Analysis of Indole Alkaloids from Rhazya stricta Hairy Roots by Ultra-Performance Liquid Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Results
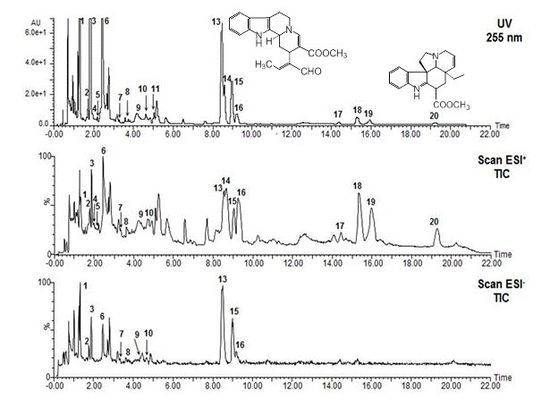
2.1. UPLC-Photodiode Array (PDA) Method Test
2.2. Alkaloid Composition
2.2.1. Analysis of Reference Substances by UPLC-PDA-MS
| Pure Substance | tR (min) | UV (nm) λmax | MW | [M + H]+ m/z | Fragments m/z (rel. int. %) |
|---|---|---|---|---|---|
| Vincanine | 2.45 | 246, 300, 365 | 292 | 293 (100) | - |
| Yohimbine | 3.34 | 227, 279 | 354 | 355 (100) | 212 (18), 144 (20), 224 (5) |
| Vincamine | 5.02 | 228, 280 | 354 | 355 (100) | 337 (90) |
| Tabersonine | 19.19 | 229, 298, 330 | 336 | 337 (100) | 305 (50) |
2.2.2. Identification of Alkaloids by UPLC-PDA-MS
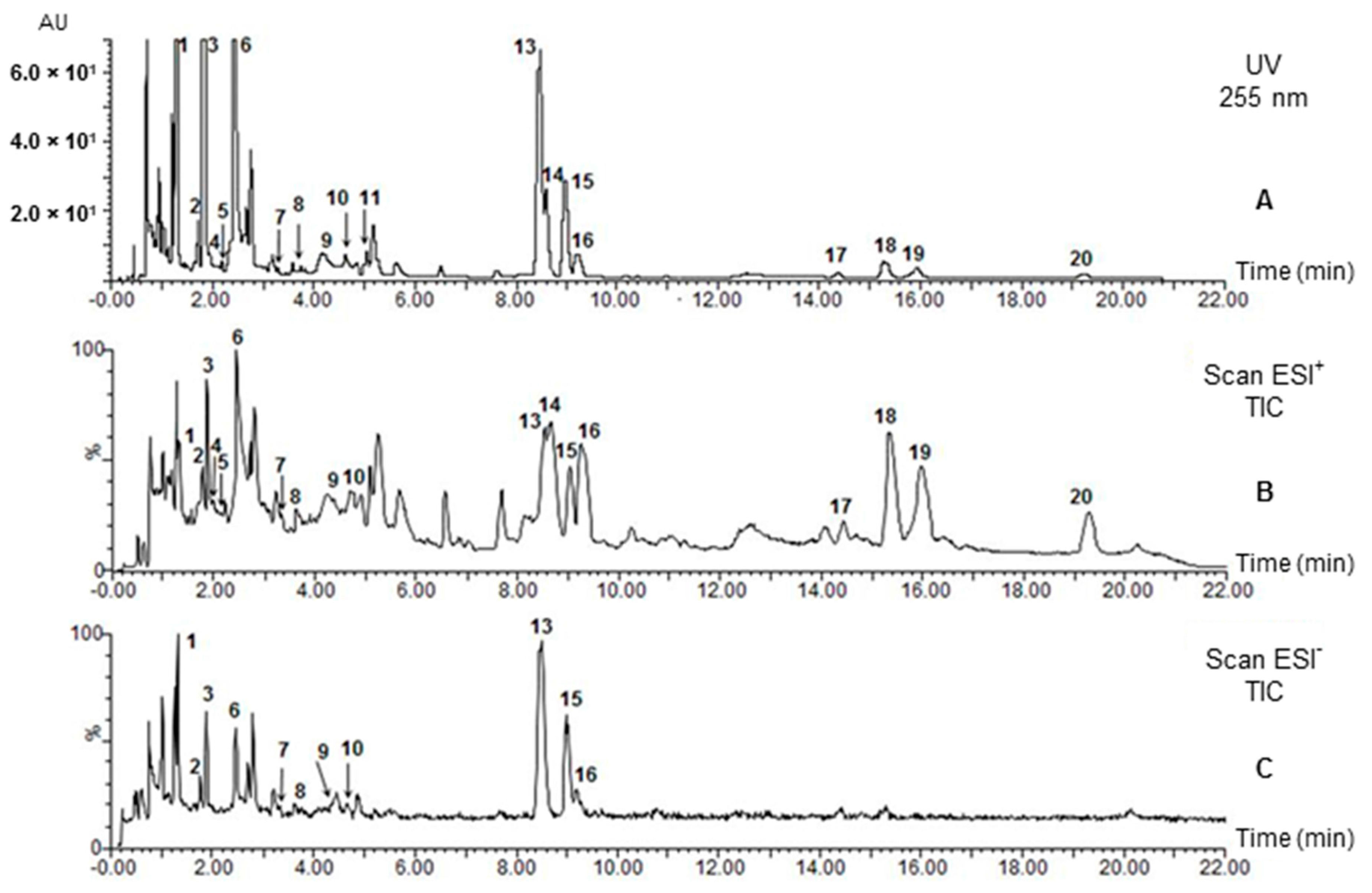
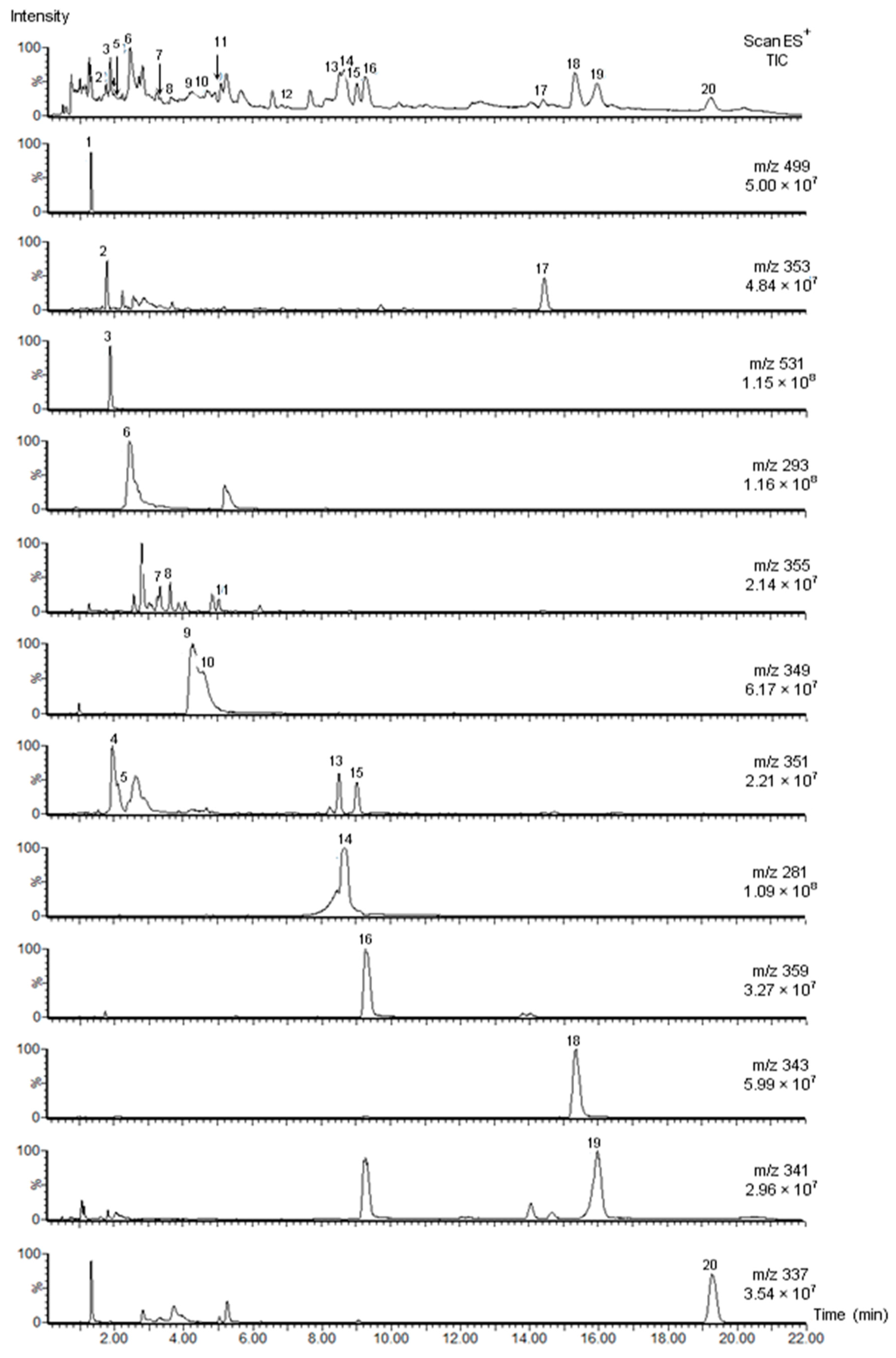
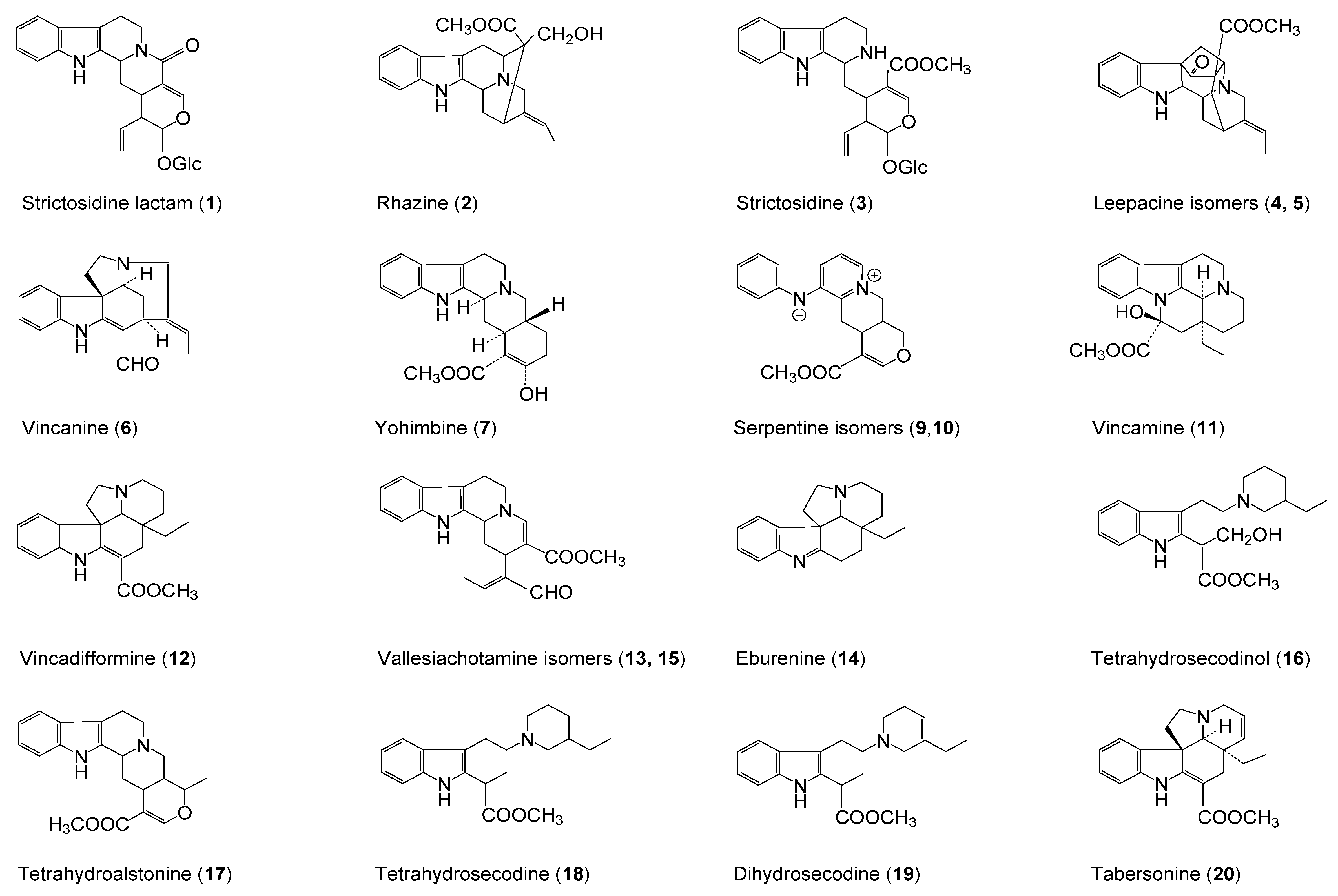
| No | Compound | tR (min) | UV (nm) λmax | MW | ESI+ (m/z) (rel. int. %) | ESI− (m/z) (rel. int. %) | References |
|---|---|---|---|---|---|---|---|
| 1 | Strictosidine lactam | 1.31 | 217 (sh), 227, 283, 290 | 498 | 337 (100), 499 (98), 267 (12) | 497 (100), 335 (tr), 557(71) | [9,11,12,13,14] |
| 2 | Rhazine | 1.77 | 227, 279, 291 (sh) | 352 | 353 (100), 323 (14), 307 (10), 230 (5) | 351 (100), 411 (15) a, 319 (12) | [15,16] |
| 3 | Strictosidine | 1.87 | 228, 270, 280, 290 | 530 | 531 (100), 514 (37), 369 (4) | 529 (20), 589 (100) a | [11] |
| 4 | Leepacine isomer I | 1.95 | 208, 252, 305 | 350 | 351 (100) | 349 (tr) | [1,9] |
| 5 | Leepacine isomer II | 2.14 | 206, 250, 305 | 350 | 351 (100) | 349 (tr) | [1,9] |
| 6 | Vincanine | 2.46 | 246, 300, 365 | 292 | 293 (100) | 291 (100) | [17,18] |
| 7 | Yohimbine | 3.33 | 227, 279 | 354 | 355 (100), 212 (6), 144 (11), 224 (4) | 353 (100), 413 (88) a | [7,19] |
| 8 | Yohimbine isomer | 3.63 | 226, 279 | 354 | 355 (100), 212 (11), 144 (21), 224 (2) | 353 (100), 413 (34) a | [7,19] |
| 9 | Serpentine isomer I | 4.28 | 210, 248, 307, 368 | 348 | 349 (100) | 347 (tr) | [9,20,21,22] |
| 10 | Serpentine isomer II | 4.60 | 210, 248, 307, 368 | 348 | 349 (100) | 347 (tr) | [9,20,21,22] |
| 11 | Vincamine | 5.03 | 228, 280 | 354 | 355 (100), 337 (82) | n.d | [18,23] |
| 12 | Vincadifformione | 7.01 | 227, 297, 328 | 338 | 339 (100), 307 (43) | n.d. | [24] |
| 13 | Vallesiachotamine isomer I | 8.47 | 222, 292 | 350 | 319 (100), 281 (83) c, 351 (50), 170 (35), 373 (18) b | 349 (100) | [25,26,27,28,29] |
| 14 | Eburenine | 8.96 | 222, 262 | 280 | 281 (100) | n.d | [30,31] |
| 15 | Vallesiachotamine isomer II | 8.99 | 223, 291 | 350 | 319 (100), 351 (56), 170 (49), 281 (30) c, 373 (20) b | 349 (100) | [25,26,27,28,29] |
| 16 | Tetrahydrosecodinol | 9.27 | 222, 283, 290 | 358 | 126 (100), 359 (62), 341 (49), 246 (30) | 357 (tr) | [32] |
| 17 | Tetrahydroalstonine | 14.45 | 227, 270 (sh), 282, 290 | 352 | 353 (100), 144 (60) | n.d | [30,33] |
| 18 | Tetrahydrosecodine | 15.34 | 224, 283, 290 (sh) | 342 | 126 (100), 343 (90), 230 (53) | n.d | [32] |
| 19 | Dihydrosecodine | 15.99 | 224, 280 (sh), 290, 305 | 340 | 126 (100), 341 (51), 228 (26) | n.d | [32] |
| 20 | Tabersonine | 19.25 | 229, 298, 331 | 336 | 337 (100), 305 (68), 228 (2) | n.d | [21,22,24] |
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Plant Material
4.3. Extraction
4.4. UPLC-PDA-MS
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rahman, A.U.; Qureshi, M.M.; Zaman, K.; Malik, S.; Ali, S.S. The alkaloids of Rhazya stricta and R. orientalis. A review. Fitoterapia 1989, 60, 291–322. [Google Scholar]
- Gilani, S.A.; Kikuchi, A.; Shinwari, Z.K.; Khattak, Z.I.; Watanabe, K.N. Phytochemical, pharmacological and ethnobotanical studies of Rhazya stricta Decne. Phytother. Res. 2007, 21, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, I.; Sheludko, Y.; Unger, M.; Stöckigt, J. Development of an efficient system for the separation of indole alkaloids by high performance liquid chromatography and its applications. Phytochem. Anal. 2001, 12, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Stöckigt, J.; Sheludko, Y.; Unger, M.; Gerasimenko, I.; Warzecha, H.; Stöckigt, D. High-performance liquid chromatographic, capillary electrophoretic and capillary electrophoretic-electrospray ionisation mass spectrometric analysis of selected alkaloid groups. J. Chromatogr. A 2002, 967, 85–113. [Google Scholar] [CrossRef]
- Han, Z.; Zheng, Y.; Chen, N.; Luan, L.; Zhou, C.; Gan, L.; Wu, Y. Simultaneous determination of four alkaloids in Lindera aggregata by ultra-high-pressure liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1212, 76–81. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, L.; Xiong, A.; Zhao, S.; Wang, Z.; Hu, Z. Simultaneous quantification of four indole alkaloids in Catharanthus roseus cell line C20hi by UPLC-MS. Anal. Sci. 2011, 27, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Baker, A.; Chen, P. Profiling the indole alkaloids in yohimbe bark with ultra-performance liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Wren, S.A.C.; Tchelitcheff, P. Use of ultra-performance liquid chromatography in pharmaceutical development. J. Chromatogr. A 2006, 1119, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Akhgari, A.; Yrjönen, T.; Laakso, I.; Vuorela, H.; Oksman-Caldentey, K.-M.; Rischer, H. Establishment of transgenic Rhazya stricta hairy roots to modulate terpenoid indole alkaloid production. Plant Cell Rep. 2015, 34, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Akhgari, A.; Laakso, I.; Seppänen-Laakso, T.; Yrjönen, T.; Vuorela, H.; Oksman-Caldentey, K.-M.; Rischer, H. Determination of terpenoid indole alkaloids in hairy roots of Rhazya stricta (Apocynaceae) by gas chromatography-mass spectrometry. Phytochem. Anal. 2015, 26, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Sudo, H.; Yamazaki, M.; Aimi, N.; Saito, K. Camptothecin biosynthetic genes in hairy roots of Ophiorrhiza pumila: Cloning, characterization and differential expression in tissues and by stress compounds. Plant Cell Physiol. 2003, 44, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Dhooghe, L.; Mesia, K.; Kohtala, E.; Tona, L.; Pieters, L.; Vlietinck, A.J.; Apers, S. Development and validation of an HPLC-method for the determination of alkaloids in the stem bark extract of Nauclea pobeguinii. Talanta 2008, 76, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Nana, H.M.; Ngane, R.A.; Gatsing, D.; Tchinda, A.T.; Rovito, R.; Cagno, V.; Cagliero, C.; Boyom, F.F.; Rubiolo, P.; et al. In vitro anti-Herpes simplex virus activity of crude extract of the roots of Nauclea latifolia Smith (Rubiaceae). BMC Complement. Altern. Med. 2013, 13, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xiao, W.; Dai, C.; Xie, L.; Ding, G.; Wang, G.; Meng, Z.; Zhang, J.; Kang, A.; Xie, T.; et al. Structural identification of the metabolites for strictosamide in rats bile by an ion trap-TOF mass spectrometer and mass defect filter technique. J. Chromatogr. B 2011, 879, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cao, X.; Dong, L.; Wang, L.; Cheng, B.; Shi, Q.; Luo, X.; Bai, G. Bioactivity-based liquid chromatography-coupled electrospray ionization tandem ion trap/time of flight mass spectrometry for β2AR agonist identification in alkaloidal extract of Alstonia scholaris. J. Chromatogr. A 2012, 1227, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Yuan, L.; Liu, X.; Gu, J.F.; Zhang, M.H.; Wang, Y. A combination of alkaloids and triterpenes of Alstonia scholaris (Linn.) R. Br. leaves enhances immunomodulatory activity in C57BL/6 mice and induces apoptosis in the A549 cell line. Molecules 2013, 18, 13920–13939. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Fatima, K.; Occolowitz, J.L.; Solheim, B.A.; Clardy, J.; Garnick, R.L.; le Quesne, P.W. Structure and absolute configuration of strictamine and strictalamine from Rhazya stricta. Stereochemistry of the picralima alkaloids. J. Am. Chem. Soc. 1977, 99, 1943–1946. [Google Scholar] [CrossRef]
- Shakirov, R.; Telezhenetskaya, M.V.; Bessonova, I.A.; Aripova, S.F.; Israilov, I.A.; Sultankhodzhaev, M.N.; Vinogradova, V.I.; Akhmedzhanova, V.I.; Tulyaganov, T.S.; Salimov, B.T.; et al. Alkaloids, plants, structure, properties. Chem. Nat. Compd. 1996, 32, 216–334. [Google Scholar] [CrossRef]
- Zanolari, B.; Ndjoko, K.; Ioset, J.R.; Marston, A.; Hostettmann, K. Qualitative and quantitative determination of yohimbine in authentic yohimbe bark and in commercial aphrodisiacs by HPLC-UV-API/MS methods. Phytochem. Anal. 2003, 14, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Pereira, D.M.; Valentão, P.; Oliveira, J.M.; Faria, J.; Gaspar, L.; Sottomayor, M.; Andrade, P.B. Simple and reproducible HPLC-DAD-ESI-MS/MS analysis of alkaloids in Catharanthus roseus roots. J. Pharm. Biomed. Anal. 2010, 51, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, W.; Zhang, Y.; Chen, J.; Chen, Z. Identification and quantification of active alkaloids in Catharanthus roseus by liquid chromatography-ion trap mass spectrometry. Food Chem. 2013, 139, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Suttipanta, N.; Pattanaik, S.; Kulshrestha, M.; Patra, B.; Singh, S.K.; Yuan, L. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011, 157, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, V.; Kompiš, I. Alkaloids from Vinca minor L. XXIII. Mass spectrometry of eburnamine-type alkaloids. Collect. Czech. Chem. Commun. 1969, 34, 2809–2818. [Google Scholar]
- Pawelka, K.H.; Stöckigt, J. Major indole alkaloids produced in cell suspension cultures of Rhazya stricta Decaisne. Z. Naturforsch. C. 1986, 41, 385–390. [Google Scholar]
- Djerassi, C.; Monteiro, H.J.; Walser, A.; Durham, L.J. Alkaloid studies. LVI. The constitution of vallesiachotamine. J. Am. Chem. Soc. 1966, 88, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Handy, G.A.; Funayama, S.; Cordell, G.A. Anticancer indole alkaloids of Rhazya stricta. J. Nat. Prod. 1981, 44, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Atta-ur-Rahman; Malik, S. Isolation of isovallesiachotamine from legumes of Rhazya stricta. J. Nat. Prod. 1984, 47, 388–389. [Google Scholar] [CrossRef]
- Kostenyuk, I.A.; Lyubarets, O.F.; Endress, S.; Gleba, Y.Y.; Stöckigt, J. Alkaloids isolated from somatic hybrid cell cultures of the species combination Rauwolfia serpentina × Rhazya stricta. Nat. Prod. Lett. 1995, 5, 303–307. [Google Scholar]
- Waterman, P.G.; Zhong, S. Vallesiachotamine and isovallesiachotamine from the seeds of Strychnos tricalysioides. Planta Med. 1982, 45, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Budzikiewicz, H.; Djerassi, C.; Williams, D.H. Alkaloids. In Structure Elucidation of Natural Products by Mass Spectrometry; Holden-Day, Inc.: San Fransisco, CA, USA, 1964; Volume 1, pp. 77–86, 98–105. [Google Scholar]
- Smith, G.F.; Wahid, M.A. 760. The isolation of (±)- and (+)-vincadifformine and of (+)-1,2-dehydroaspidospermidine from Rhazya stricta. J. Chem. Soc. 1963, 4002–4004. [Google Scholar] [CrossRef]
- Cordell, G.A.; Smith, G.F.; Smith, G.N. The isolation of monomeric secodine-type alkaloids from Rhazya species. J. Chem. Soc. D 1970, 4, 189–190. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Malik, S. Tetrahydroalstonine from fruits of Rhazya stricta. Planta Med. 1984, 50, 283. [Google Scholar]
- Hisiger, S.; Jolicoeur, M. Analysis of Catharanthus roseus alkaloids by HPLC. Phytochem. Rev. 2007, 6, 207–234. [Google Scholar] [CrossRef]
- Runguphan, W.; Maresh, J.J.; O’Connor, S.E. Silencing of tryptamine biosynthesis for production of non-natural alkaloids in plant culture. Proc. Natl. Acad. Sci. USA 2009, 106, 13673–13678. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.L.; Chen, L.H.; Yan, D.; Zhang, P.; Tan, M.R.; Du, X.X.; Xiao, X.H. Simultaneous determination of six quaternary ammonium alkaloids in Coptidis rhizoma by UPLC. Chin. Herb. Med. 2012, 4, 164–169. [Google Scholar]
- Buckingham, J.; Baggaley, K.H.; Roberts, A.D.; Szabó, L.F. (Eds.) Dictionary of Alkaloids, 2nd ed.; CRC Press/Taylor & Francis Group: London, UK, 2010.
- Smith, G.N. Strictosidine: A key intermediate in the biogenesis of indole alkaloids. Chem. Commun. 1968, 15, 912–914. [Google Scholar] [CrossRef]
- O’Connor, S.E.; Maresh, J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006, 23, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; Wiley: New York, NY, USA, 2002; pp. 350–358. [Google Scholar]
- Zhou, J.L.; Li, P.; Li, H.J.; Jiang, Y.; Ren, M.T.; Liu, Y. Development and validation of a liquid chromatography/electrospray ionization time-of-flight mass spectrometry method for relative and absolute quantification of steroidal alkaloids in Fritillaria species. J. Chromatogr. A. 2008, 1177, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Kurz, W.G.; Chatson, K.B.; Constabel, F.; Kutney, J.P.; Choi, L.S.; Kolodziejczyk, P.; Sleigh, S.K.; Stuart, K.L.; Worth, B.R. Alkaloid production in Catharanthus roseus cell cultures VIII. Planta Med. 1981, 42, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, L.; Balsevich, J.; Kurz, W.G.W. Indole alkaloid production by hairy root cultures of Catharanthus roseus. Plant Cell Tiss. Org. Cult. 1989, 18, 79–93. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ghosal, C.R.; Chaudhury, N.A.; Ghosal, S. Alkaloids of Rhazya stricta Decaisne. Chem. Ind. 1961, 1034–1035. [Google Scholar]
- Rahman, A.U.; Zaman, K.; Perveen, S.; Rehman, H.U.; Muzaffar, A.; Choudhary, M.I.; Pervin, A. Alkaloids from Rhazya stricta. Phytochemistry 1991, 30, 1285–1293. [Google Scholar] [CrossRef]
- Ahmad, Y.; Le Quesne, P.W.; Neuss, N. Sewarine, a new phenolic akuammicine alkaloid from Rhazya stricta. J. Pharm. Sci. 1971, 60, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Stauffacher, D. Alkaloide aus Diplorrhyncus condylocarpon (Muell. Arg.) Pichon ssp. mossambicensis (Benth.) Duvign. Helv. Chim. Acta. 1961, 44, 2006–2015. [Google Scholar] [CrossRef]
- Stöckigt, J.; Pfitzner, A.; Firl, J. Indole alkaloids from cell suspension cultures of Rauwolfia serpentina Benth. Plant Cell Rep. 1981, 1, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Stöckigt, D.; Unger, M.; Belder, D.; Stöckigt, J. Analysis of alkaloids by capillary electrophoresis and capillary electrophoresis-electrospray mass spectrometry. Nat. Prod. Lett. 1997, 9, 265–272. [Google Scholar]
- Van der Heijden, R.; Jacobs, D.I.; Snoeijer, W.; Hallared, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Sheludko, Y.; Gerasimenko, I.; Unger, M.; Kostenyuk, I.; Stöckigt, J. Induction of alkaloid diversity in hybrid plant cell cultures. Plant Cell Rep. 1999, 18, 911–918. [Google Scholar] [CrossRef]
- Sheludko, Y.; Gerasimenko, I.; Platonova, O. Divergence of the indole alkaloid pattern in two somatic hybrid plant cell subcultures of Rauvolfia serpentina × Rhazya stricta. Planta Med. 2000, 66, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Cordell, G.A.; Smith, G.F.; Smith, G.N. Presecamines, a new group of dimeric indole alkaloids from Rhazya species, and their thermally derived monomers, secodine and 15,20-dihydrosecodine. Chem. Commun. 1970, 4, 191–192. [Google Scholar] [CrossRef]
- Evans, D.A.; Smith, G.F.; Smith, G.N.; Stapleford, K.S.J. Rhazya alkaloids: The secamines, a new group of indole alkaloids. Chem. Commun. 1968, 15, 859–861. [Google Scholar] [CrossRef]
- Zsadon, B.; Szilasi, M.; Tamás, J.; Kaposi, P. Dimeric alkaloids of secodine-type from Amsonia tabernaemontana roots. Phytochemistry 1975, 14, 1438–1439. [Google Scholar] [CrossRef]
- Zsadon, B.; Vegh, A.; Hubay, R. Tabersonine isolated from Rhazya orientalis seeds. Magy. Kem. Foly. 1971, 77, 52–53. [Google Scholar]
- Rischer, H.; Orešič, M.; Seppänen-Laakso, T.; Katajamaa, M.; Lammertyn, F.; Ardiles-Diaz, W.; van Montagu, M.C.; Inzé, D.; Oksman-Caldentey, K.-M.; Goossens, A. Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc. Natl. Acad. Sci. USA 2006, 103, 5614–5619. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Hairy root samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhgari, A.; Laakso, I.; Seppänen-Laakso, T.; Yrjönen, T.; Vuorela, H.; Oksman-Caldentey, K.-M.; Rischer, H. Analysis of Indole Alkaloids from Rhazya stricta Hairy Roots by Ultra-Performance Liquid Chromatography-Mass Spectrometry. Molecules 2015, 20, 22621-22634. https://doi.org/10.3390/molecules201219873
Akhgari A, Laakso I, Seppänen-Laakso T, Yrjönen T, Vuorela H, Oksman-Caldentey K-M, Rischer H. Analysis of Indole Alkaloids from Rhazya stricta Hairy Roots by Ultra-Performance Liquid Chromatography-Mass Spectrometry. Molecules. 2015; 20(12):22621-22634. https://doi.org/10.3390/molecules201219873
Chicago/Turabian StyleAkhgari, Amir, Into Laakso, Tuulikki Seppänen-Laakso, Teijo Yrjönen, Heikki Vuorela, Kirsi-Marja Oksman-Caldentey, and Heiko Rischer. 2015. "Analysis of Indole Alkaloids from Rhazya stricta Hairy Roots by Ultra-Performance Liquid Chromatography-Mass Spectrometry" Molecules 20, no. 12: 22621-22634. https://doi.org/10.3390/molecules201219873