Dihydrochalcone Compounds Isolated from Crabapple Leaves Showed Anticancer Effects on Human Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Separation and Purification of Dihydrochalcone Monomers in Malus Crabapples var. “Radiant”
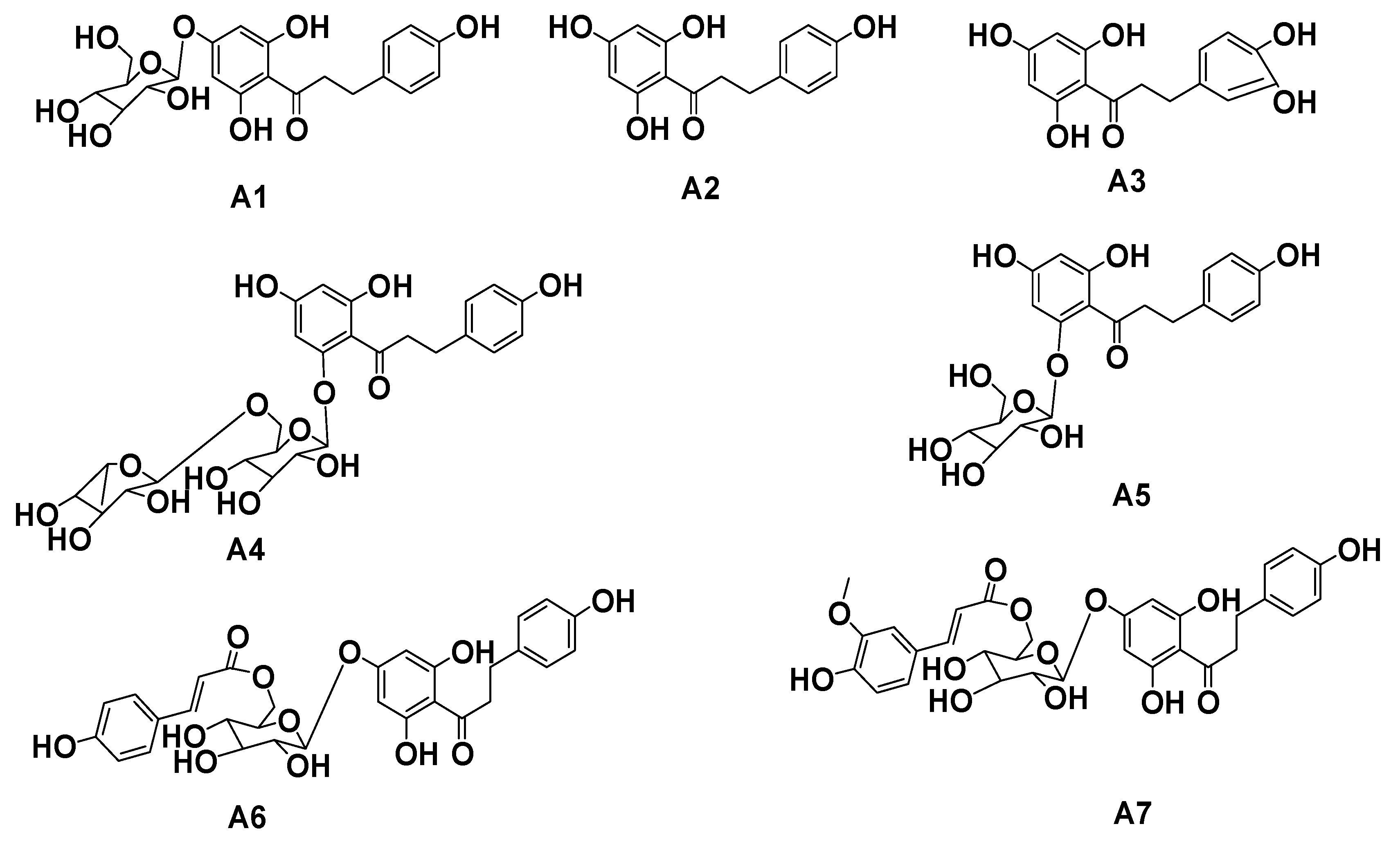
| C | A1 | A2 | A3 | A4 | A5 |
|---|---|---|---|---|---|
| 1 | 133.82 | 132.6 | 133.43 | 132.51 | 132.47 |
| 2 | 130.3 | 128.93 | 114.89 | 129.04 | 128.99 |
| 3 | 116.1 | 114.7 | 143.9 | 114.71 | 114.68 |
| 4 | 156.43 | 155.03 | 144.69 | 154.93 | 154.97 |
| 5 | 116.1 | 114.7 | 115.16 | 114.71 | 114.68 |
| 6 | 130.3 | 128.93 | 119.24 | 139.04 | 128.99 |
| 1′ | 106.87 | 103.91 | 103.91 | 105.5 | 105.37 |
| 2′ | 165.32 | 164.74 | 164.44 | 166.12 | 166.17 |
| 3′ | 96.41 | 94.34 | 94.33 | 97.04 | 96.93 |
| 4′ | 164.96 | 164.44 | 164.72 | 164.44 | 164.51 |
| 5′ | 96.41 | 94.34 | 94.33 | 94.24 | 94.01 |
| 6′ | 165.32 | 164.74 | 164.44 | 160.84 | 160.91 |
| α | 47.49 | 45.93 | 45.85 | 45.54 | 45.58 |
| β | 31.79 | 30.09 | 30.23 | 29.46 | 29.43 |
| C=O | 207.01 | 205 | 205.03 | 205.2 | 205.15 |
| Sugar-1′′ | 101.49 | 100.87 | 100.66 | ||
| 2′′ | 74.61 | 75.79 | 73.3 | ||
| 3′′ | 78.24 | 78.08 | 77.08 | ||
| 4′′ | 71.13 | 70.95 | 69.67 | ||
| 5′′ | 77.88 | 73.32 | 77.01 | ||
| 6′′ | 62.36 | 66.28 | 61.01 | ||
| 1′′′ | 100.61 | ||||
| 2′′′ | 77.06 | ||||
| 3′′′ | 70.62 | ||||
| 4′′′ | 72.64 | ||||
| 5′′′ | 69.78 | ||||
| CH3 | 16.52 |
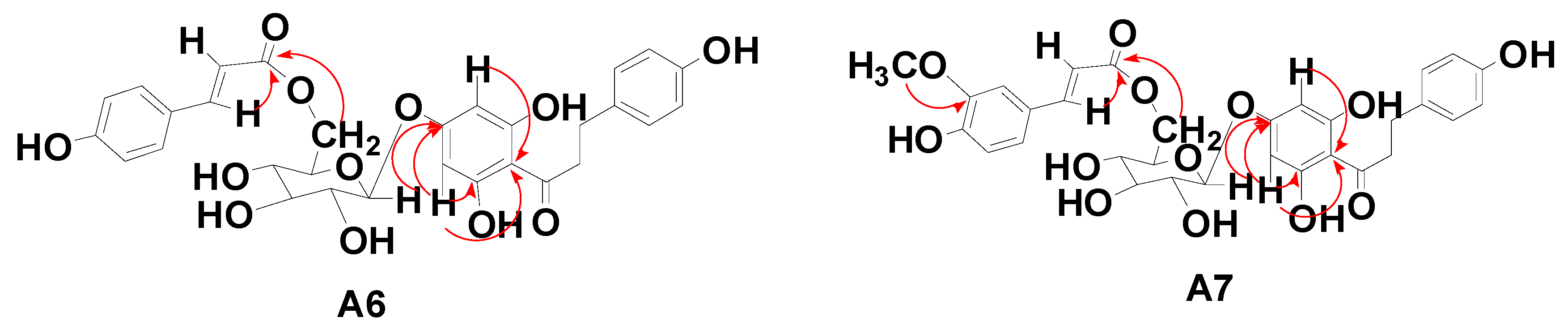
| C | A6 | A7 |
|---|---|---|
| 1 | 133.85 | 132.47 |
| 2 | 130.27 | 128.94 |
| 3 | 116.07 | 114.72 |
| 4 | 156.24 | 155.02 |
| 5 | 116.07 | 114.72 |
| 6 | 130.27 | 128.94 |
| 1′ | 106.87 | 105.52 |
| 2′ | 165.18 | 163.32 |
| 3′ | 96.47 | 95.14 |
| 4′ | 164.59 | 163.92 |
| 5′ | 96.47 | 95.14 |
| 6′ | 165.18 | 163.36 |
| α | 47.43 | 46.12 |
| β | 31.05 | 29.7 |
| C=O | 206.94 | 205.61 |
| Glc-1′′ | 100.7 | 99.49 |
| 2′′ | 74.51 | 74.28 |
| 3′′ | 78.83 | 76.56 |
| 4′′ | 71.79 | 70.51 |
| 5′′ | 75.52 | 73.21 |
| 6′′ | 64.56 | 63.28 |
| coumaroyl 1′′′ | 114.86 | 113.78 |
| 2′′′ | 127.15 | 126.35 |
| 3′′′ | 131.19 | 149.19 |
| 4′′′ | 161.03 | 147.89 |
| 5′′′ | 131.19 | 128.94 |
| 6′′′ | 127.15 | 114.99 |
| 7′′′ | 116.69 | 113.78 |
| 8′′′ | 146.85 | 145.73 |
| 9′′′ | 169.11 | 167.7 |
2.2. in Vitro Cytotoxicity of the Seven Dihydrochalcone Monomer Compounds
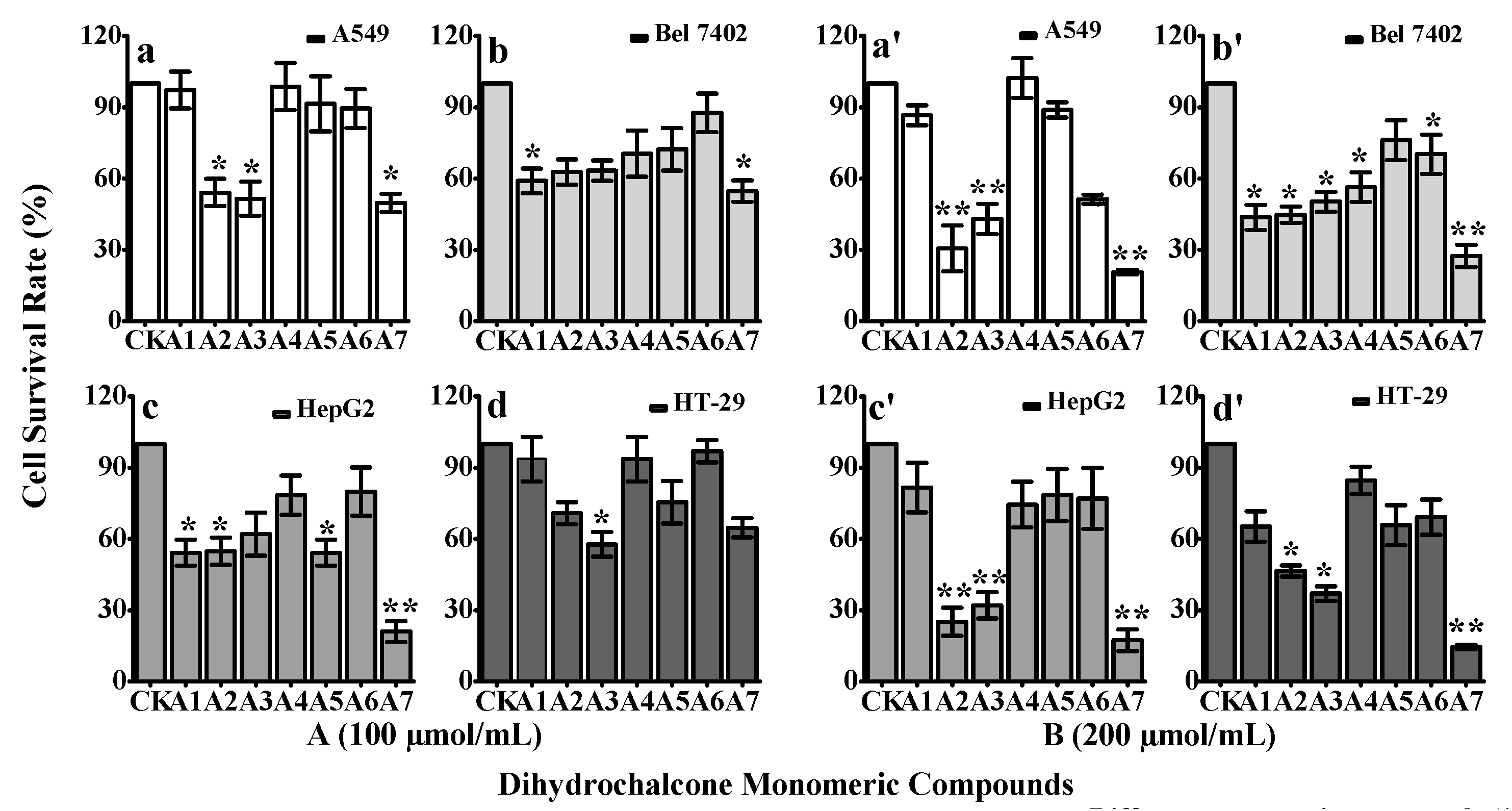
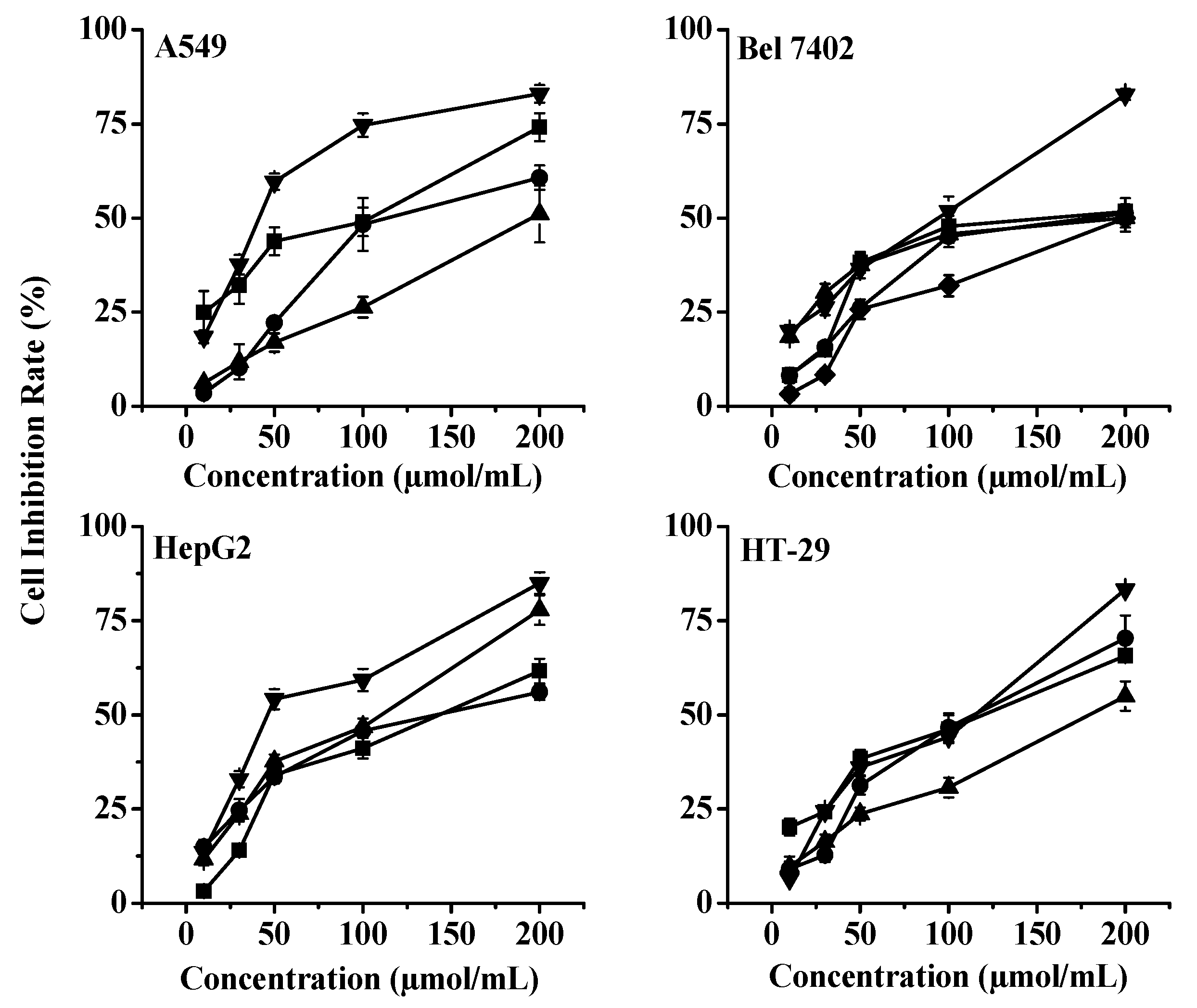
2.3. Cytotoxic Effects of Dihydrochalcone Monomeric Compounds on Four Human Cancer Cell Lines
| Compound | A549 | BEL7402 | HepG2 | HT29 |
|---|---|---|---|---|
| A1 | >150 | 76.86 ± 4.97 | >150 | >150 |
| A2 | 26.86 ± 3.11 | 37.08 ± 5.04 | 37.43 ± 1.23 | 33.20 ± 1.94 |
| A3 | 39.83 ± 4.23 | 45.17 ± 8.02 | 37.79 ± 4.04 | 35.37 ± 2.53 |
| A4 | >150 | >150 | >150 | >150 |
| A5 | >150 | >150 | >150 | >150 |
| A6 | 109.1 ± 18.31 | 86.61 ± 17.95 | 62.46 ± 7.20 | 99.87 ± 3.72 |
| A7 | 39.79 ± 5.72 | 59.28 ± 5.06 | 49.36 ± 3.04 | 65.09 ± 2.77 |
3. Experimental Section
3.1. Materials and Instruments
3.2. Purification of Dihydrochalcones from Crabapple “Radiant” Leaves
3.3. MS System and Conditions
3.4. Cancer Cell Growth Inhibition
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gosch, C.; Halbwirth, H.; Kuhn, J.; Miosic, S.; Stich, K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.). Plant Sci. 2009, 176, 223–231. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, M.; de Bernonville, T.D.; Lohou, D.; Guyot, S.; Guillemette, T.; Brisset, M.-N.; Dat, J.F. Histolocalization and physico-chemical characterization of dihydrochalcones: Insight into the role of apple major flavonoids. Phytochemistry 2013, 90, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, M.; Hernández-Revelles, J.; Hernández-Cassou, S.; Saurina, J. Determination of polyphenols in the pear pulp matrix by solvent extraction and liquid chromatography with UV-Vis detection. Anal. Methods 2014, 6, 9769–9776. [Google Scholar] [CrossRef]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Oike, H. Phloridzin reduces blood glucose levels and alters hepatic gene expression in normal BALB/c mice. Food Chem. Toxicol. 2012, 50, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Ziaullah, Z.; Rupasinghe, H.V. Phloridzin fatty acid esters induce apoptosis and alters gene expression in human liver cancer cells (261.2). FASEB J. 2014, 28 (Suppl. 1), 261.2. [Google Scholar] [CrossRef]
- Bissinger, R.; Fischer, S.; Jilani, K.; Lang, F. Stimulation of erythrocyte death by phloretin. Cell. Physiol. Biochem. 2014, 34, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, antioxidant and antimicrobial activity of a new phloridzin derivative for dermo-cosmetic applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Huang, W.-C.; Liou, C.-J. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, J.R.; Lewis, N.G.; Ronald Kahn, C.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, H. Exogenous polyamines alleviate the lipid peroxidation induced by cadmium chloride stress in Malus hupehensis Rehd. Sci. Hortic. 2008, 116, 442–447. [Google Scholar] [CrossRef]
- Park, H.; Lee, M.; Young, H.; Choi, J. Phytochemical study for botanical utilization of the fruits of Malus baccata. Korean J. Pharmacogn. 1993, 24, 282–282. [Google Scholar]
- Li, N.; Shi, J.; Wang, K. Profile and antioxidant activity of phenolic extracts from ten crabapples (Malus wild species). J. Agric. Food Chem. 2014, 62, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Zhu, X.-F.; Wang, X.-N.; Shen, T.; Xiang, F.; Lou, H.-X. Flavonoids from Malus hupehensis and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Phytochemistry 2013, 87, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, F.; Lu, L.; Zhang, X.; Xu, X.; Li, D. Phenolic profile and changes in the antioxidant activity of crabapple (Malus domestica cv Royalty) fruit during maturation on the tree. Int. J. Food Sci. Technol. 2014, 49, 1680–1688. [Google Scholar] [CrossRef]
- Qin, X.-D.; Liu, J.-K. A new sweet dihydrochalcone-glucoside from leaves of Lithocarpus pachyphyllus (Kurz) Rehd.(Fagaceae). Z. Naturforsch. C 2003, 58, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Cseh, R.; Hetzer, M.; Wolf, K.; Kraus, J.; Bringmann, G.; Benz, R. Interaction of phloretin with membranes: on the mode of action of phloretin at the water-lipid interface. Eur. Biophys. J. 2000, 29, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Constitution of some chemical components of apple seed. Food Chem. 1998, 61, 29–33. [Google Scholar] [CrossRef]
- Wang, L.; Wu, M.; Wang, J.; Jiang, J.; Chen, Y.; Li, T. A new chalcone glycoside from combretum griffithii. Chem. Nat. Compd. 2014, 50, 258–260. [Google Scholar] [CrossRef]
- Hilt, P.; Schieber, A.; Yildirim, C.; Arnold, G.; Klaiber, I.; Conrad, J.; Beifuss, U.; Carle, R. Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC-PDA-MS/MS and NMR spectroscopy. J. Agric. Food Chem. 2003, 51, 2896–2899. [Google Scholar] [CrossRef] [PubMed]
- Xü, K.; Lü, H.; Qü, B.; Shan, H.; Song, J. High-speed counter-current chromatography preparative separation and purification of phloretin from apple tree bark. Sep. Purif. Technol. 2010, 72, 406–409. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Allerdings, E.; Ralph, J.; Steinhart, H.; Bunzel, M. Isolation and structural identification of complex feruloylated heteroxylan side-chains from maize bran. Phytochemistry 2006, 67, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Mortensen, S.; Brimijoin, S.; Hooper, M.; Padilla, S. Comparison of thein VitroSensitivity of rat acetylcholinesterase to chlorpyrifos-oxon: What do tissue IC50 values represent? Toxicol. Appl. Pharmacol. 1998, 148, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Dai, Q.; Yin, Q.; Zhao, Y.; Guo, R.; Li, Z.; Ma, S.; Lu, N. III-10, a newly synthesized flavonoid, induces cell apoptosis with the involvement of reactive oxygen species-mitochondria pathway in human hepatocellular carcinoma cells. Eur. J. Pharmacol. 2015, 764, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-I.; Park, H.-S.; Kim, M.-K.; Hong, G.-E.; Nagappan, A.; Lee, H.-J.; Yumnam, S.; Lee, W.-S.; Won, C.-K.; Shin, S.-C. Flavonoids identified from Korean citrus aurantium L. inhibit non-small cell lung cancer growth in vivo and in vitro. J. Funct. Foods 2014, 7, 287–297. [Google Scholar] [CrossRef]
- Sample Availability: not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Xing, Y.F.; Zhou, Z.; Yao, Y. Dihydrochalcone Compounds Isolated from Crabapple Leaves Showed Anticancer Effects on Human Cancer Cell Lines. Molecules 2015, 20, 21193-21203. https://doi.org/10.3390/molecules201219754
Qin X, Xing YF, Zhou Z, Yao Y. Dihydrochalcone Compounds Isolated from Crabapple Leaves Showed Anticancer Effects on Human Cancer Cell Lines. Molecules. 2015; 20(12):21193-21203. https://doi.org/10.3390/molecules201219754
Chicago/Turabian StyleQin, Xiaoxiao, Yun Feng Xing, Zhiqin Zhou, and Yuncong Yao. 2015. "Dihydrochalcone Compounds Isolated from Crabapple Leaves Showed Anticancer Effects on Human Cancer Cell Lines" Molecules 20, no. 12: 21193-21203. https://doi.org/10.3390/molecules201219754





