Studies on Chromatographic Fingerprint and Fingerprinting Profile-Efficacy Relationship of Saxifraga stolonifera Meerb.
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Fingerprints
2.1.1. Analysis of HPLC Fingerprints and Similarities
2.1.2. Results of HCA

| Sample No. | Sources | Acquisition Time | Similarity | Sample No. | Sources | Acquisition Time | Similarity |
|---|---|---|---|---|---|---|---|
| S1 | Haozhou city, Medicine Market, Anhui | May, 2014 | 0.984 | S13 | Kaiyang county, Medicine Market, Guizhou | May, 2014 | 0.949 |
| S2 | Baiyun town, Guizhou | April, 2014 | 0.978 | S14 | Censong town, Guizhou | April, 2014 | 0.979 |
| S3 | Qingchang town, Guizhou | June, 2014 | 0.027 | S15 | Majiangxiasi town, Guizhou | October, 2012 | 0.981 |
| S4 | Yanxia town, Guizhou | July, 2014 | 0.972 | S16 | Dujiangyan city Medicine Market, Sichuan | April, 2014 | 0.758 |
| S5 | Guilin city, Medicine Market, Guangxi | May, 2014 | 0.983 | S17 | Benzhuang town, Guizhou | March, 2014 | 0.956 |
| S6 | Qingping, Medicine Market, Guangdong | March, 2014 | 0.972 | S18 | Qitan town, Guizhou | April, 2014 | 0.990 |
| S7 | Huaguoyuan, Medicine Market, Guizhou | May, 2014 | 0.979 | S19 | Shidong town, Guizhou | March, 2014 | 0.983 |
| S8 | Dongfeng town, Guizhou | July, 2014 | 0.599 | S20 | Zhenfeng county, Medicine Market, Guizhou | April, 2014 | 0.986 |
| S9 | Shuitian town, Guizhou | March, 2014 | 0.935 | S21 | Liutong town, Guizhou | March, 2014 | 0.942 |
| S10 | Guizhou, Botanical Garden, Guizhou | April, 2014 | 0.973 | S22 | Liutun town, Guizhou | July, 2014 | 0.981 |
| S11 | Baoding city, Medicine Market, Hebei | May, 2014 | 0.981 | S23 | Zhazuo town, Guizhou | July, 2014 | 0.941 |
| S12 | Gaopo town, Guizhou | March, 2014 | 0.970 | S24 | Banqiao town, Guizhou | June, 2014 | 0.967 |

2.1.3. Results of PCA
| Component | Initial Eigenvalues | Extraction Sums of Squared Loadings | |||
|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | |
| 1 | 4.324 | 24.022 | 24.022 | 4.324 | 24.022 |
| 2 | 3.075 | 17.085 | 41.107 | 3.075 | 17.085 |
| 3 | 2.741 | 15.228 | 56.335 | 2.741 | 15.228 |
| 4 | 2.192 | 12.179 | 68.513 | 2.192 | 12.179 |
| 5 | 1.569 | 8.716 | 77.230 | 1.569 | 8.716 |
| 6 | 0.947 | 5.261 | 82.490 | 0.947 | 5.261 |
| 7 | 0.816 | 4.533 | 87.023 | 0.816 | 4.533 |
| 8 | 0.699 | 3.882 | 90.906 | 0.699 | 3.882 |
| 9 | 0.407 | 2.264 | 93.169 | 0.407 | 2.264 |
| 10 | 0.346 | 1.922 | 95.092 | 0.346 | 1.922 |
| 11 | 0.267 | 1.485 | 96.577 | 0.267 | 1.485 |
| 12 | 0.221 | 1.231 | 97.807 | 0.221 | 1.231 |
| 13 | 0.159 | 0.882 | 98.689 | 0.159 | 0.882 |
| 14 | 0.099 | 0.552 | 99.241 | 0.099 | 0.552 |
| 15 | 0.081 | 0.451 | 99.692 | 0.081 | 0.451 |
| 16 | 0.040 | 0.220 | 99.913 | 0.040 | 0.220 |
| 17 | 0.013 | 0.071 | 99.984 | 0.013 | 0.071 |
| 18 | 0.003 | 0.016 | 100.000 | 0.003 | 0.016 |
2.2. Results of Screening Differences Samples
2.3. Results of Anti-Benign Prostatic Hyperplasia Activities
2.3.1. Effect of AESS on Prostate Index
2.3.2. Effect of Aqueous Extracts of S. stolonifera on Serum DHT Concentration
2.3.3. Effect of AESS on Serum ACP Activity
| Groups | Prostatic Index (mg/100 g Body Weight) | Serum DHT Concentration (nmol/L) | Serum ACP Activity (IU/L) | Serum PACP Concentration (ng/L) | Serum SRD5A2 Concentration (pg/L) |
|---|---|---|---|---|---|
| control group | 36.42 ± 2.36 ## | 130.50 ± 15.41 ## | 47.89 ± 5.90 ## | 647.85 ± 54.63 ## | 78.23 ± 9.26 ## |
| BPH model control group | 51.52 ± 5.56 | 187.54 ± 29.75 | 69.79 ± 8.45 | 816.66 ± 60.85 | 125.23 ± 9.69 |
| Finasteride control group | 35.15 ± 5.33 ## | 126.47 ± 15.42 ## | 46.63 ± 8.49 ## | 698.06 ± 32.38 ## | 90.72 ± 9.54 ## |
| QKPT control group | 36.11 ± 3.46 ## | 128.43 ± 18.00 ## | 50.60 ± 7.84 ## | 675.98 ± 55.81 ## | 89.86 ± 12.60 ## |
| group A | 37.47 ± 4.67 ## | 127.02 ± 14.80 ## | 50.23 ± 4.89 ## | 708.86 ± 40.00 ## | 101.04 ± 13.99 ## |
| group B | 37.22 ± 7.34 ## | 133.97 ± 7.79 ## | 54.23 ± 5.91 ## | 750.58 ± 47.51 # | 105.10 ± 6.61 # |
| group C | 38.57 ± 5.84 ## | 153.06 ± 28.35 ## | 55.18 ± 5.39 ## | 878.04 ± 50.05 # | 106.87 ± 19.10 # |
| group D | 38.09 ± 6.07 ## | 125.02 ± 13.57 ## | 48.76 ± 5.46 ## | 755.04 ± 42.58 # | 99.89 ± 8.35 ## |
| group E | 38.10 ± 5.03 ## | 143.39 ± 17.74 ## | 48.95 ± 6.24 ## | 755.50 ± 51.82 # | 102.15 ± 11.32 ## |
| group F | 41.07 ± 5.37 ## | 151.88 ± 22.64 ## | 48.92 ± 6.28 ## | 776.68 ± 68.20 | 106.97 ± 15.05 # |
| group G | 41.57 ± 7.43 # | 129.50 ± 8.10 ## | 48.46 ± 6.57 ## | 769.71 ± 98.94 | 108.68 ± 9.03 # |
| group H | 36.53 ± 5.32 ## | 162.25 ± 15.47 # | 52.14 ± 7.95 ## | 792.84 ± 36.84 | 109.76 ± 10.09 |
| group I | 39.70 ± 5.24 ## | 165.30 ± 19.39 # | 53.78 ± 8.57 ## | 746.88 ± 19.32 ## | 114.82 ± 7.74 |
2.3.4. Effect of AESS on Serum PACP Concentration
2.3.5. Effect of AESS on Serum SRD5A2 Concentration
2.4. Analysis of Spectrum-Effect Relationship
2.4.1. Results of Grey Relational Analysis
| NO. | Y1 | Y2 | Y3 | Y4 | Y5 | Average | Order |
|---|---|---|---|---|---|---|---|
| A1 | 0.573 | 0.651 | 0.584 | 0.641 | 0.573 | 0.604 | 9 |
| A2 | 0.522 | 0.525 | 0.496 | 0.620 | 0.522 | 0.537 | 17 |
| A3 | 0.557 | 0.630 | 0.536 | 0.640 | 0.557 | 0.584 | 13 |
| A4 | 0.626 | 0.698 | 0.595 | 0.673 | 0.626 | 0.644 | 5 |
| A5 | 0.566 | 0.621 | 0.539 | 0.659 | 0.566 | 0.590 | 12 |
| A6 | 0.532 | 0.583 | 0.534 | 0.641 | 0.532 | 0.564 | 15 |
| A7 | 0.567 | 0.638 | 0.564 | 0.644 | 0.567 | 0.596 | 11 |
| A8 | 0.525 | 0.587 | 0.514 | 0.608 | 0.525 | 0.552 | 16 |
| A9 | 0.619 | 0.615 | 0.650 | 0.636 | 0.619 | 0.628 | 8 |
| A10 | 0.588 | 0.644 | 0.546 | 0.655 | 0.588 | 0.604 | 9 |
| A11 | 0.579 | 0.581 | 0.548 | 0.702 | 0.579 | 0.598 | 10 |
| A12 | 0.555 | 0.622 | 0.538 | 0.646 | 0.555 | 0.583 | 14 |
| A13 | 0.623 | 0.641 | 0.611 | 0.716 | 0.623 | 0.643 | 6 |
| A14 | 0.724 | 0.733 | 0.669 | 0.774 | 0.724 | 0.725 | 2 |
| A15 | 0.631 | 0.622 | 0.661 | 0.621 | 0.631 | 0.633 | 7 |
| A16 | 0.656 | 0.635 | 0.650 | 0.656 | 0.656 | 0.651 | 4 |
| A17 | 0.736 | 0.658 | 0.704 | 0.677 | 0.736 | 0.702 | 3 |
| A18 | 0.869 | 0.744 | 0.838 | 0.819 | 0.869 | 0.828 | 1 |
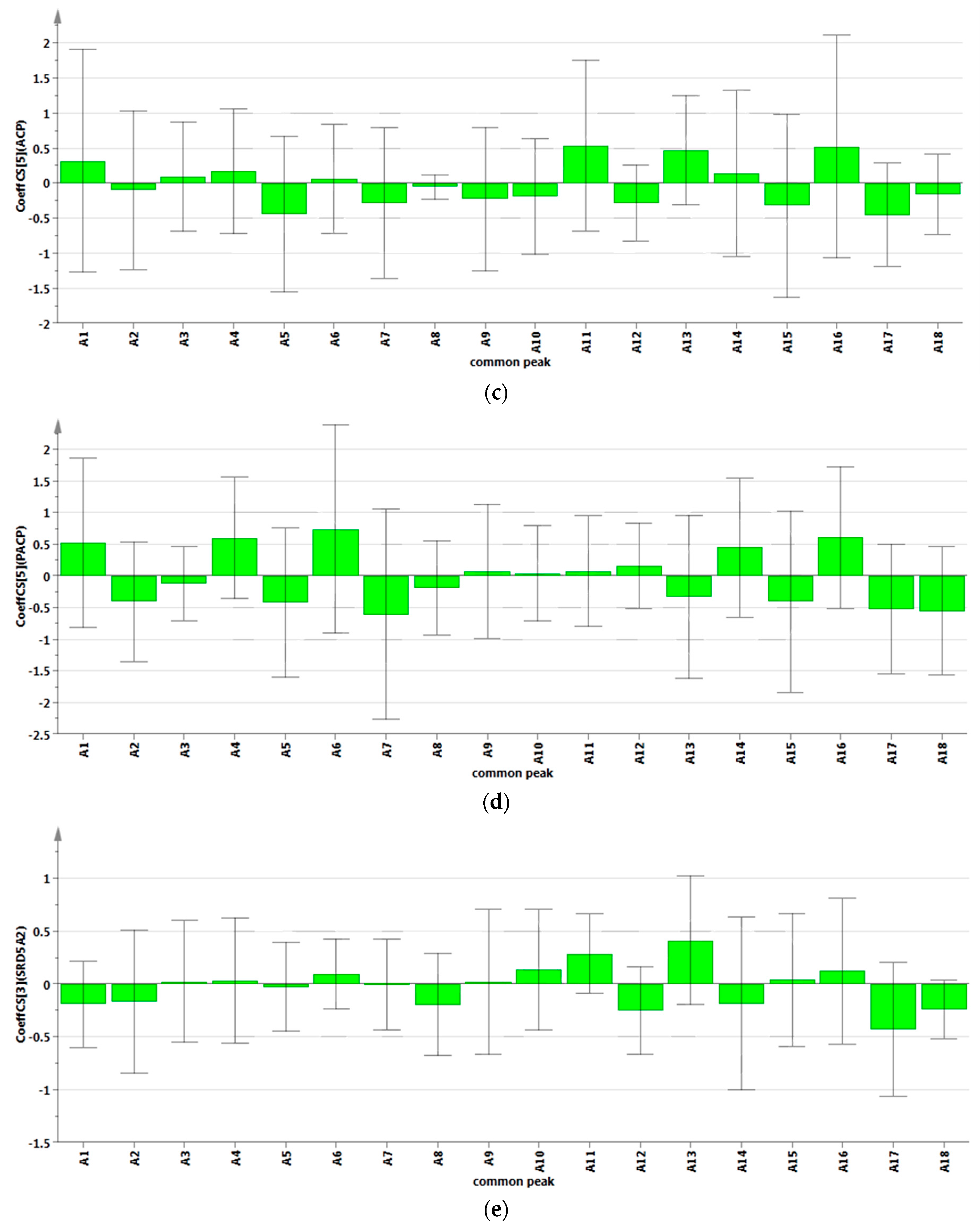
2.4.2. Results of Partial Least Squares Regression Analysis

3. Experimental Section
3.1. Materials
3.2. Instruments
3.3. Determination of HPLC Fingerprints
3.3.1. Chromatographic Separation
3.3.2. Solution’s Preparation
3.3.3. Similarity Analysis (SA)
3.3.4. Hierarchical Clustering Analysis (HCA)
3.3.5. Principal Component Analysis
3.3.6. Screening for Differences between Samples
3.4. Anti-Benign Prostatic Hyperplasia Experimentation
3.4.1. Castration Procedure
3.4.2. Induction of BPH and Treatments
3.4.3. Determination of Prostatic Index (PI)
3.4.4. Immunohistochemical Analysis
3.4.5. Statistical Analysis
3.5. Spectrum-Effect Relationship Analysis
3.5.1. Grey Relational Analysis
3.5.2. Partial Least Squares Regression
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pagano, E.; Laudato, M.; Griffo, M.; Capasso, R. Phytotherapy of benign prostatic hyperplasia. A minireview. Phytother Res. 2014, 28, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, H.G.; Huang, Z.J.; Yang, S.L. Screening of anti-prostate-tumor parts from Saxifraga stolonifera. Chin. Pharm. Bulletin. 2013, 29, 867–870. [Google Scholar]
- Chen, Z.; Liu, Y.M.; Yang, S.; Song, B.A.; Xu, G.F.; Bhadury, P.S. Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L.) Meeb. Bioorg. Med. Chem. 2008, 16, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, Q.Y. Saxifrage research on biological activity and pharmacological effects. Chin. J. Anhui Agric. Sci. 2014, 42, 5422–5424. [Google Scholar]
- Liu, J.; Jiao, S.R.; Sun, X.; Tang, Y.M.; Feng, H. The extraction of antioxidants from Saxifraga stolonifera and component analysis. Chin. J. Bioprocess Eng. 2012, 10, 42–46. [Google Scholar]
- Daniel, K.A.; George, A.; Asare, K.B.; Samuel, A.; Lin, J.M.; Peng, J.; Hong, Z.F. Treatment of benign prostatic hyperplasia with croton membranaceus in an experimental animal model. J. Ethnopharmacol. 2014, 157, 90–98. [Google Scholar]
- Fourcade, R.O.; Theret, N.; Taieb, C. Profile and management of patients treated for the first time for lower urinary tract symptoms/benign prostatic hyperplasia in four European countries. BJU Int. 2008, 101, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Nahata, A.; Dixit, V.K. Protective effects of Echinops echinatus on testosterone-induced prostatic hyperplasia in rats. Eur. J. Integr. Med. 2012, 4, 177–185. [Google Scholar] [CrossRef]
- Zabkowski, T. Evaluation of the clinical indications, adverse drug reactions, and finasteride use in patients with benign prostatic hyperplasia in Poland. Pharmacol. Rep. 2014, 66, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.L.; Wahane, V.D. Current status of 5a-reductase inhibitors in the treatment of benign hyperplasia of prostate. Indian. J. Med. Sci. 2008, 62, 167–175. [Google Scholar]
- William, D.S. 5α-Reductase activity in the prostate. Urology 2001, 58, 17–24. [Google Scholar]
- Harkaway, R.C.; Issa, M.M. Medical and minimally invasive therapies for the treatment of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2006, 9, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Gilling, T.L.; Morrill, B.; Wilson, T.H.; Rittmaster, R.S. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia the enlarged prostate international comparator study (EPICS). BJU Int. 2011, 108, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kobayashi, Y.; Lin, Y.; Rauwald, H.W.; Fang, L.; Qiao, H.; Kuchta, K. A phytosterol enriched refined extract of Brassica campestris L. pollen significantly improves benign prostatic hyperplasia (BPH) in a rat model as compared to the classical TCM pollen preparation Qianlie Kang Pule’an Tablets. Phytomedicine 2015, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zambra, F.M.; Biolchi, V.; Brum, I.S.; Chies, J.A. CCR2 and CCR5 genes polymorphisms in benign prostatic hyperplasia and prostate cancer. Hum. Immunol. 2013, 74, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Lee, M.Y.; Jung, D.Y.; Seo, C.S.; Ha, H.K.; Shin, H.K. Ursolic acid reduces prostate size and dihydrotestosterone level in a rat model of benign prostatic hyperplasia. Food Chem. Toxicolo. 2012, 50, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Li, Y.D.; Shi, Y.P.; Jin, L.; Chen, J. Quality control of traditional Chinese medicines a review. Chin. J. Nat. Med. 2013, 11, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Peng, L.X.; Wang, S.; Hu, Y.B.; Zou, L. HPLC fingerprint-antioxidant properties study of buckwheat. J. Integr. Agri. 2012, 11, 1111–1118. [Google Scholar] [CrossRef]
- Kong, W.; Wang, J.; Zang, Q.; Xing, X.; Zhao, Y.; Liu, W.; Jin, C.; Li, Z.; Xiao, X. Fingerprint-efficacy study of artificial Calculus bovis in quality control of Chinese materia medica. Food Chem. 2011, 127, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bi, K.; Han, F.; Guan, J.; Tang, Z.; Chen, K.; Zhao, L.; Li, Q.; Yin, R.; Hou, X. Simultaneous determination of two iridoid glycosides, two anthraquinones and four flavonoid glycosides of Zhi-Zi-Da-Huang decoction in rat plasma by UFLC-MS/MS: Application to a comparative pharmacokinetic study in normal and cholestatic liver injury rats. J. Chromatogr. B 2014, 960, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Chun, E.H.; Hyun, J.H.; Choi, S.W.; Su, S.T.; Kim, W.; Kim, D.O.; Kim, B.Y.; Baik, M.Y. Optimization of hot water extraction and ultra high pressure extraction for deer antler. Food Sci. Biotechnolo. 2015, 24, 507–512. [Google Scholar] [CrossRef]
- State Food and Drug Administration of China. Technical requirements for chromatographic fingerprints of traditional Chinese medicinal injection. Chin. Tradit. Pat. Med. 2000, 22, 671–678. [Google Scholar]
- Wang, X.W.; Zang, W.Y.; Bi, K.R. Approaching the study on the similarity analysis of HPLC fingerprints spectra for traditional Chinese medicines. J. Shenyang Pharm. Univ. 2003, 20, 360–366. [Google Scholar]
- Calhoun, V.D.; Silva, R.F.; Adali, T.; Rachakonda, S. Comparison of PCA approaches for very large group ICA. NeuroImage 2015, 118, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Hakimzadeh, N.; Parastar, H.; Fattahi, M. Combination of multivariate curve resolution and multivariate classification techniques for comprehensive high-performance liquid chromatography-diode array absorbance detection fingerprints analysis of Salvia reuterana extracts. J. Chromatogr. A 2014, 1326, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef]
- Goto, T.; Kawano, H.; Akiyama, T.; Shinoda, Y.; Okuma, T.; Kobayashi, H.; Nemoto, T.; Funata, N. Serum acid phosphatase can be a useful tumour marker for giant cell tumour of bone. Arch. Orthop. Trauma Surg. 2009, 129, 1641–1644. [Google Scholar] [CrossRef] [PubMed]
- Eri, L.M.; Tveter, K.J. Effects of bicalutamide and leuprolide on prostate-specific antigen (PSA) acid phosphatase (ACP). Prostate Cancer Prostatic Dis. 2001, 4, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.; But, B.W.; Lee, C.Y.; Lam, Y.Y.; Ng, K.L.; Tung, J.Y.; Kwan, E.Y.; Chan, Y.K.; Tsui, T.K.; Lam, A.L.; et al. Diagnosis of 5α-reductase 2 deficiency: is measurement of dihydrotestosterone essential? Clin. Chem. 2013, 59, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Kannel, P.R.; Lee, S.; Kanel, S.R.; Khan, S.P. Chemometric application in classification and assessment of monitoring locations of an urban river system. Anal. Chim. Acta 2007, 582, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhao, L.; Wang, J.B.; Liu, T.T.; Zhang, B.; Gong, M. Spectrum-effect relationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J. Ethnopharmacol. 2014, 153, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Coppenolle, F.V.; Bourhis, X.; Carpentier, F.; Delaby, G.; Cousse, H.; Raynaud, J.P.; Prevarskaya, N. Pharmacological effects of the lipidosterolic extract of Serenoa repens (Permixon) on rat prostate hyperplasia induced by hyperprolactinemia: comparison with finasteride. Prostate 2000, 43, 49–58. [Google Scholar] [CrossRef]
- Wei, H.; Lin, L.; Zhang, Y.; Wang, L.; Chen, Q. Research on the application of grey system theory in the pattern recognition for chromatographic fingerprints of traditional Chinese medicine. Chin. J. Chromatogr. 2013, 31, 127–132. [Google Scholar] [CrossRef]
- Abhang, L.B.; Hameedullah, M. Determination of optimum parameters for multi-performance characteristics in turning by using grey relational analysis. Int. J. Adv. Manuf. Tech. 2012, 63, 13–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, Y.; Lei, J.W.; Xie, C.X.; Fan, M.Y.; Hu, X.L. Rapid determination of water-extract in jingutongxiao pills by near-infrared spectroscopy. Chin. J. Exp. Trad. Med. Formul. 2015, 21, 52–55. [Google Scholar]
- Li, F.; Mistele, B.; Hu, Y.; Chen, X.U. Reflectance estimation of canopy nitrogen content in winter wheat using optimised hyperspectral spectral indices and partial least squares. Eur. J. Agron. 2014, 52, 198–209. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Molfetta, F.A.; Bruni, A.T.; Rosselli, F.P.; Silva, A.B.F. A partial least squares and principal component regression study of quinone compounds with trypanocidal activity. Struct. Chem. 2006, 18, 49–57. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds Chlorogenic acid, Quercetin 5-O-β-d-glucopyranoside and Quercetin 3-O-β-l-rhamnopyranoside are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.-D.; Chen, H.-G.; Zhou, X.; Huang, Y.; Hu, E.-M.; Jiang, Z.-M.; Zhao, C.; Gong, X.-J.; Deng, Q.-F. Studies on Chromatographic Fingerprint and Fingerprinting Profile-Efficacy Relationship of Saxifraga stolonifera Meerb. Molecules 2015, 20, 22781-22798. https://doi.org/10.3390/molecules201219882
Wu X-D, Chen H-G, Zhou X, Huang Y, Hu E-M, Jiang Z-M, Zhao C, Gong X-J, Deng Q-F. Studies on Chromatographic Fingerprint and Fingerprinting Profile-Efficacy Relationship of Saxifraga stolonifera Meerb. Molecules. 2015; 20(12):22781-22798. https://doi.org/10.3390/molecules201219882
Chicago/Turabian StyleWu, Xing-Dong, Hua-Guo Chen, Xin Zhou, Ya Huang, En-Ming Hu, Zheng-Meng Jiang, Chao Zhao, Xiao-Jian Gong, and Qing-Fang Deng. 2015. "Studies on Chromatographic Fingerprint and Fingerprinting Profile-Efficacy Relationship of Saxifraga stolonifera Meerb." Molecules 20, no. 12: 22781-22798. https://doi.org/10.3390/molecules201219882






