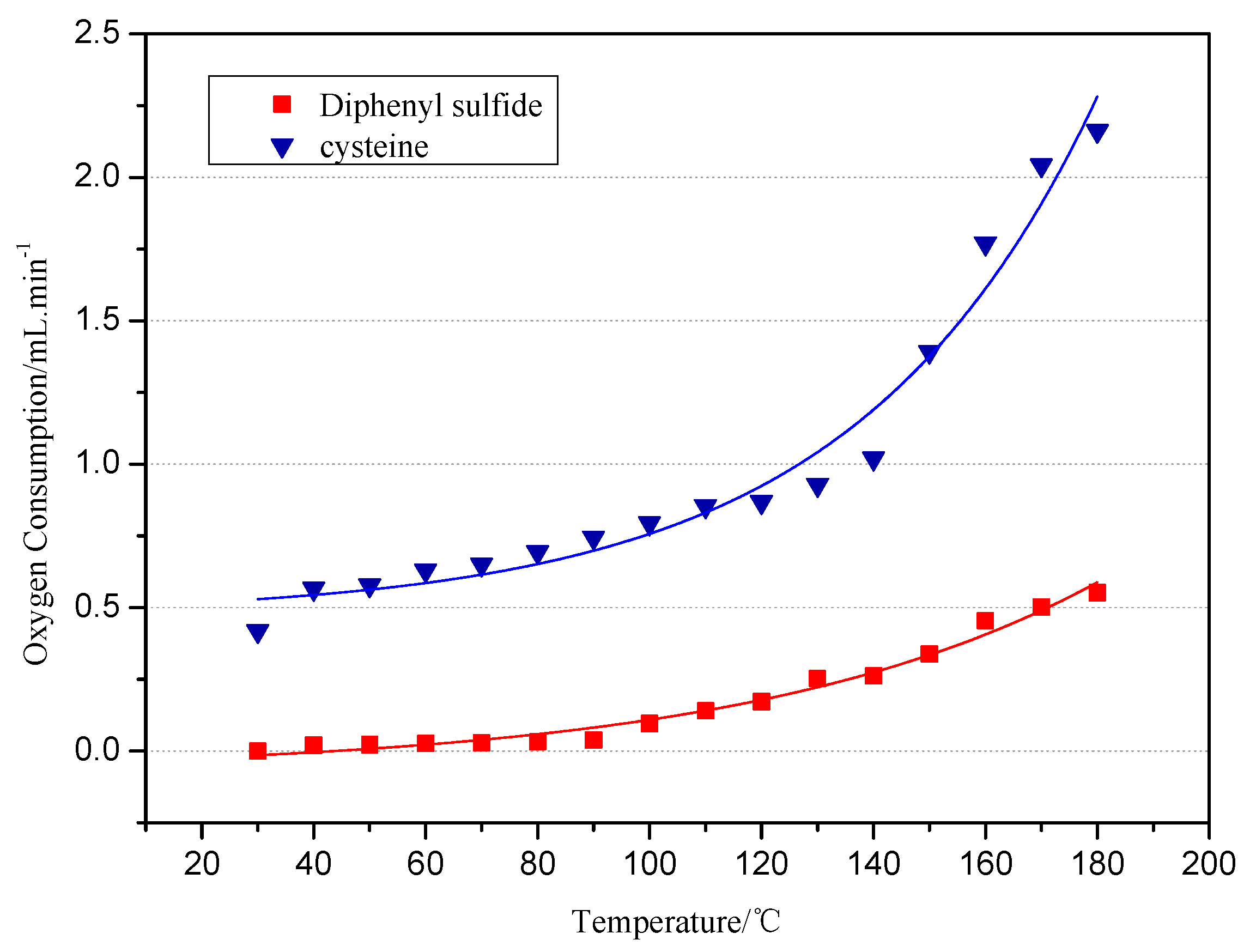
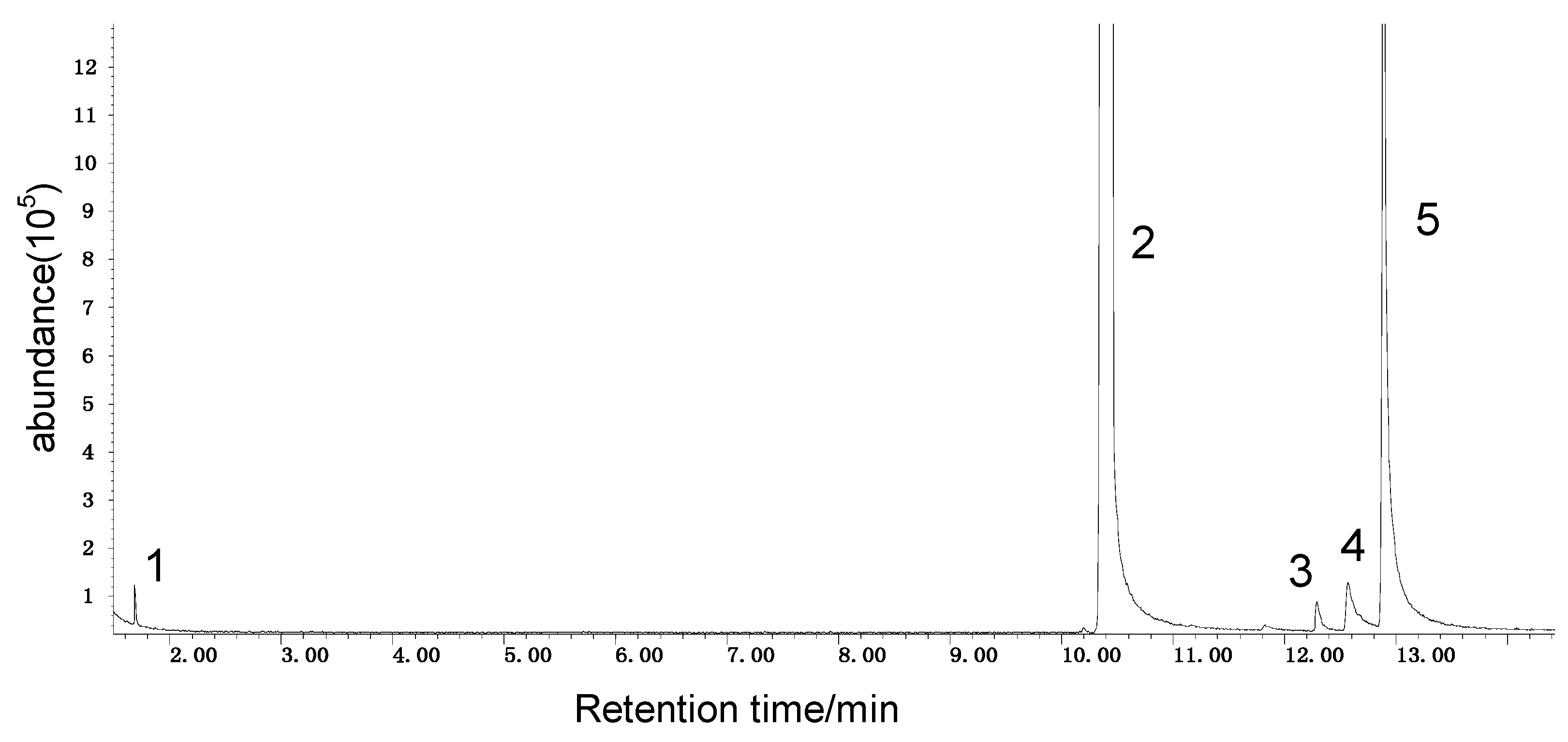
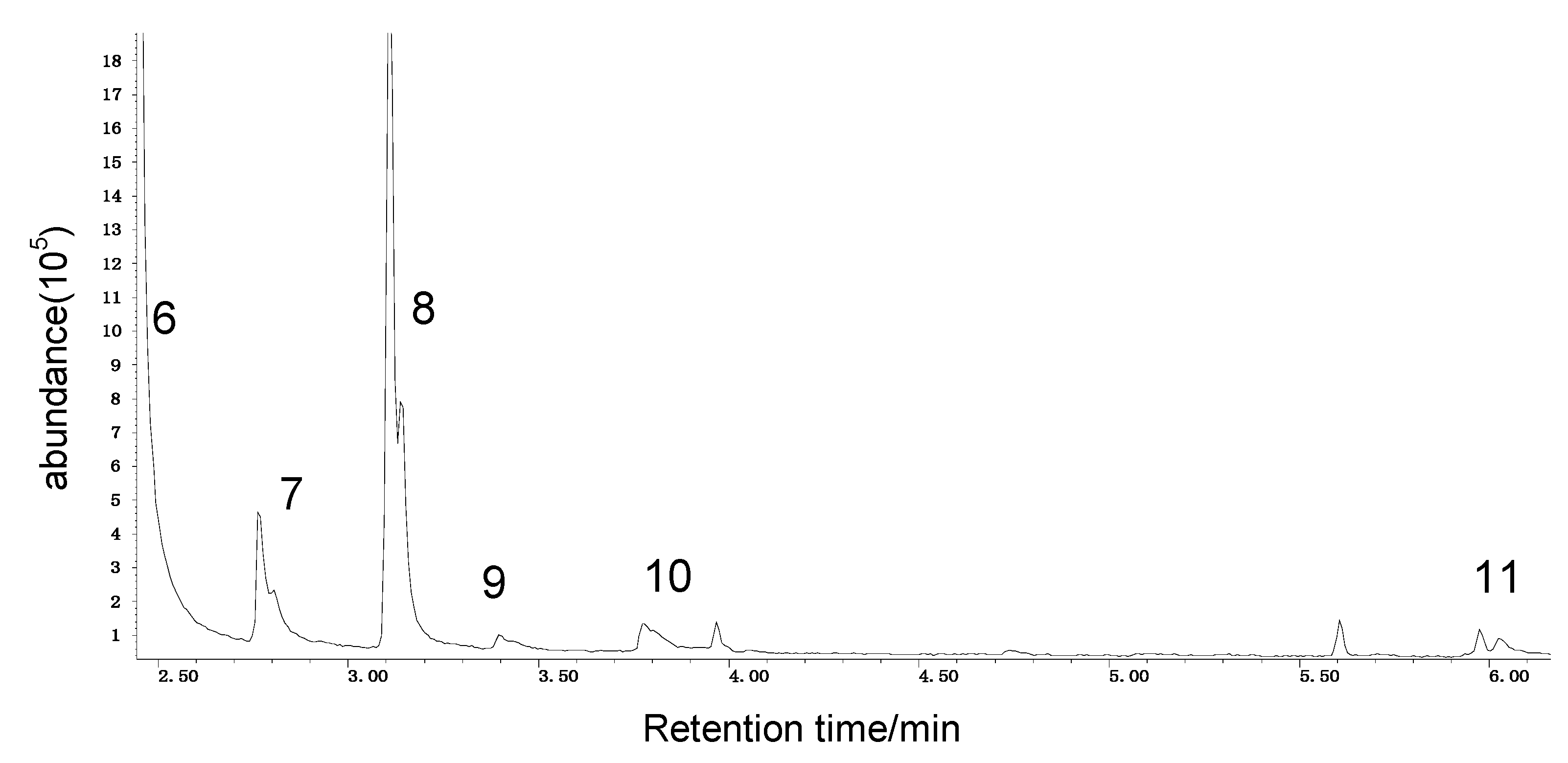
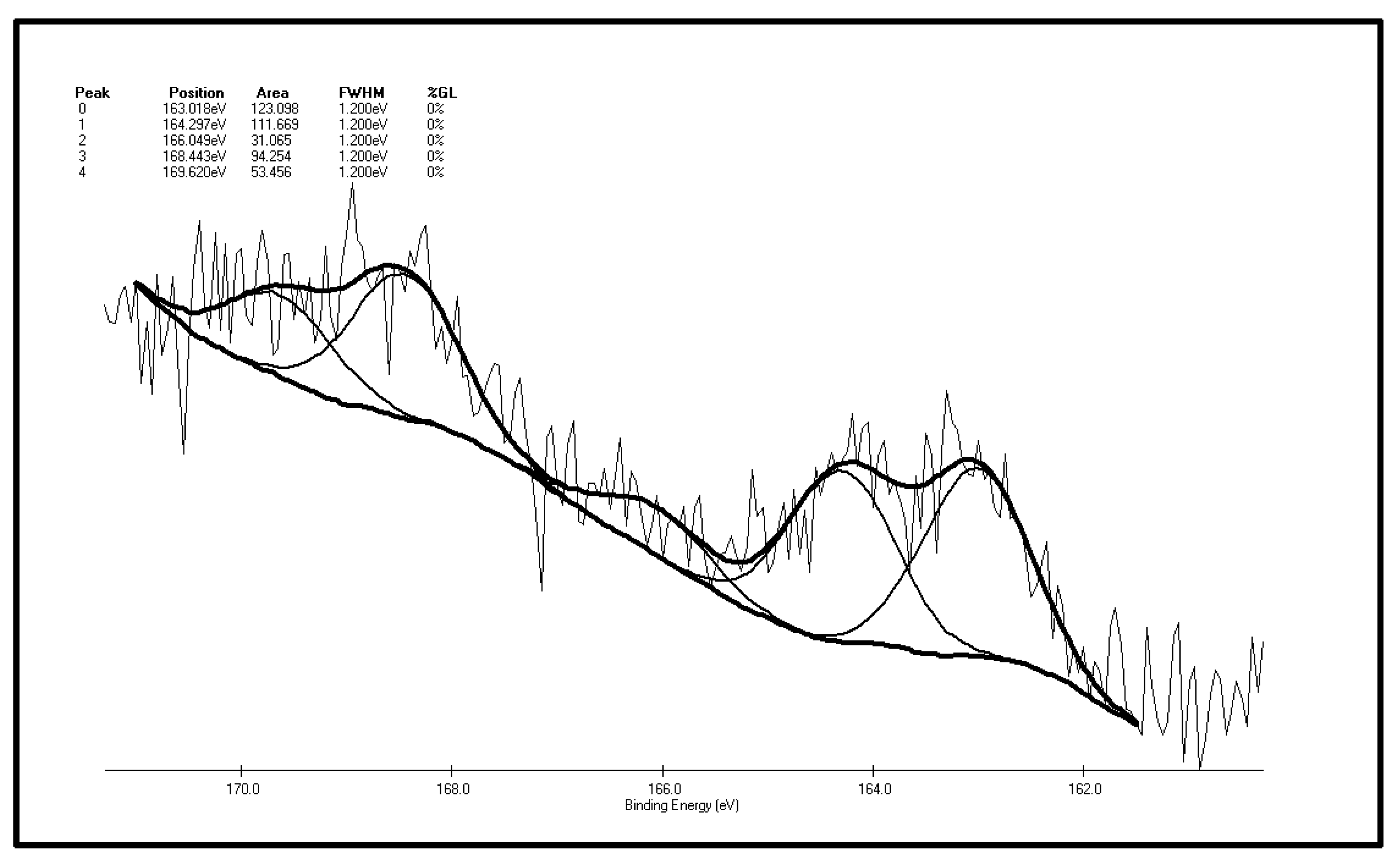
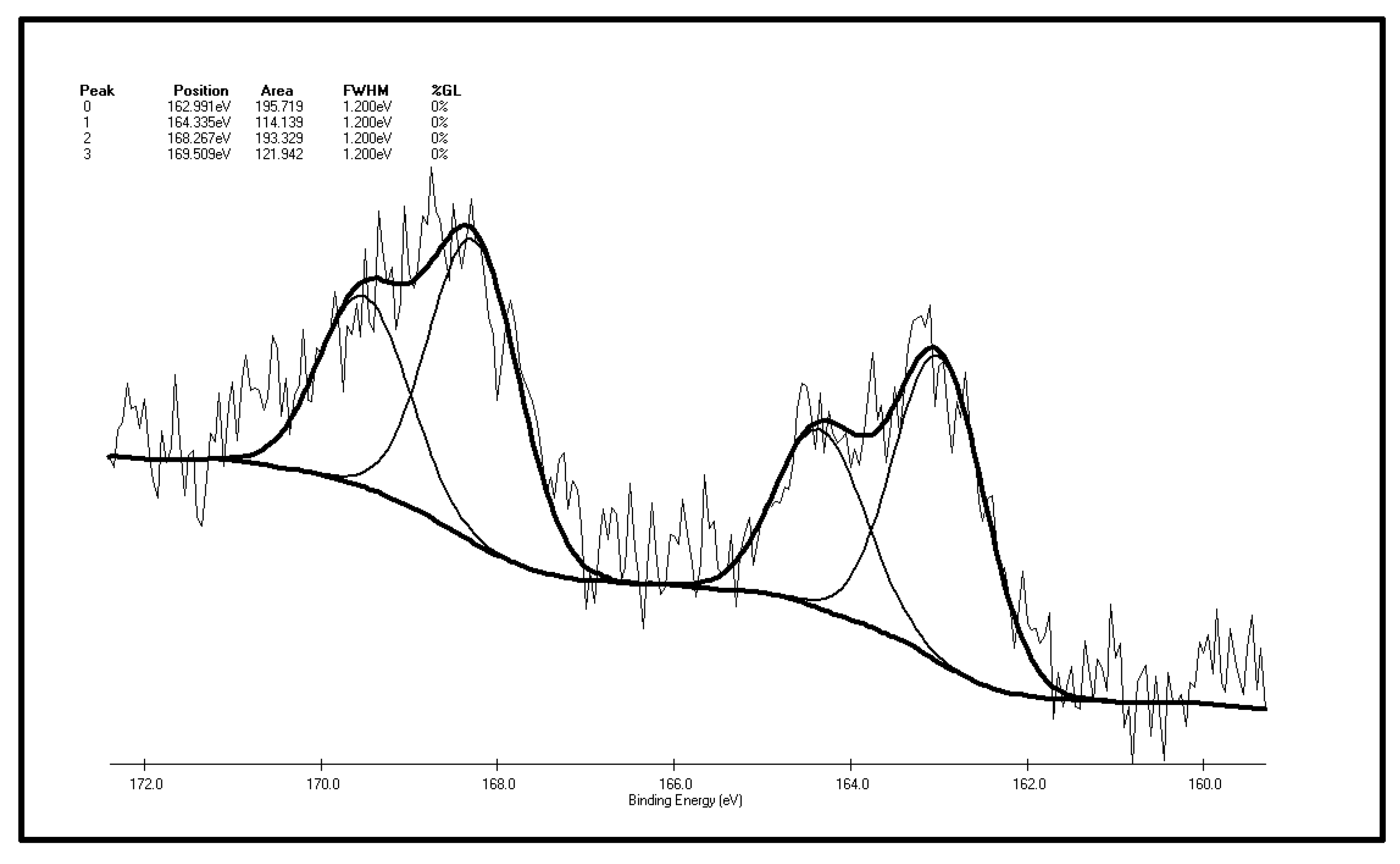
Spontaneous coal combustion is a severe disaster ocurring widely in coal industry. It not only occurs during the coal exploitation process, but also during the processes of coal transport and storage. Meanwhile, it causes great economical losses, as well as safety and environmental issues [
1,
2,
3]. Some scholars believe that deep coal beds have a high sulfur content while shallow coal beds have low sulfur content [
4]. As massive scale exploitation and utilization of coal resources continues, the sulfur content in coal is increasing. Sulfur in coal can be classified into two categories: inorganic and organic sulfur. Inorganic sulfur is present in the form of pyrite and sulfate, mainly the former. Often, organic sulfur and the macromolecular structure of coal join together in the form of covalent bonds with complex structure and hard to separate. It mainly includes the form of mercaptans, sulfide, disulphides, thioethers, sulfoxides, sulphones, thiophene, sulfoacids,
etc. [
5,
6,
7]. It is generally acknowledged that pyrite as the main component of inorganic sulfur exerts an important effect on coal spontaneous combustion. In the presence of water, pyrite reacts with oxygen to form sulfate, H
2O
2, and hydroperoxides, and thereby oxidation is initiated [
8,
9,
10,
11]. Nearly all coals contain more or less organic sulfur. According to Chinese statistics, low sulfur coal whose total sulfur content is lower than 0.5%, mainly contains organic sulfur. Organic sulfur in high sulfur coal, whose average sulfur content registers 2.76%, accounts for 1.04% of the total amount and 37.7% of the total sulfur [
12,
13,
14]. The organic sulfur content in some superhigh organosulfur coals such as Spanish lignite from Mequinenza and Croatian coal from Raša is over 10%, while, the organic sulfur content in Zelanian coal from Charming Creek (New Zealand) and Indian coal from Tipong is 5% [
15]. Because organic sulfur in coal has a complex structure and cannot be separated as it often exists in macromolecular form, its form and content cannot be determined directly so far [
16]. People usually use destructive methods to study the forms of organic sulfur in coal. The principle is that HS is produced by pyrolysis and catalytic reduction or SO by oxidation reaction [
5]. The analyses mainly involve temperature programmed reduction (TPR) [
17], temperature programmed pyrolysis (TPP) [
17], temperature programmed oxidation (TPO) [
18,
19], fast pyrolysis [
20]
etc. Nowadays, some non-destructive technologies such as XANS [
21], or XPS [
22,
23] are widely applied in the determination of sulfur forms in coal. It is noteworthy that the information about surface sulfur in coal samples detected by surface-analysis technologies is not identical to forms of bulk sulfur found [
22,
24,
25].
The oxidation characteristics of organic sulfur and inorganic sulfur in coal during the coal spontaneous combustion process are different. People often ignore the role of organic sulfur during the process of coal spontaneous combustion. From modern chemistry knowledge, knowing that sulfur uses 3p orbital overlap with other atoms to form π bonds, the π bond overlap among 2p and 3p orbitals or between 3p orbitals is smaller, the distance between π electrons and the nucleus is also farther, and C-S bond and S-H bonds are prone to break. Besides, the atomic volume of sulfur is larger, while its electronegativity is smaller, so the valence shell is far from the nucleus, and less influenced by the nucleus. Compared to carbon atoms, sulfur atoms is more easily oxidized [
26], so the organic sulfur functional groups in coal are often more active, although the oxidation characteristics of the various forms of organic sulfur differ greatly. For example, the mercapto group in mercaptans has strong reductibility, and it can be instantly oxidized to disulfide with cryogenic air. Under moderate conditions, this reaction can take place and disulfide can be further oxidized to a sulfonic acid [
27]. Sulfides such as thioethers also can be oxidized by air to sulfoxides and sulphones. However, –SO
2 bond connected with aliphatic and aromatic functional groups are prone to C–S bond breakage under low temperature heating conditions, and this releases SO
2 gas. Under a higher temperature sulfoxides and sulphones with β hydrogens, can be oxidized to sulfinic acids and sulfonic acids [
28]. Organic sulfur in thiophene is very stable, even at 500 °C [
29]. LaCount
et al., reported that the model compound thiophene could release SO
2 at 450 °C [
30] and the reaction can take place slowly with water at 300 °C [
31]. From the perspective of dissociation energy, in the structure of R–S–H, the dissociation energy of C–S bond is 20 kcal lower than that of the S–H bond, because the sulfur atom function weakens the bond energy. The C–S bond energy when connected with a phenyl is higher than in the corresponding aliphatic hydrocarbon compound. This is due to the fact that the unpaired electrons and the aromatic structure produce a conjugation effect [
32]. Dark
et al., claimed that at 20–25 °C the C–S bond easily breaks and produces free radicals, so radicals appear during low-temp oxidation. As the aliphatic carbon atom content increases, the C–S bond dissociation energy decreases significantly [
33]. On the contrary, the dissociation energy of an aromatic disulfide S–S bond is lower than an aliphatic disulfide one because it is undermined by the phenyls [
32]. The technology for oxidative desulphurization of organic sulfur in coal is based on the characteristic that C–S bonds and S–S bonds are easy to break during low-temp oxidation. At present, this technology has been widely applied in the coal chemical industry. Borah
et al., believe that desulphurization of organic sulfur in coal is due to the release of sulfur free radicals under 50 °C, but when temperature reaches 50 °C, along with free radicals, volatile sulfur compounds begins to participate [
6]. After oxidation pretreatment, the organic sulfur in coal will be broken down into smaller molecules, thus making further desulphurization easier. They also believe that low-temp oxidation can convert organic sulfur in coal to S=O and –SO
2 groups [
6,
30,
34]. Gorbaty
et al., conducted oxidation experiments on three coal samples with air in 125 °C, the XPS and XANS results showed that aliphatic sulfides are more easily oxidized than aromatic sulfides. The aliphatic sulfides were then converted into sulfoxides, sulfones and sulfonic acids. One important thing is that sulfonic acid is easier to produce in solution than by air oxidation [
35,
36]. Pietrzak and Grzybek did series of oxidation experiments using different rank coals by using O
2/Na
2CO
3, PPA, and their XPS results showed that the oxidation sequence of the organic sulfur in coal is: sulphide → sulfoxide → sulfone. They also found the sulfur enrichment on the surface of coal during oxidation is probably due to the opening and expansion of coal pores [
23,
37,
38].
All in all, the organic sulfur groups in coal show strong reactivity, and some organic sulfur presents itself as mercaptan, thioether and other groups, which can be oxidized at normal temperature. During oxidation, free radicals can be produced. Due to the easily-oxidized features of organic sulfur, people have conducted plenty of studies on desulphurization that have yielded fruitful results [
34,
39], but scholars have seldom researched the changes in organic sulfur functional groups during the process of coal low-temp oxidation in air [
6], let alone the effect of organic sulfur on the characteristics of spontaneous coal combustion. In light of the above, this paper selects two representative organic sulfur compounds as model compounds. By analyzing the chromatograms of their gaseous low-temp oxidation products and GC/MS scans of the extractable matter in the oxidation residues, we tried to figure out what happens to these sulfur model compounds during low temp-oxidation. On this basis, we conducted an experiment against coal samples containing organic sulfur from Xingcheng, Guizhou Province (China). By adopting XPS technology, we investigated the sulfur form changes in coal samples before and after low-temp oxidation so as to further study the role organic sulfur plays in spontaneous coal combustion.