Preparation of a Titania/X-Zeolite/Porous Glass Composite Photocatalyst Using Hydrothermal and Drop Coating Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystalline Phases of the Composites
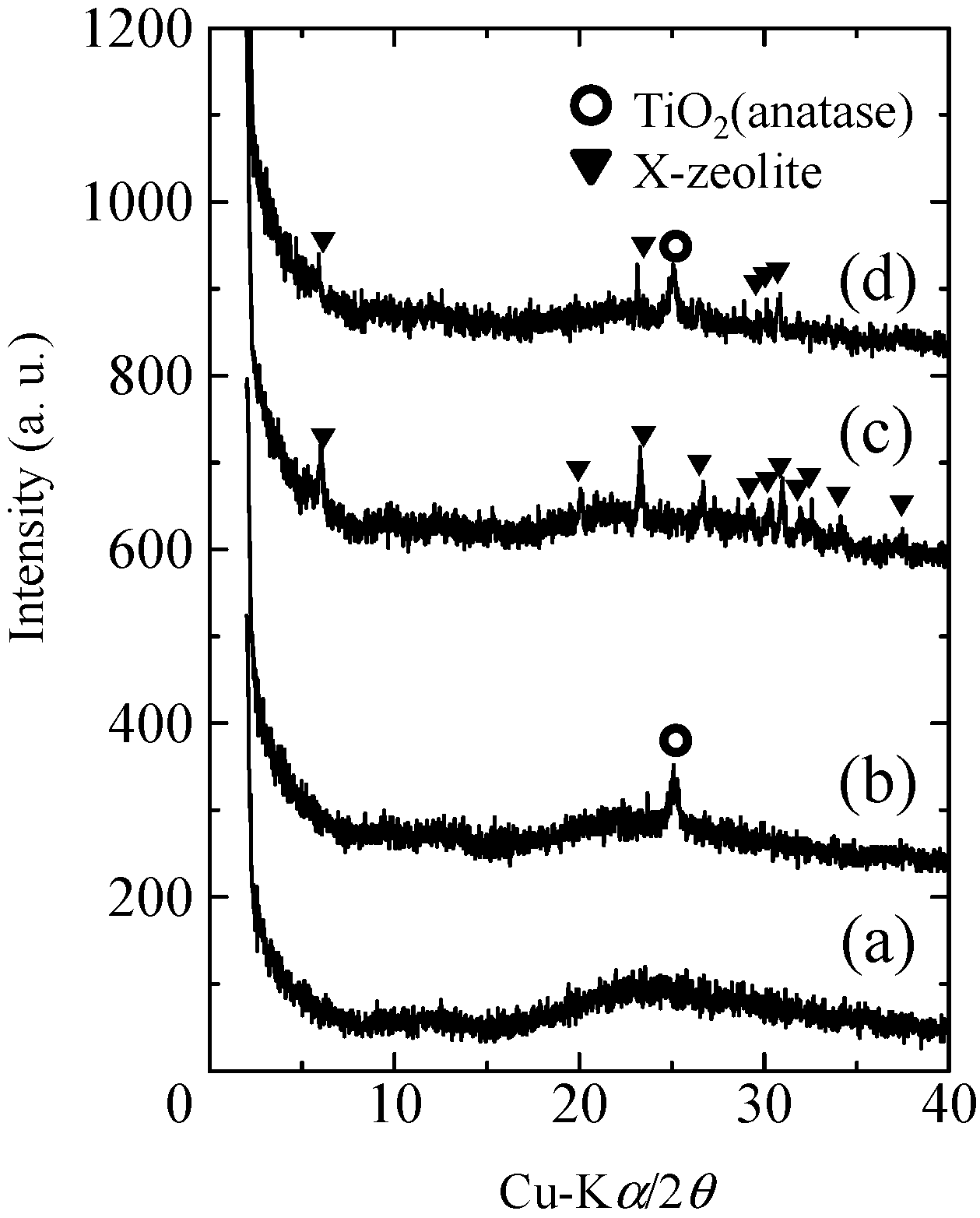
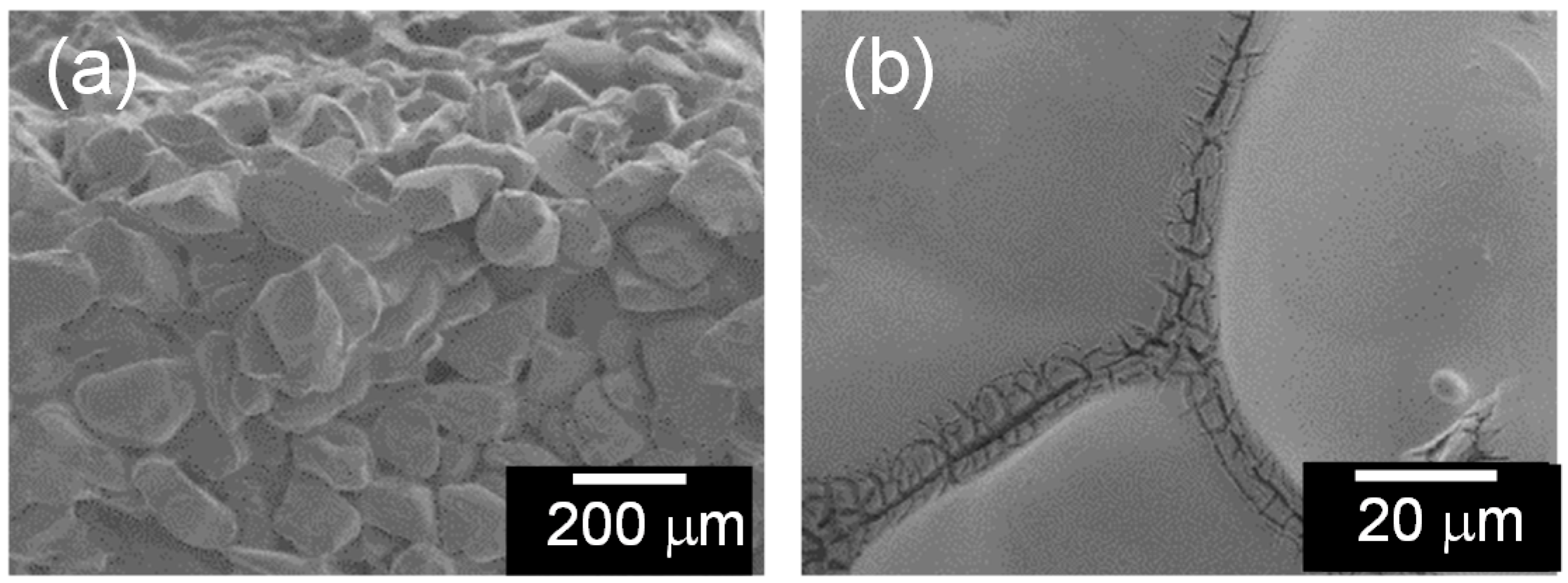
2.2. Micro Textures of the Composites
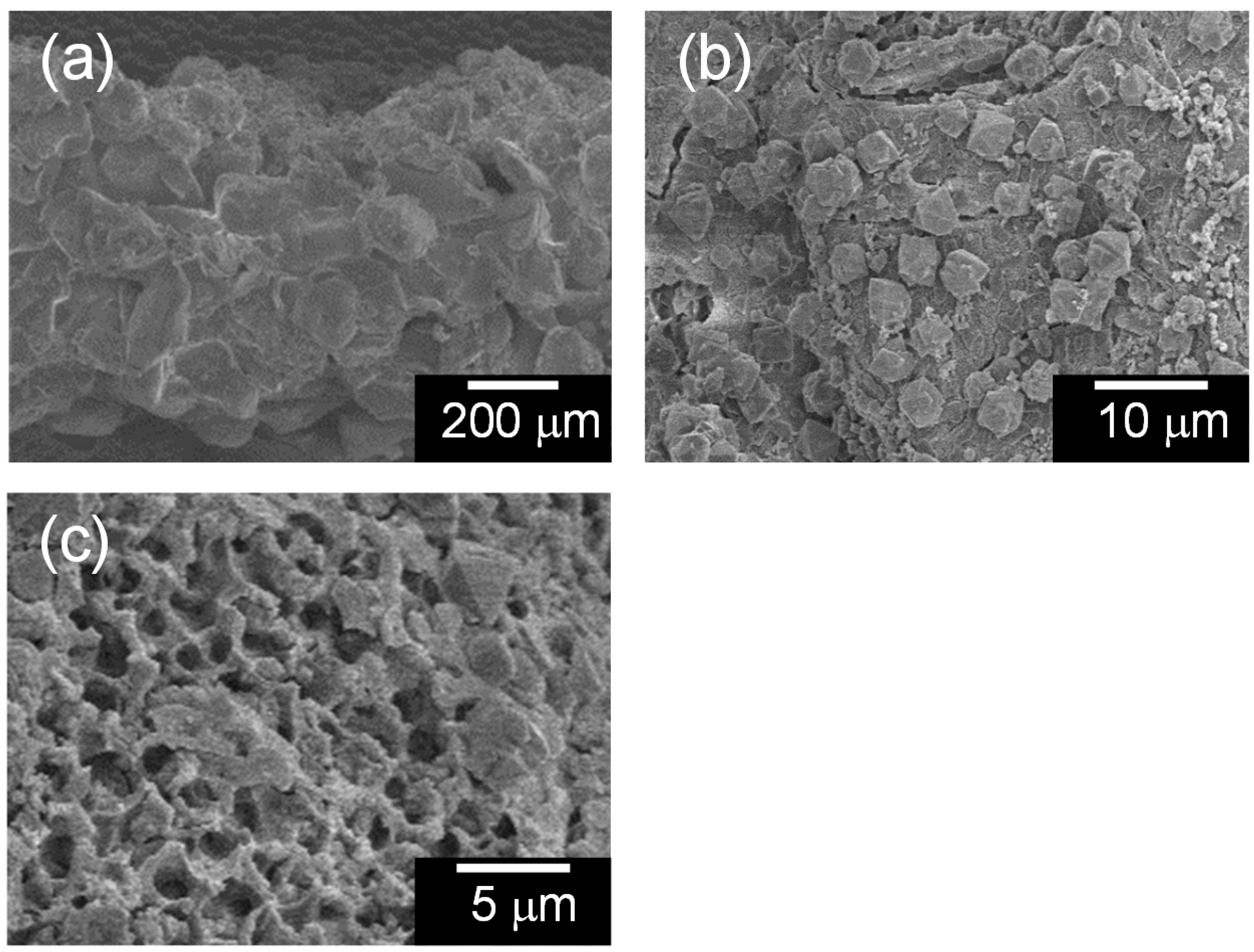
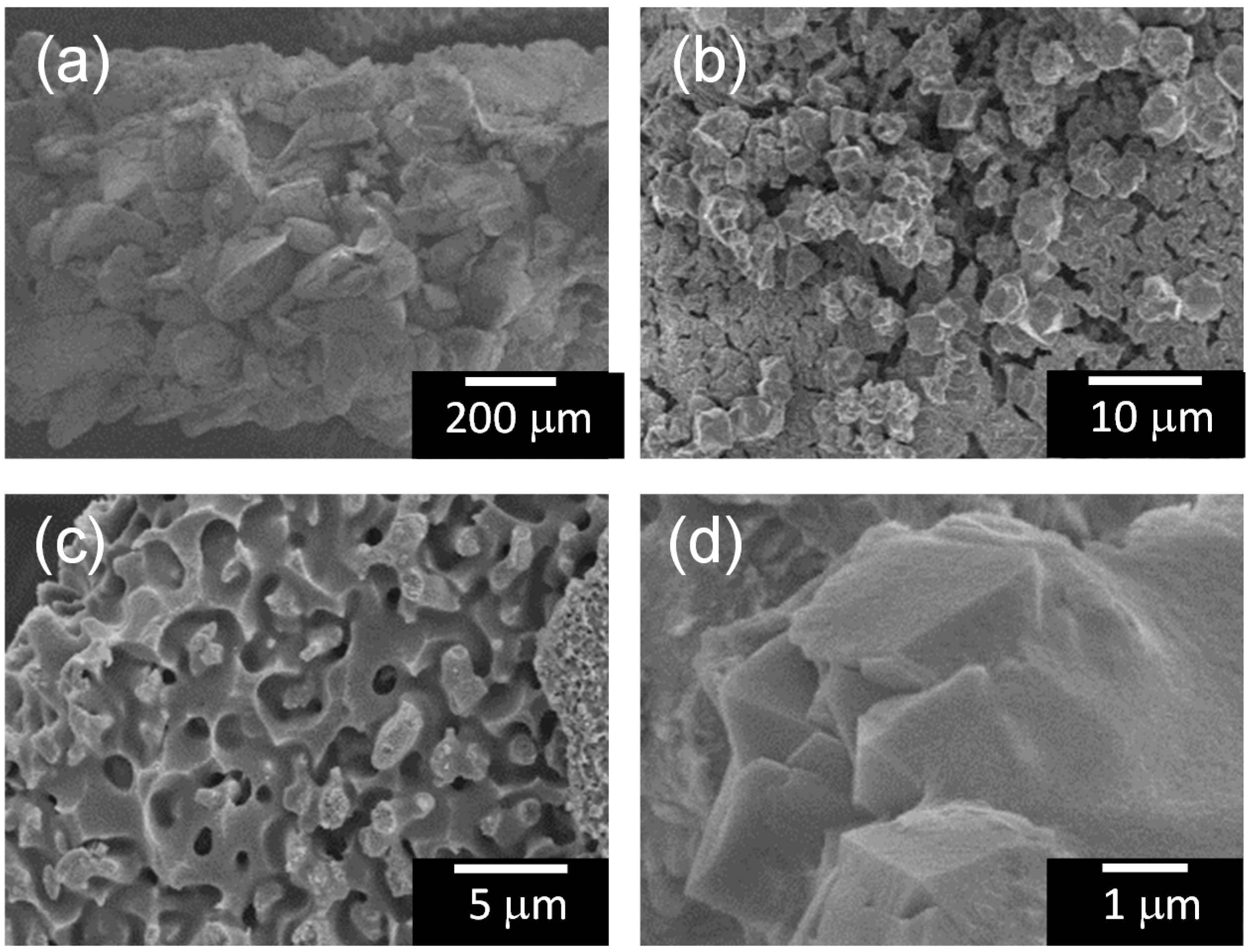
2.3. Adsorption Characteristics of the Composites
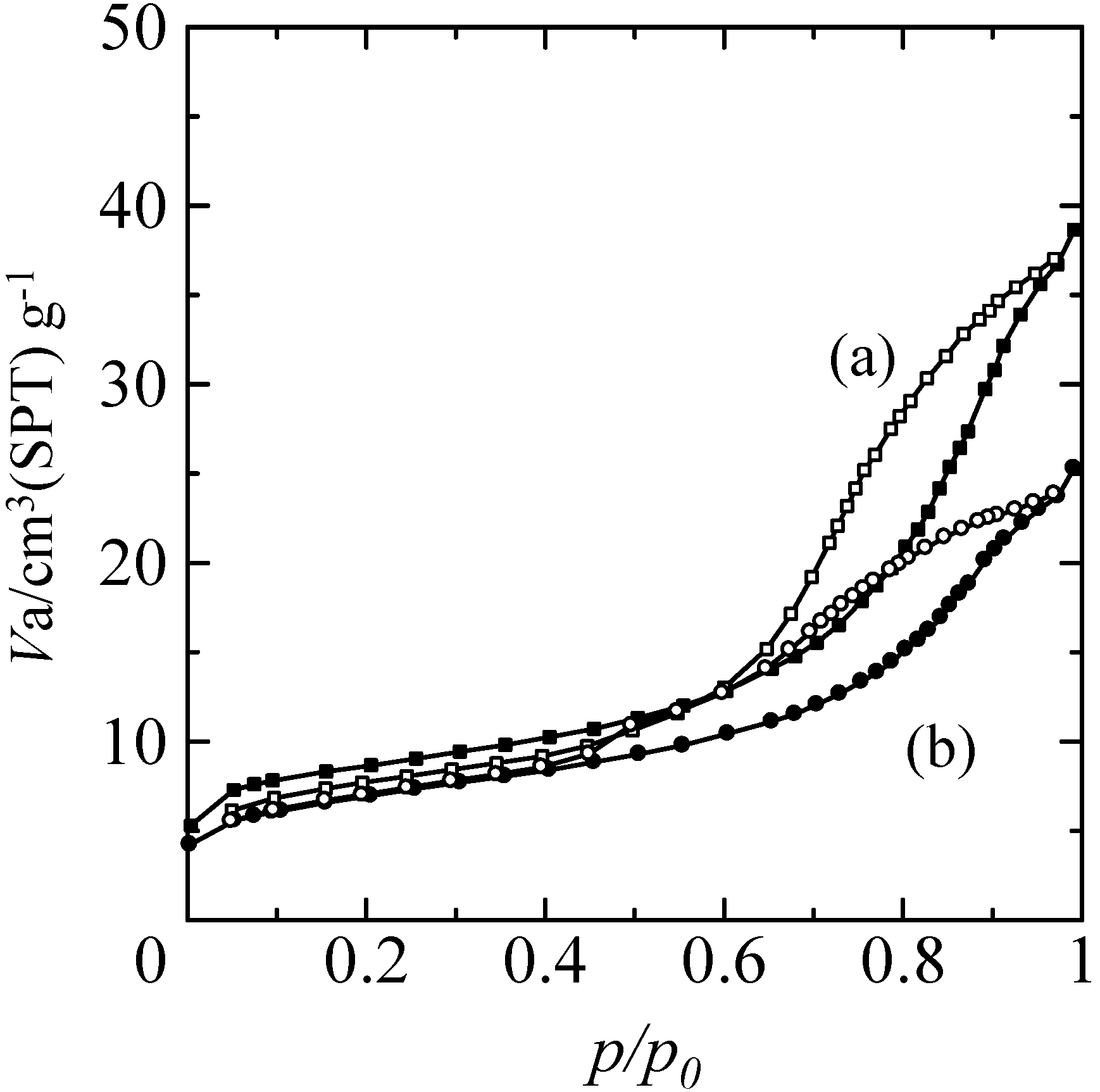
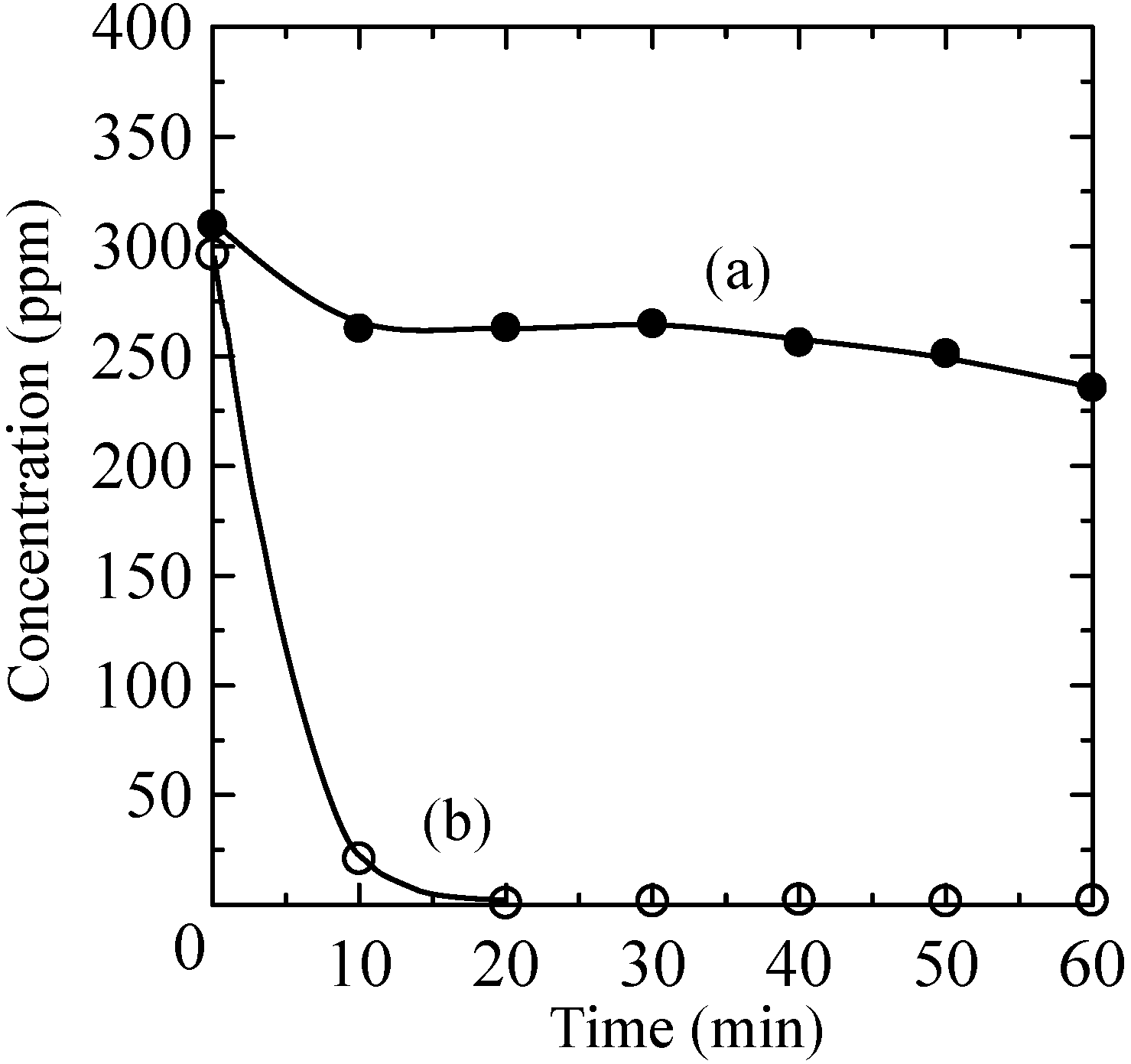
2.4. Photocatalytic Properties of the Composites


3. Experimental Section
3.1. Preparation of Partially Sintered Glass Supports
3.2. Synthesis of the Zeolite on the Partially Sintered Glass Support
3.3. TiO2 Coating of the Samples
3.4. Characterizations of the Samples
3.5. Gas Adsorption Ability and Photocatalytic Activity of the Samples
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Peral, J.; Ollis, D.F. Heterogeneous photocatalytic oxidation of gas-phase organics for air purification Acetone, 1-butanol, butyraldehyde, formaldehyde, and m-xylene oxidation. J. Catal. 1992, 136, 554–565. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photchem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium Dioxide Photocatalysis in Atmospheric Chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.F.; Kim, J.T. Photocatalytic Oxidation for Maintenance of Indoor Environmental Quality. Indoor Built Environ. 2013, 22, 39–51. [Google Scholar] [CrossRef]
- Chapter 4: Summary of the guidelines. In Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Office for Europe, WHO Regional Publications, European Series: Copenhagen, Demark, 2000; pp. 32–33.
- Shaham-Waldmann, N.; Paz, Y. Modified Photocatalysts. In Photocatalysis and Water Purification; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 107–111. [Google Scholar]
- Kato, K. Photocatalytic Property of TiO2 Anchored on Porous Alumina Ceramic Support by the Alkoxide Method. J. Ceram. Soc. Jpn. 1993, 101, 245–249. [Google Scholar] [CrossRef]
- Sauer, M.L.; Ollis, D.F. Acetone Oxidation in a Photocatalytic Monolith Reactor. J. Catal. 1994, 149, 81–91. [Google Scholar] [CrossRef]
- Obee, T.N.; Brown, R.T. TiO2 Photocatalysis for Indoor Air Applications Effects of Humidity and Trace Contaminant Levels on the Oxidation Rates of Formaldehyde, Toluene, and 1,3-Butadiene. Environ. Sci. Technol. 1995, 29, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Yasumori, A.; Ishizu, K.; Hayashi, S.; Okada, K. Preparation of a TiO2 based multiple layer thin film photocatalyst. J. Mater. Chem. 1998, 8, 2521–2524. [Google Scholar] [CrossRef]
- Yasumori, A.; Shinoda, H.; Kameshima, Y.; Hayashi, S.; Okada, K. Photocatalytic and photoelectrochemical propertiesof TiO2-based multiple layer thin film prepared by sol–gel and reactive-sputtering methods. J. Mater. Chem. 2001, 11, 1253–1257. [Google Scholar] [CrossRef]
- Anpo, M.; Aikawa, N.; Kubokawa, Y. Photoluminescence and photocatalytic activity of highly dispersed titanium oxide anchored onto porous Vycor glass. J. Phys. Chem. 1985, 89, 5017–5021. [Google Scholar] [CrossRef]
- Yamashita, H.; Honda, M.; Harada, M.; Ichihashi, Y.; Anpo, M.; Hirao, T.; Itoh, N.; Iwamoto, N. Preparation of Titanium Oxide Photocatalysts Anchored on Porous Silica Glass by a Metal Ion-Implantation Method. J. Phys. Chem. B 1998, 102, 10707–10711. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.C. Photocatalyst TiO2 supported on glass fiber for indoor air purification effect of NO on the photodegradation of CO and NO2. J. Photochem. Photobiol. A Chem. 2003, 156, 171–177. [Google Scholar] [CrossRef]
- Ho, W.H.; Yu, J.C.; Yu, J. Photocatalytic TiO2/Glass Nanoflake Array Films. Langmuir 2005, 21, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Machida, F.; Daiko, Y.; Mineshige, A.; Kobune, M.; Toyoda, N.; Yamada, I.; Yazawa, T. Structures and Photocatalytic Properties of Crystalline Titanium Oxide-Dispersed Nanoporous Glass-Ceramics. J. Am. Ceram. Soc. 2010, 93, 461–464. [Google Scholar] [CrossRef]
- Takeda, N.; Torimoto, T.; Sampath, S.; Kuwabata, S.; Yoneyama, H. Effect of Inert Supports for Titanium Dioxide Loading on Enhancement of Photodecomposition Rate of Gaseous Propionaldehyde. J. Phys. Chem. 1995, 99, 9986–9991. [Google Scholar] [CrossRef]
- Torimoto, T.; Okawa, Y.; Takeda, N.; Yoneyama, H. Effect of Activated Carbon Content in TiO2-loaded Activated Carbon on Photodegradation Behaviors of Dichloromethane. J. Photochem. Photobiol. A Chem. 1997, 103, 153–157. [Google Scholar] [CrossRef]
- Huang, B.; Saka, S. Photocatalytic activity of TiO2 crystallite-activated carbon composites prepared in supercritical isopropanol for the decomposition of formaldehyde. J. Wood Sci. 2003, 49, 79–85. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Combination effect of activated carbon with TiO2 for the photodegradation of binary pollutants at typical indoor air level. J. Photochem. Photobiol. A Chem. 2004, 161, 131–140. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Indoor air purification by photocatalyst TiO2 immobilized on an activated carbon filter installed in an air cleaner. Chem. Eng. Sci. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-Based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Carbon Support. J. Phys. Chem. Lett. 2010, 1, 520–527. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.; Fu, X.; Xu, Y. TiO2−Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2−Graphene Truly Different from Other TiO2−Carbon Composite Materials. ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Yamahana, D.; Yamashita, H. Graphene Coating of TiO2 Nanoparticles Loaded on Mesoporous Silica for Enhancement of Photocatalytic Activity. J. Phys. Chem. C 2011, 114, 15049–15053. [Google Scholar] [CrossRef]
- Domen, K.; Sakata, Y.; Kudo, A.; Maruya, K.; Onishi, T. The Photocatalytic Activity of a Platinized Titanium Dioxide Catalyst Supported over Silica. Bull. Chem. Soc. Jpn. 1988, 61, 359–362. [Google Scholar] [CrossRef]
- Yasumori, A.; Yamazaki, K.; Shibata, S.; Yamane, M. Preparation of TiO2 Fine Particles Supported on Silica Gel as Photocatalyst. J. Ceram. Soc. Jpn. 1994, 102, 702–707. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. An Improved Photocatalyst of TiO2/SiO2 Prepared by a Sol-Gel Synthesis. J. Phys. Chem. 1995, 99, 9882–9885. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Lu, G.Q.; Yue, P.; Greenfield, P.F. Novel Silica Gel Supported TiO2 Photocatalyst Synthesized by CVD Method. Langmuir 2000, 16, 6216–6222. [Google Scholar] [CrossRef]
- Ismail, A.A.; Ibrahim, I.A.; Ahmed, M.S.; Mohamed, R.M.; El-Shall, H. Sol–gel synthesis of titania–silica photocatalyst for cyanide photodegradation. J. Photochem. Photobiol. A Chem. 2004, 163, 445–451. [Google Scholar] [CrossRef]
- Yu, J.C.; Wang, X.; Fu, X. Pore-Wall Chemistry and Photocatalytic Activity of Mesoporous Titania Molecular Sieve Films. Chem. Mater. 2004, 16, 1523–1530. [Google Scholar] [CrossRef]
- Li, H.; Bian, Z.; Zhu, J.; Huo, Y.; Li, H.; Lu, Y. Mesoporous Au-TiO2 Nanocomposites with Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 4538–4539. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.K.; Kumar, M.K.; An, W.W.; Gao, H. Transparent, Well-Aligned TiO2 Nanotube Arrays with Controllable Dimensions on Glass Substrates for Photocatalytic Applications. Appl. Mater. Interfaces 2010, 2, 498–503. [Google Scholar] [CrossRef]
- Chu, S.; Inoue, S.; Wada, K.; Li, D.; Haneda, H.; Awatsu, S. Highly Porous (TiO2-SiO2-TeO2)/Al2O3/TiO2 Composite Nanostructures on Glass with Enhanced Photocatalysis Fabricated by Anodization and Sol-Gel Process. J. Phys. Chem. B 2003, 107, 6586–6589. [Google Scholar] [CrossRef]
- Qian, X.F.; Kamegawa, T.; Mori, K.; Li, H.X.; Yamashita, H. Calcium Phosphate Coatings Incorporated in Mesoporous TiO2/SBA-15 by a Facile Inner-pore Sol-gel Process toward Enhanced Adsorption-photocatalysis Performances. J. Phys. Chem. C 2013, 117, 19544–19551. [Google Scholar] [CrossRef]
- Sircar, S.; Myers, A.L. 22 Gas Separation by Zeolites. Part V Applications. In Handbook of Zeolite Science and Technology; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; CRC Press: New York, NY, USA, 2003; pp. 1063–1104. [Google Scholar]
- Corma, A.; Garcia, H. Zeolite-based photocatalysts. Chem. Commun. 2004, 1443–1459. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamashita, H. Efficient photocatalytic degradation of organics diluted in water and air using TiO2 designed with zeolites and mesoporous silica materials. J. Mater. Chem. 2011, 21, 2407–2416. [Google Scholar] [CrossRef]
- Sampath, S.; Uchida, H.; Yoneyama, H. Photocatalytic Degradation of Gaseous Pyridine over Zeolite-Supported Titanium Dioxide. J. Catal. 1994, 149, 189–194. [Google Scholar] [CrossRef]
- Torimoto, T.; Ito, S.; Kuwabata, S.; Yoneyama, H. Effects of Adsorbents Used as Supports for Titanium Dioxide Loading on Photocatalytic Degradation of Propyzamide. Environ. Sci. Technol. 1996, 30, 1275–1281. [Google Scholar] [CrossRef]
- Xu, Y.; Langford, C.H. Photoactivity of Titanium Dioxide Supported on MCM41, Zeolite X, and Zeolite Y. J. Phys. Chem. B 1997, 101, 3115–3121. [Google Scholar] [CrossRef]
- Ichiura, H.; Kitaoka, T.; Tanaka, H. Preparation of composite TiO2-zeolite sheets using a papermaking technique and their application to environmental improvement Part I Removal of acetaldehyde with and without UV irradiation. J. Mater. Sci. 2002, 37, 2937–2941. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kimura, T.; Hidaka, M.; Rakhmawaty, D.; Anpo, M. Photocatalytic oxidation of acetaldehyde with oxygen on TiO2/ZSM-5 photocatalyst: Effect of hydrophobicity of zeolites. J. Catal. 2007, 246, 235–240. [Google Scholar] [CrossRef]
- Donphai, W.; Kamegawa, T.; Careonpanich, M.; Nueangnoraj, K.; Mishihara, H.; Kyotani, T.; Yamashita, H. Photocatalytic performance of TiO2–zeolite templated carbon composites in organic contaminant degradation. Phys. Chem. Chem. Phys. 2014, 16, 25004–25007. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Ishiguro, Y.; Kido, R.; Yamashita, H. Design of Composite Photocatalyst of TiO2 and Y-Zeolite for Degradation of 2-Propanol in the Gas Phase under UV and Visible Light Irradiation. Molecules 2014, 19, 16477–16488. [Google Scholar] [CrossRef] [PubMed]
- Ichiura, H.; Kitaoka, T.; Tanaka, H. Preparation of composite TiO2-zeolite sheets using a papermaking technique and their application to environmental improvement, Part II Effect of zeolite coexisting in the composite sheet on NOx removal. J. Mater. Sci. 2003, 38, 1611–1615. [Google Scholar] [CrossRef]
- Jan, Y.; Lin, L.; Karthik, M.; Bai, H. Titanium Dioxide/Zeolite Catalytic Adsorbent for the Removal of NO and Acetone Vapors. J. Air Waste Manag. Assoc. 2009, 59, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.F. Introduction to the Structural Chemistry of Zeolites. Part II Synthesis and Structure. In Handbook of Zeolite Science and Technology; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; CRC Press: New York, NY, USA, 2003; pp. 65–89. [Google Scholar]
- Ohko, Y.; Kashimoto, K.; Fujishima, A. Kinetics of Photocatalytic Reactions under Extremely Low-Intensity UV Illumination on Titanium Dioxide Thin Films. J. Phys. Chem. A 1997, 101, 8057–8062. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Inumaru, K.; Oumi, Y.; Sano, T.; Yamanaka, S. Photocatalytic decomposition of 2-propanol in air by mechanical mixtures of TiO2 crystalline particles and silicalite adsorbent: The complete conversion of organic molecules strongly adsorbed within zeolitic channels. Microporous Mesoporous Mater. 2009, 117, 350–355. [Google Scholar] [CrossRef]
- Robert, D.; Keller, V.; Keller, N. Immobilization of a Semiconductor Photocatalyst on Solid Suppors: Methods, Materials, and Applications. In Photocatalysis and Water Purification; Pichat, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 145–178. [Google Scholar]
- Yazawa, T.; Tanaka, H.; Eguchi, K.; Yokoyama, S. Novel alkali-resistance porous glass prepared from a mother glass based on the SiO2-B2O3-RO-ZrO2 (R=Mg, Ca, Sr, Ba and Zn) system. J. Mater. Sci. 1994, 29, 3433–3440. [Google Scholar] [CrossRef]
- Subotić, B.; Bronić, J. 5 Theoretical and Practical Aspects of Zeolite Crystal Growth. Part II Synthesis and Structure. In Handbook of Zeolite Science and Technology; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; CRC Press: New York, NY, USA, 2003; pp. 129–203. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquěrol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1986, 57, 603–619. [Google Scholar]
- Yang, Y.; Burke, N.; Zhang, J.; Huang, S.; Lim, S.; Zhu, Y. Influence of charge compensating cations on propane adsorption in X zeolites: Experimental measurement and mathematical modeling. RSC Adv. 2014, 4, 7279–7287. [Google Scholar] [CrossRef]
- Bickley, R.I.; Munuera, G.; Stone, F.S. Photoadsorption and Photocatalysis at Rutile Surfaces II. Photocatalytic Oxidation of Isopropanol. J. Catal. 1973, 31, 398–407. [Google Scholar] [CrossRef]
- Raillard, C.; Héquet, V.; Cloirec, P.L.; Legrand, J. Kinetic study of ketones photocatalytic oxidation in gas phase using TiO2-containing paper: Effect of water vapor. J. Photochem. Photobiol. A Chem. 2004, 163, 425–431. [Google Scholar] [CrossRef]
- Cheung, O.; Hedin, N. Zeolites and related sorbents with narrow pores for CO2 separation from flue gas. RSC Adv. 2014, 4, 14480–14494. [Google Scholar] [CrossRef]
- Doremus, R.H. 4 Phase Separation. Part One Formation and Structure of Glasses. In Glass Science; John Wiley & Sons: New York, NY, USA, 1973; pp. 44–73. [Google Scholar]
- Kűhl, G.H. Crystallization of low-silica faujasite (SiO2/Al2O3~2.0). Zeolites 1987, 7, 451–457. [Google Scholar] [CrossRef]
- Sample Availability: Melt-quenched glass samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasumori, A.; Yanagida, S.; Sawada, J. Preparation of a Titania/X-Zeolite/Porous Glass Composite Photocatalyst Using Hydrothermal and Drop Coating Processes. Molecules 2015, 20, 2349-2363. https://doi.org/10.3390/molecules20022349
Yasumori A, Yanagida S, Sawada J. Preparation of a Titania/X-Zeolite/Porous Glass Composite Photocatalyst Using Hydrothermal and Drop Coating Processes. Molecules. 2015; 20(2):2349-2363. https://doi.org/10.3390/molecules20022349
Chicago/Turabian StyleYasumori, Atsuo, Sayaka Yanagida, and Jun Sawada. 2015. "Preparation of a Titania/X-Zeolite/Porous Glass Composite Photocatalyst Using Hydrothermal and Drop Coating Processes" Molecules 20, no. 2: 2349-2363. https://doi.org/10.3390/molecules20022349




