1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of db/db Mice
Abstract
:1. Introduction
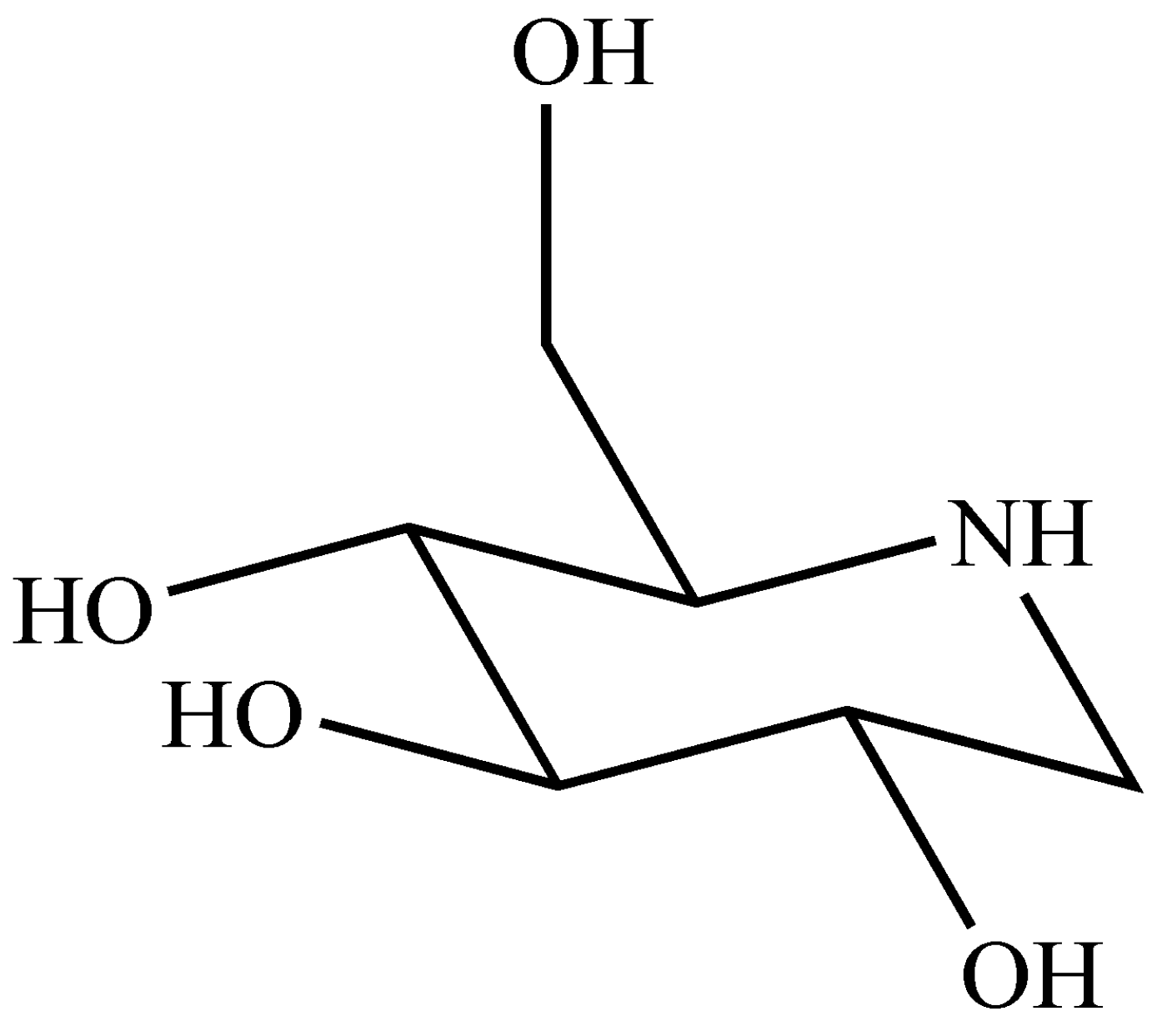
2. Results and Discussion
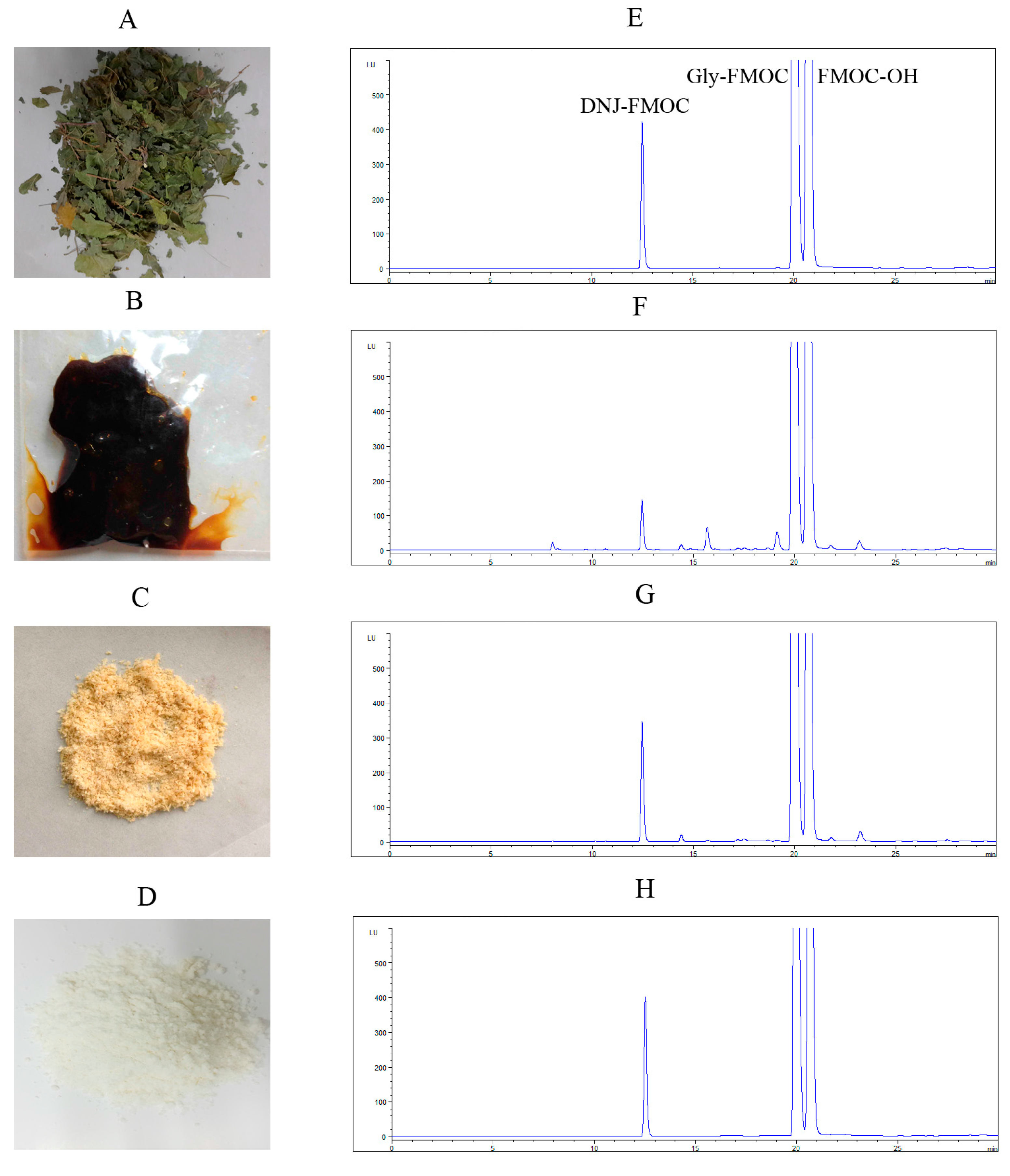
2.1. Preparation of DNJ
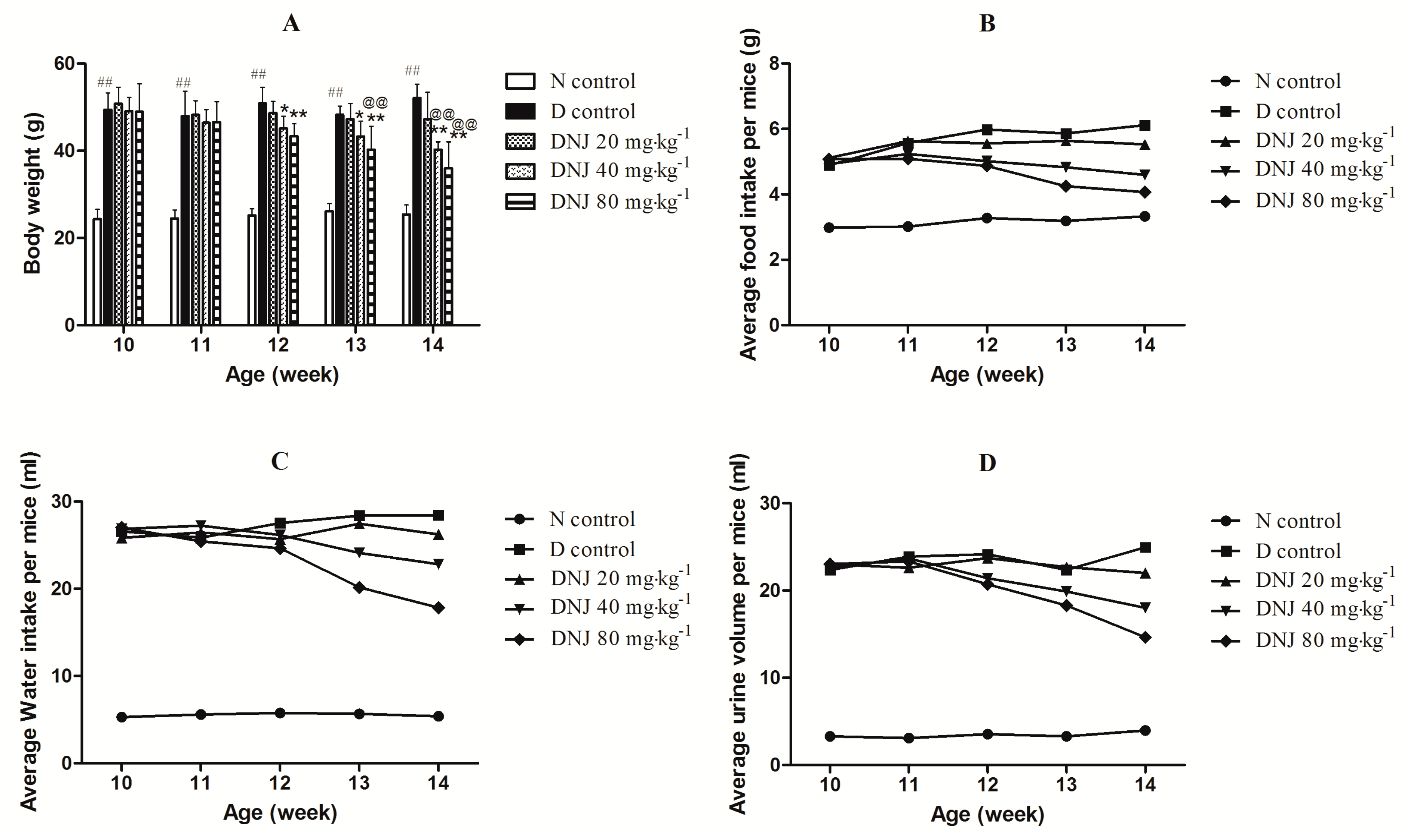
2.2. Effectsof DNJ on Body Weight and Average Food Intake, Water Intake, and Urine Output
2.3. DNJ Protecteddb/db Mice against the Onset of Type 2 Diabetes

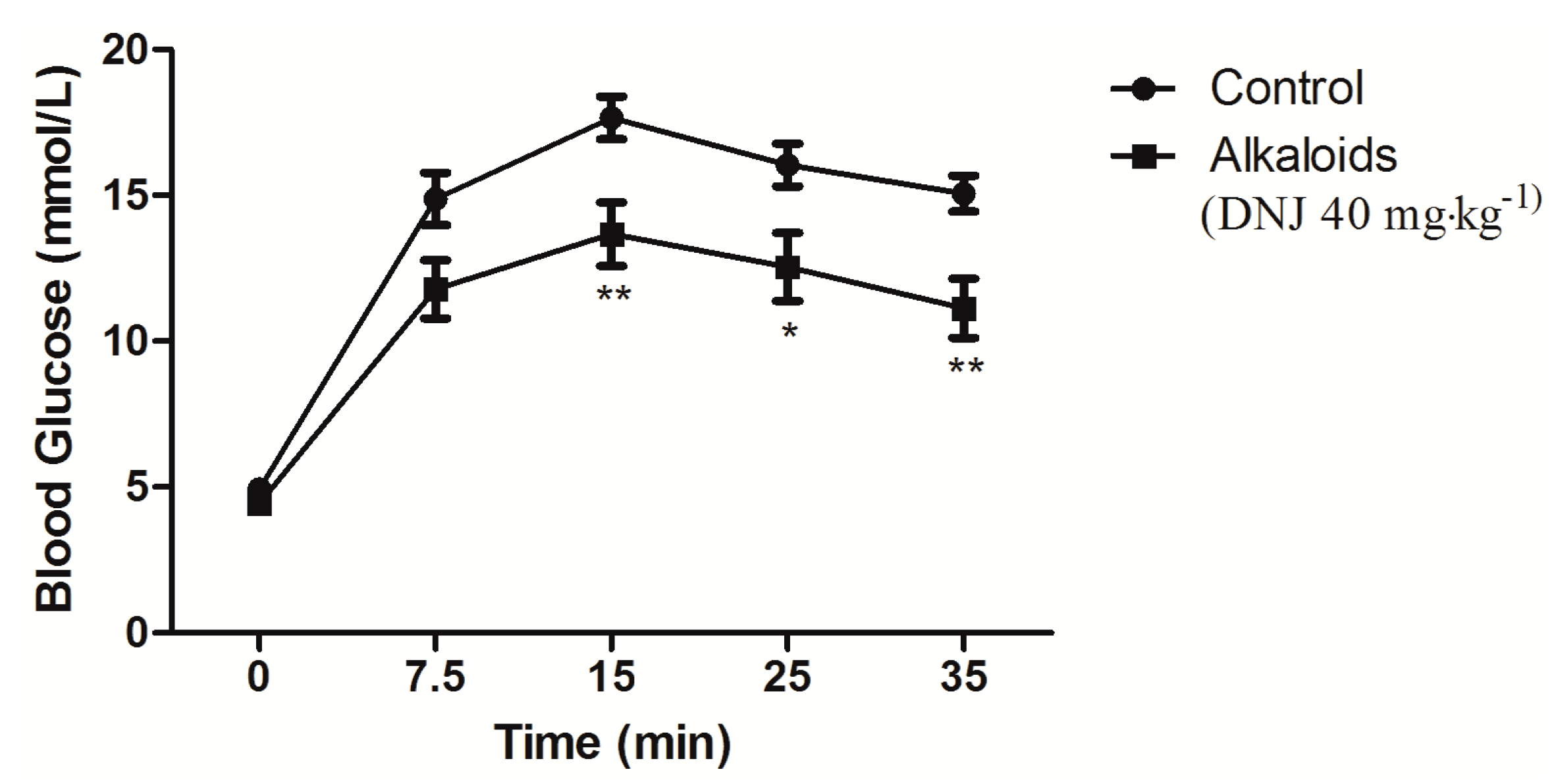
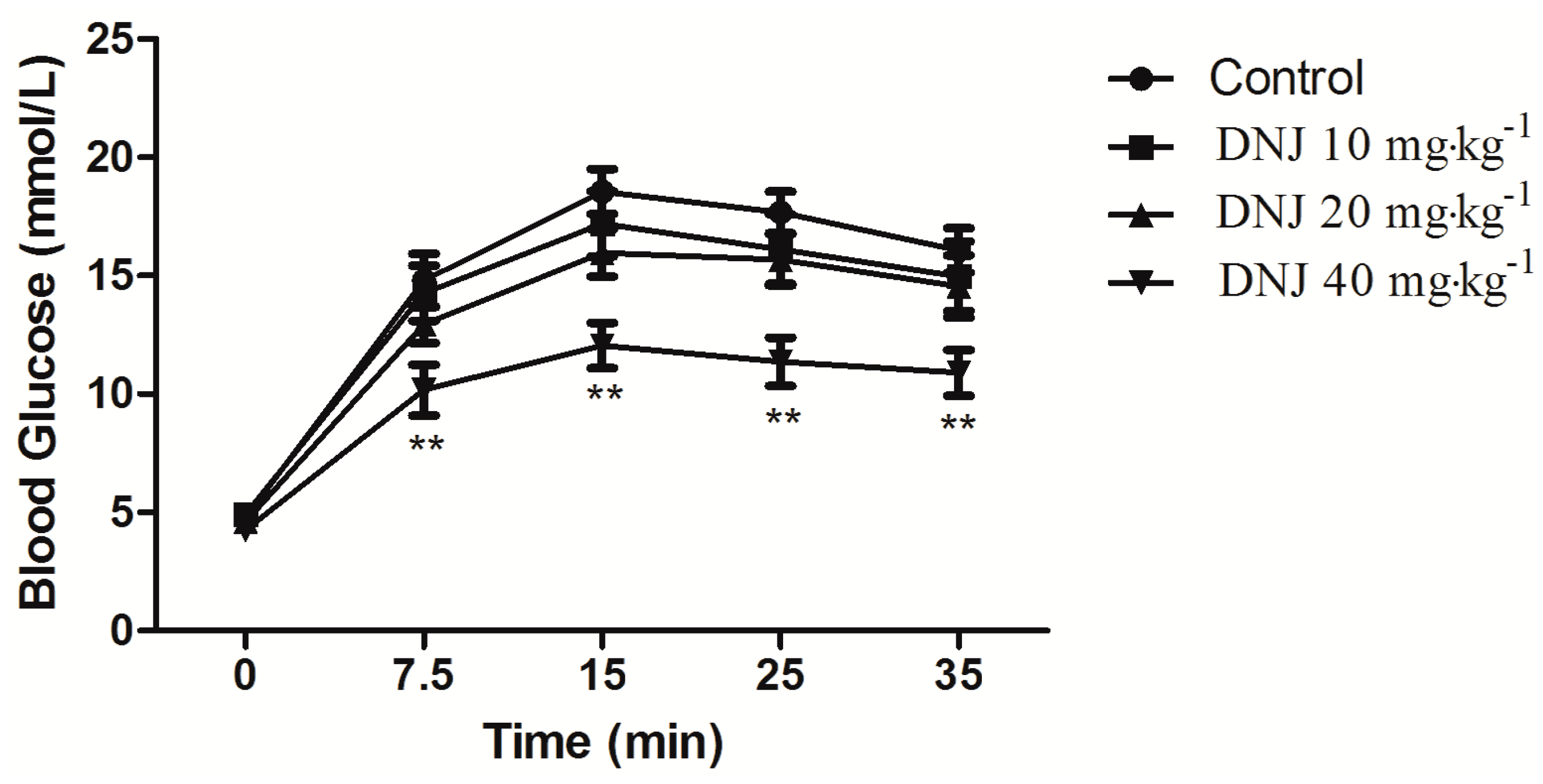
2.4. Effect of DNJ on IPGTT and IPITT

2.5. Effect of DNJ onGLUT4 Protein Expression and Translocation

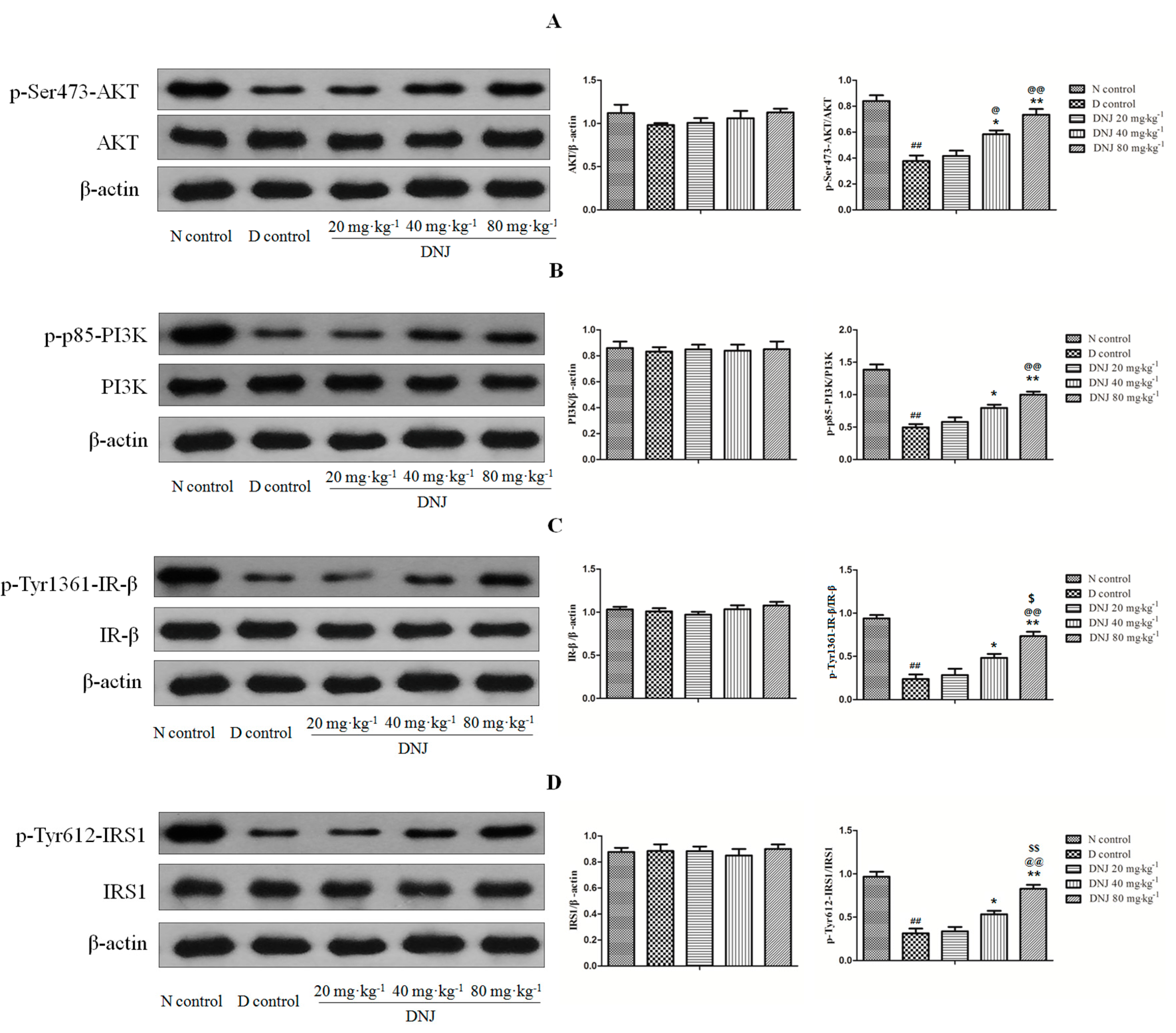
2.6. DNJ Up-Regulated Phosphorylation of AKT, PI3K, IR-β, and IRS1
3. Experimental Section
3.1. Materials
3.2. Preparation of DNJ
3.3. Quantitative Determination of DNJ
3.4. Animals
3.5. Experimental Design
3.6. Body Weight and Average Food Intake, Water Intake, Urine Output
3.7. Glucose and Serum Insulin Measurements
3.8. Intraperitoneal Glucose Tolerance Test (IPGTT) and Intraperitoneal Insulin Tolerance Test (IPITT)
3.9. Extraction of Membrane Protein from Skeletal Muscle
3.10. Western Blot
3.11. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Zheng, T.; Shu, G.; Yang, Z.; Mo, S.; Zhao, Y.; Mei, Z. Antidiabetic effect of total saponins from Entada phaseoloides (L.) Merr. in type 2 diabetic rats. J. Ethnopharmacol. 2012, 139, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L.; Barbour, E.K.; Abulnaja, K.O.; Moselhy, S.S. Management of hyperglycaemia by ethyl acetate extract of Balanites aegyptiaca (desert date). Molecules 2015, 20, 14425–14434. [Google Scholar] [CrossRef] [PubMed]
- Beit-Yannai, E.; Ben-Shabat, S.; Goldschmidt, N.; Chapagain, B.P.; Liu, R.H.; Wiesman, Z. Antiproliferative activity of steroidal saponins from Balanites aegyptiaca—An in vitro study. Phytochem. Lett. 2011, 4, 43–47. [Google Scholar] [CrossRef]
- Whiting, D.R.; Guariquata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Nolan, T.; Beagley, J.; Linnenkamp, U.; Jacqmain, O. IDF Diabetes Atlas, 6th ed.; International Diabetes Federation: Brussels, Belgium, 2013; p. 160. [Google Scholar]
- Bakar, M.H.A.; Sarmidi, M.R.; Cheng, K.K.; Khan, A.A.; Chua, L.S.; Huri, H.Z.; Yaakob, H. Metabolomics—The complementary field in systems biology: A review on obesity and type 2 diabetes. Mol. Biosyst. 2015, 11, 1742–1774. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Tinshun, Z.; Nakamura, T.; Ashida, H. Fermented tea improves glucose intolerance in mice by enhancing translocation of glucose transporter 4 in skeletal muscle. J. Agric. Food Chem. 2012, 60, 11366–11371. [Google Scholar] [CrossRef] [PubMed]
- Kooij, R.; Branderhorst, H.M.; Bonte, S.; Wieclawska, S.; Martin, N.I.; Pieters, R.J. Glycosidase inhibition by novel guanidinium and urea iminosugar derivatives. Med. Chem. Comm. 2013, 4, 387–393. [Google Scholar] [CrossRef]
- Tsuduki, T.; Kikuchi, I.; Kimura, T.; Nakagawa, K.; Miyazawa, T. Intake of mulberry 1-deoxynojirimycin prevents diet-induced obesity through increases in adiponectin in mice. Food Chem. 2013, 139, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Chen, F. Antihyperglycemic effects of N-containing sugars derived from mulberry leaves is streptozocin-in-duced diabetic mice. Wakan Iyakugaku Zasshi 1995, 12, 214–219. [Google Scholar]
- Yagi, M.; Kouno, T.; Aoyagi, Y.; Murai, H. The structure of moranoline, a peperidine alkaloid from Morus species. Nippon Nogei Kagaku Kaishi 1976, 50, 571–572. [Google Scholar] [CrossRef]
- Zechel, D.L.; Boraston, A.B.; Gloster, T.; Boraston, C.M.; Macdonald, J.M.; Tilbrook, D.M.; Stick, R.V.; Davies, G.J. Iminosugar glycosidase inhibitors: Structural and thermodynamic dissection of the binding of isofagomine and 1-deoxynojirimycin to β-glucosidases. J. Am. Chem. Soc. 2003, 125, 14313–14323. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, B.; Vlahos, C.J.; Cheatham, L.; Wang, L.; Blenis, J.; Kahn, C.R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell Biol. 1994, 14, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Le Marchand-Brustel, Y.; Gautier, N.; Cormont, M.; van Obberghen, E. Wortmannin inhibits the action of insulin but not that of okadaic acid in skeletal muscle: Comparison with fat cells. Endocrinology 1995, 136, 3564–3570. [Google Scholar] [PubMed]
- Nakagawa, K.; Kubota, H.; Kimura, T.; Yamashita, S.; Tsuzuki, T.; Oikawa, S.; Miyazawa, T. Occurrence of orally administered mulberry 1-deoxynojirimycin in rat plasma. J. Agric. Food Chem. 2007, 55, 8928–8933. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L. Current antihyperglycemic treatment guidelines and algorithms for patients with type 2 diabetes mellitus. Am. J. Med. 2010, 123, 12–18. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes: Metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997, 5, 177–269. [Google Scholar] [CrossRef]
- Lanza, I.R.; Sreekumaran Nair, K. Regulation of skeletal muscle mitochondrial function: Genes to proteins. Acta Physiol. 2010, 199, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; Defronzo, R.A.; Shulman, R.G. Quantitation of muscle glycogen-synthesis in normal subjects and subjects with non-insulin-dependent diabetes by c-13 nuclear magnetic-resonance spectroscopy. N. Engl. J. Med. 1990, 322, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Akira, A.; Nakaqawa, K.; Hiquchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar]
- Wang, T.; Li, C.Q.; Zhang, H.; Li, J.W. Response surface optimized extraction of 1-deoxynojirimycin from mulberry leaves (Morus alba L.) and preparative separation with resins. Molecules 2014, 19, 7040–7056. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.H.; Oh, S.H.; Ahn, Y.R.; Kim, K.W.; Kim, J.H.; Seo, S.W. Antiobesity effects and improvement of insulin sensitivity by 1-deoxynojirimycin in animal models. J. Agric. Food Chem. 2008, 56, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Jung, H.; Karuppasamy, S.; Lee, J.Y.; Kang, K.D.; Hwang, K.Y.; Seong, S.I.; Suh, J.G. Anti-hyperlipidemic effect of soybean extract fermented by Bacillus subtilis MORI in db/db mice. Lab. Anim. Res. 2012, 28, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Forte, T.M.; Taniguchi, S.; Ishida, B.Y.; Oka, K.; Chan, L. The db/db mouse, a model for diabetic dyslipidemia: Molecular characterization and effects of Western diet feeding. Metabolism 2000, 49, 22–31. [Google Scholar] [CrossRef]
- Burra, P. Liver abnormalities and endocrine diseases. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Kwon, H.J.; Chung, J.Y.; Kim, J.Y.; Kwon, O. Comparison of 1-deoxynojirimycinand aqueous mulberry leaf extract with emphasis on postprandial hypoglycemic effects: In vivo and in vitro studies. J. Agric. Food Chem. 2011, 59, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Lv, Z.Q.; Lin, T.B. Cooperative anti-diabetic effects of deoxynojirimycin-polysaccharide by inhibiting glucose absorption and modulating glucose metabolism in streptozotocin-induced diabetic mice. PLoS ONE 2013, 8, e65892. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Lv, Z.Q.; Lin, T.B.; Chen, S.; Hu, G.Y. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in alloxan-induced diabetic mice. J. Ethnopharmacol. 2011, 134, 961–970. [Google Scholar] [PubMed]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Lin, T.B.; Lv, Z.Q.; Hu, G.Y.; Wang, X. 1-deoxynojirimycin inhibits glucose absorption and accelerates glucose metabolism in streptozotocin-induced diabetic mice. Sci. Rep. 2013, 3, 1377. [Google Scholar] [CrossRef] [PubMed]
- Benalla, W.; Bellahcen, S.; Bnouham, M. Antidiabetic medicinal plants as a source of α-glucosidase inhibitors. Curr. Diabetes Rev. 2010, 6, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jwa, H.; Yanagawa, Y.; Park, T. Extract from Dioscorea batatas ameliorates insulin resistance in mice fed a high-fat diet. J. Med. Food 2012, 15, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Cha, B.Y.; Saito, K.; Yamakawa, H.; Choi, S.S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.K.; Xu, X.G.; Wong, Y.C.; Sim, S.Z.; Lee, K.O. Des-aspartate-angiotensin I exerts hypoglycemic action via glucose transporter-4 translocation in type 2 diabetic kkay mice and gk rats. Endocrinology 2007, 148, 5925–5932. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Thirone, A.C.; Huang, C.; Klip, A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol. Metab. 2006, 17, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sargis, R.M.; Neel, B.A.; Brock, C.O.; Lin, Y.; Hickey, A.T.; Carlton, D.A.; Brady, M.J. The novel endocrine disruptor tolylfluanid impairs insulin signaling in primary rodent and human adipocytes through a reduction in insulin receptor substrate-1 levels. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Liu, K.; Huang, F.; Guo, X.; Wang, M.; Liu, B. Opposite effects of quercetin, luteolin, and epigallocatechin gallate on insulin sensitivity under normal and inflammatory conditions in mice. Inflammation 2013, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, Y.B. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J. Intern. Med. 2010, 25, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Lee, J.J.; Kim, Y.; Kim, I.S.; Han, J.H.; Lee, S.G.; Ahn, M.J.; Jung, S.H.; Myung, C.S. Effect of eriodictyol on glucose uptake and insulin resistance in vitro. J. Agric. Food Chem. 2012, 60, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Kolnes, A.J.; Ingvaldsen, A.; Bolling, A.; Stuenaes, J.T.; Kreft, M.; Zorec, R.; Shepherd, P.R.; Jensen, J. Caffeine and theophylline block insulin-stimulated glucose uptake and PKB phosphorylation in rat skeletal muscles. Acta Physiol. 2010, 200, 65–74. [Google Scholar] [CrossRef] [PubMed]
- McClenaghan, N.H. Physiological regulation of the pancreatic β-cell: Functional insights for understanding and therapy of diabetes. Exp. Physiol. 2007, 92, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Giani, J.F.; Mayer, M.A.; Munoz, M.C.; Silberman, E.A.; Hocht, C.; Taira, C.A.; Gironacci, M.M.; Turyn, D.; Dominici, F.P. Chronic infusion of angiotensin-(1–7) improves insulin resistance and hypertension induced by a high-fructose diet in rats. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E262–E271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Zhang, X.H.; Yu, Y.; Poulev, A.; Ribnicky, D.; Floyd, Z.E.; Cefalu, W.T. Bioactives from bitter melon enhance insulin signaling and modulate acyl carnitine content in skeletal muscle in high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, S.U.; Lee, H.S.; Kim, I.; Ahn, M.Y.; Ryu, K.S. Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2003, 1002, 93–99. [Google Scholar] [CrossRef]
- Hagberg, C.E.; Mehlem, A.; Falkevall, A.; Muhl, L.; Fam, B.C.; Ortsater, H.; Scotney, P.; Nyqvist, D.; Samén, E.; Lu, L.; et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 2012, 490, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Huang, C.F.; Chen, Y.W.; Yen, Y.P.; Wu, C.T.; Uang, B.J.; Yang, R.S.; Liu, S.H. Antidiabetic effects of pterosin A, a small-molecular-weight natural product, on diabetic mouse models. Diabetes 2013, 62, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ok, H.M.; Kwon, O. Protective effects of Korean red ginseng against alcohol-induced fatty liver in rats. Molecules 2015, 20, 11604–11616. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Nagaoka, M.; Yonezawa, T.; Tanabe, R.; Woo, J.T.; Kato, H.; Chung, U.I.; Yagasaki, K. Regulatory mechanism for the stimulatory action of genistein on glucose uptake in vitro and in vivo. J. Nutr. Biochem. 2012, 23, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of 1-Deoxynojirimycin is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Peng, G. 1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of db/db Mice. Molecules 2015, 20, 21700-21714. https://doi.org/10.3390/molecules201219794
Liu Q, Li X, Li C, Zheng Y, Peng G. 1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of db/db Mice. Molecules. 2015; 20(12):21700-21714. https://doi.org/10.3390/molecules201219794
Chicago/Turabian StyleLiu, Qingpu, Xuan Li, Cunyu Li, Yunfeng Zheng, and Guoping Peng. 2015. "1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of db/db Mice" Molecules 20, no. 12: 21700-21714. https://doi.org/10.3390/molecules201219794




