. [Google Scholar] [CrossRef]
- Welch, R.M. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Kerridge, G.H.; Ruhl, E.H.; Nicholas, P.R. Shiraz berry size in relation to seed number and implications for juice and wine composition. Aust. J. Grape Wine Res. 2005, 11, 2–8. [Google Scholar] [CrossRef]
- Williams, L.E.; Ayars, J.E. Water use of Thompson Seedless grapevines as affected by the application of gibberellic acid (GA3) and trunk girdling—practices to increase berry size. Agric. For. Meteorol. 2005, 129, 85–94. [Google Scholar] [CrossRef]
- Kohno, M.; Takato, H.; Horiuchi, H.; Fujita, K.; Suzuki, S. Auxin-nonresponsive grape Aux/IAA19 is a positive regulator of plant growth. Mol. Biol. Rep. 2012, 39, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, C.; Keyzers, R.A.; Boss, P.K.; Davies, C. Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3–1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J. Exp. Bot. 2010, 61, 3615–3625. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.D.S.; de Souza Veras Silani, I.; Toaldo, I.M.; Correa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagne, S.; Saucier, C.; Geny, L. Composition and cellular localization of tannins in Cabernet Sauvignon skins during growth. J. Agric. Food Chem. 2006, 54, 9465–9471. [Google Scholar] [CrossRef] [PubMed]
- Hanlin, R.L.; Downey, M.O. Condensed Tannin Accumulation and Composition in Skin of Shiraz and Cabernet Sauvignon Grapes during Berry Development. Am. J. Enol. Vitic. 2009, 60, 13–23. [Google Scholar]
- Darne, G. New Hypotheses on Anthocyanin Biosynthesis in Berries and Leaves of The Grapevine. Vitis 1993, 32, 77–85. [Google Scholar]
- Kaya, C.; Higgs, D. Response of tomato (Lycopersicon esculentum L.) cultivars to foliar application of zinc when grown in sand culture at low zinc. Sci. Hortic. 2002, 93, 53–64. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.H.; Xin, H.P.; Wang, N.; Guan, L.; Wu, B.H.; Li, S.H. Anthocyanin profile and gene expression in berry skin of two red Vitis vinifera grape cultivars that are sunlight dependent versus sunlight independent. Aust. J. Grape Wine Res. 2013, 19, 238–248. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.B.; Howard, S.; Chen, S.; Wang, Y.; Yu, O.; Kovacs, L.G.; Qiu, W. Berry skin development in Norton grape: Distinct patterns of transcriptional regulation and flavonoid biosynthesis. BMC Plant Biol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Ge, H.; Howard, S.; Qiu, W. Transcriptional expression of Stilbene synthase genes are regulated developmentally and differentially in response to powdery mildew in Norton and Cabernet Sauvignon grapevine. Plant Sci. 2012, 197, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.L.; Wan, S.B.; Zhang, J.H.; Zhang, P.; Zhan, J.C.; Huang, W.D. Chalcone isomerase in grape vine: Gene expression and localization in the developing fruit. Biol. Plant. 2012, 56, 545–550. [Google Scholar] [CrossRef]
- Muir, S.R.; Collins, G.J.; Robinson, S.; Hughes, S.; Bovy, A.; de Vos, C.H.R.; van Tunen, A.J.; Verhoeyen, M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 2001, 19, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.K.; Shirley, B.W. Analysis of flavanone 3-hydroxylase in Arabidopsis seedlings—Coordinate regulation with chalcone synthase and chalcone isomerase. Plant Physiol. 1996, 111, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The Grapevine R2R3-MYB Transcription Factor VvMYBF1 Regulates Flavonol Synthesis in Developing Grape Berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 2003, 9, 110–121. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Fan, W.J.; Li, H.; Yang, J.; Huang, J.R.; Zhang, P. Functional Characterization of Dihydroflavonol-4-Reductase in Anthocyanin Biosynthesis of Purple Sweet Potato Underlies the Direct Evidence of Anthocyanins Function against Abiotic Stresses. PLoS One 2013, 8, e78484. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Peinado, J.; de Lerma, N.L.; Moreno, J.; Peinado, R.A. Antioxidant activity of different phenolics fractions isolated in must from Pedro Ximenez grapes at different stages of the off-vine drying process. Food Chem. 2009, 114, 1050–1055. [Google Scholar] [CrossRef]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Meng, J.; Fang, Y.; Gao, J.; Qiao, L.; Zhang, A.; Guo, Z.; Qin, M.; Huang, J.; Hu, Y.; Zhuang, X. Phenolics Composition and Antioxidant Activity of Wine Produced from Spine Grape (Vitis davidii Foex) and Cherokee Rose (Rosa laevigata Michx.) Fruits from South China. J. Food Sci. 2012, 77, C8–C14. [Google Scholar] [CrossRef] [PubMed]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the catechin hydrate, rutin, and methyl cellulose are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.-Z.; Liu, M.-Y.; Meng, J.-F.; Chi, M.; Xi, Z.-M.; Zhang, Z.-W. Promoting Effect of Foliage Sprayed Zinc Sulfate on Accumulation of Sugar and Phenolics in Berries of Vitis vinifera cv. Merlot Growing on Zinc Deficient Soil. Molecules 2015, 20, 2536-2554. https://doi.org/10.3390/molecules20022536
Song C-Z, Liu M-Y, Meng J-F, Chi M, Xi Z-M, Zhang Z-W. Promoting Effect of Foliage Sprayed Zinc Sulfate on Accumulation of Sugar and Phenolics in Berries of Vitis vinifera cv. Merlot Growing on Zinc Deficient Soil. Molecules. 2015; 20(2):2536-2554. https://doi.org/10.3390/molecules20022536
Chicago/Turabian StyleSong, Chang-Zheng, Mei-Ying Liu, Jiang-Fei Meng, Ming Chi, Zhu-Mei Xi, and Zhen-Wen Zhang. 2015. "Promoting Effect of Foliage Sprayed Zinc Sulfate on Accumulation of Sugar and Phenolics in Berries of Vitis vinifera cv. Merlot Growing on Zinc Deficient Soil" Molecules 20, no. 2: 2536-2554. https://doi.org/10.3390/molecules20022536





