Influence of Excipients and Spray Drying on the Physical and Chemical Properties of Nutraceutical Capsules Containing Phytochemicals from Black Bean Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extract Powder Production
2.1.1. Powder Moisture and Hygroscopicity
| Run | Total Solids in Solution Feed (%) | Maltodextrin in Total Solids (%) | Moisture Content (%) | Hygroscopicity (g/100 g) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 5 | 6 | 5.8 ± 0.3 a | 27.3 ± 2.7 a | 78.6 ± 1.4 a |
| 2 | 5 | 15 | 5.7 ± 0.1 a | 21.4 ± 1.6 b | 77.2 ± 0.6 a |
| 3 | 5 | 30 | 5.5 ± 0.2 a | 19.6 ± 1.1 b | 20.6 ± 3.8 d |
| 4 | 5 | 45 | 5.3 ± 0.1 a | 22.1 ± 1.3 b | 18.0 ± 5.7 d |
| 5 | 10 | 60 | 5.6 ± 0.0 a | 17.6 ± 1.8 c | 77.4 ± 0.5 a |
| 6 | 10 | 80 | 5.2 ± 0.1 a | 17.0 ± 1.2 c | 46.9 ± 4.3 c |
| 7 | 10 | 90 | 5.4 ± 0.1 a | 13.5 ± 1.4 d | 66.5 ± 2.9 b |
2.1.2. Powder Phytochemical Characterization
| Phytochemical | λmax Absorption | [M + H]+ (m/z) | Content (mg/100 g DB) | Recovery of Phytochemicals after Spray Drying (%): Run | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||||
| Myricetin-3O-glucoside | 260 | 358 | 481 | 2.1 ± 0.1 | 100 | 61 | 100 | 67 | 84 | 79 | 69 |
| Quercetin-3O-glucoside | 258 | 356 | 465 | 0.8 ± 0.2 | 100 | 69 | 54 | 43 | 51 | 12 | 27 |
| Kaempferol-3O-glucoside | 266 | 348 | 449 | 0.6 ± 0.1 | 100 | 74 | 63 | 47 | 56 | 78 | 47 |
| Myricetin | 256 | 274 | 319 | 1.3 ± 0.2 | 56 | 33 | 42 | 59 | 31 | 77 | 88 |
| Quercetin | 255 | 371 | 303 | 1.4 ± 0.2 | 75 | 79 | 48 | ND | ND | 83 | 41 |
| Soyasaponin Ba | - | - | 959 | 2.6 ± 0.4 | 73 | 87 | ND | ND | ND | 52 | ND |
| Kaempferol | 266 | 366 | 287 | 0.7 ± 0.1 | 100 | 93 | 48 | 27 | 58 | 74 | ND |
| Soyasaponin αg | 295 | - | 1085 | 7.4 ± 0.8 | 100 | 59 | 38 | 23 | 38 | 69 | ND |
2.2. Evaluation of the Physical Properties and Flowability of Excipients, Black Bean Extract Powders and Formulations for Placebo Capsule Production
| Materials | Particle Size (μm) 1 | Bulk Density (g/cm3) | Tapped Density (g/cm3) | True Density (g/cm3) 2 |
|---|---|---|---|---|
| Black bean spray dried powder | 49 | 0.6 ± 0.0 | 0.83 ± 0.3 a | n.d. |
| Maltodextrin | 30–40 | 0.5 ± 0.1 | 0.63 ± 0.2 b | 1.50 |
| Microcrystalline Cellulose 25 | 25 | 0.6 ± 0.1 | 0.89 ± 0.2 a | 1.56 |
| Microcrystalline Cellulose 50 | 50 | 0.6 ± 0.1 | 0.84 ± 0.2 a | 1.43 |
| Starch | 60-90 | 0.5 ± 0.1 | 0.58 ± 0.1 c | 1.48 |
| Treatment | ||||
|---|---|---|---|---|
| A | B | C | ||
| Composition (%) | Maltodextrin | 50 | 25 | 25 |
| MCC 50 | 49.1 | 24.1 | - | |
| Starch | - | 50 | 74.1 | |
| Particle size distribution (μm) | d(0.1) | 22.51 b | 26.39 a | 22.95 a |
| d(0.5) | 57.55 a | 45.51 b | 43.71 b | |
| d(0.9) | 135.6 a | 76.81 b | 81.27 b | |
| Surface area | (m2/g) | 0.07 | 0.06 | 0.06 |
| Powder flowability | CFI (%) | 52.8 c | 60.5 b | 65.0 a |
| Flow category | Fair | Fair | Good | |
| Capsule | Weight (mg) | 565.9 c | 728.6 a | 614.1 b |
| Weight variability (%) | 0.5 | 1.5 | 1.5 | |
2.3. Black Bean Powder Capsule Production
| Treatment | ||||
|---|---|---|---|---|
| A 1 | B 2 | C 3 | ||
| Particle size distribution (μm) | d(0.1) | 13.58 | 16.87 | 4.06 |
| d(0.5) | 57.29 | 57.08 | 28.05 | |
| d(0.9) | 138.27 | 139.3 | 61.84 | |
| Surface area | (m2/g) | 0.09 | 0.09 | 0.21 |
| Powder flowability | CFI (%) | 47.8 | 55.1 | 48.3 |
| Flow category | Passable | Fair | Passable | |
| Capsules using powder without previous drying | Weight (mg) | 348.3 c | 456.6 a | 391.7 b |
| Weight variability (%) | 3.1 | 3.0 | 7.4 | |
| Black bean extract amount (mg) | 192.3 b | 196.8 ab | 200.7 a | |
| Black bean extract variability (%) | 6.0 | 5.9 | 14.8 | |
| Capsules using powder with previous drying | Weight (mg) | 395.3 c | 457.2 a | 417.6 b |
| Weight variability (%) | 2.7 | 1.6 | 2.5 | |
| Black bean extract amount (mg) | 218.4 a | 196.2 b | 214.0 a | |
| Black bean extract variability (%) | 5.5 | 3.2 | 5.8 | |
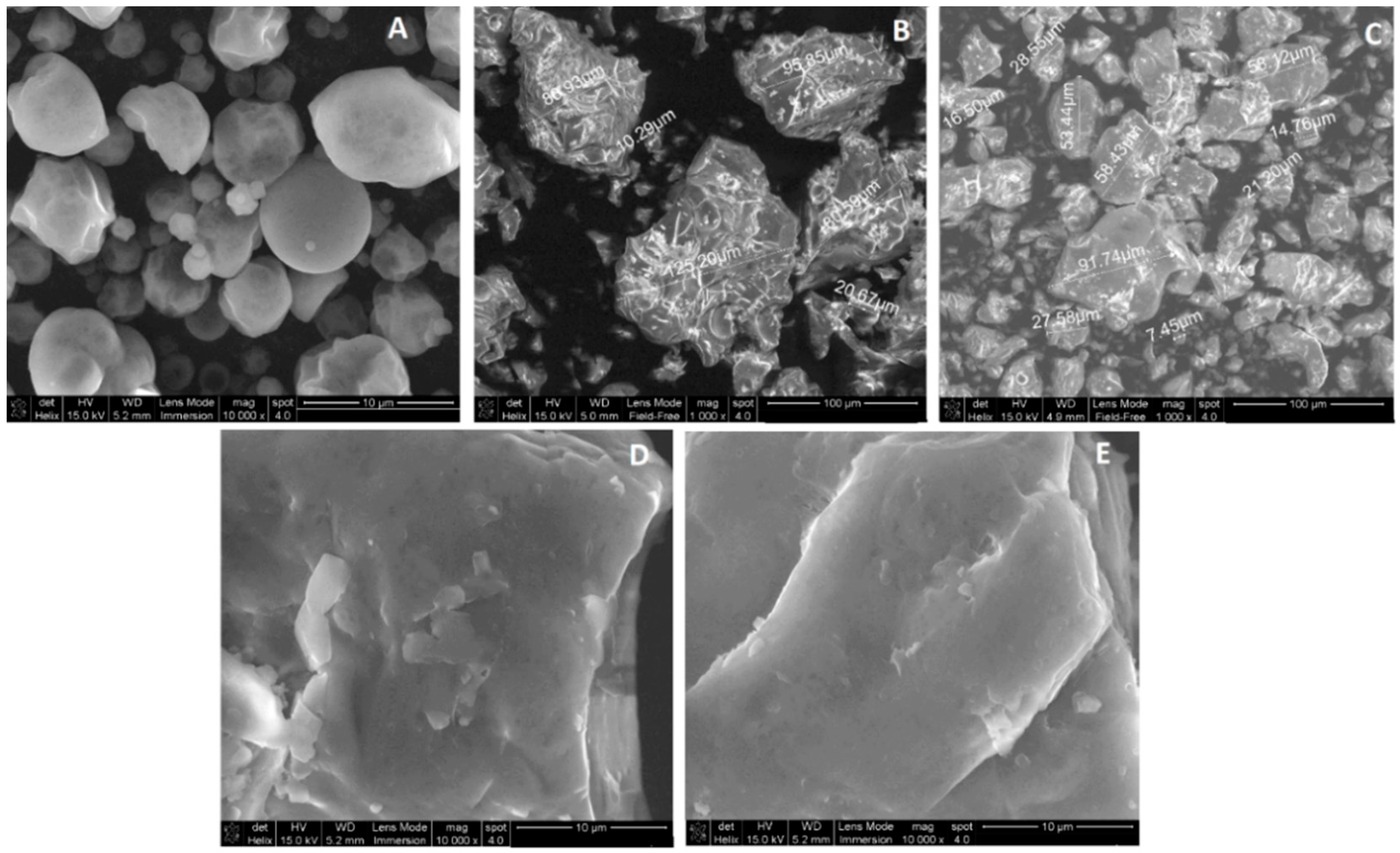
3. Experimental Section
3.1. Materials
3.2. Extraction Process
3.3. Analysis of Powder Moisture Content and Hygroscopicity
3.4. Spray drying Using Different Solutions of Black Bean Extract with Maltodextrin
3.5. Determination of Flavonols and Saponins
3.6. Excipient Formulation and Capsule Manufacture
3.7. Bulk and Tapped Density Determination
3.8. Powder Flowability Analysis
3.9. Capsule Weight Uniformity
3.10. Particle Morphology, Particle Size and Surface Area Distributions
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, P.Y.; Lai, H.M. Bioactive compounds in legumes and their germinated products. J. Agric. Food Chem. 2006, 54, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Hangen, L.; Bennik, M.R. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 60–65. [Google Scholar] [PubMed]
- Guajardo-Flores, D.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Evaluation of the antioxidant and antiproliferative activities of saponin and flavonols from germinated black beans (Phaseolus vulgaris L.) extracts. Food Chem. 2013, 141, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Thompson, H.J.; Brick, M.A.; McGinley, J.N.; Jiang, W.; Zhu, Z.; Wolfe, P. Mechanism associated with dose-dependent inhibition of rat mammary carcinogenesis by dry bean (Phaseolus vulgaris L.). J. Nutr. 2008, 138, 2091–2097. [Google Scholar]
- Harkey, M.R.; Henderson, G.L.; Gershwin, M.E.; Stern, S.S.; Hackman, R.M. Variability in commercial ginseng products: An analysis of 25 preparations. Am. J. Clin. Nutr. 2001, 73, 1101–1106. [Google Scholar]
- Souza, C.R.F.; Oliviera, W.P. Powder properties and system behavior during spray drying of Bauhinia forficata link extract. Dry Technol. 2006, 24, 735–749. [Google Scholar]
- Vidovic, S.S.; Vladic, J.Z.; Vastag, Z.G.; Zekovic, Z.P.; Popovic, L.M. Maltodextrin as a carrier of health benefic compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Auino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Saydi, D.; Hatamipour, M.S. Analysis of the effective parameters on potato powder quality produced by spray dryer. AIChE J. 2012, 9, 55–62. [Google Scholar]
- Sinija, V.R.; Mishra, M.R.; Bal, S. Process technology for production of soluble tea powder. J. Food Eng. 2007, 82, 276–283. [Google Scholar] [CrossRef]
- Moreira, G.E.G.; Maia Costa, M.G.; Rodriguez de Souza, A.C.; Sousa de Brito, E.; Dantas de Medeiros, M.F.; Azeredo, H.M.C. Physical properties of spray dried acerola pomace extract as affected by temperature and drying AIDS. LWT Food Sci. Technol. 2009, 42, 641–645. [Google Scholar] [CrossRef]
- Phoungchandang, S.; Sertwasana, A. Spray drying of ginger juice and physicochemical properties of ginger powders. Sci. Asia 2010, 36, 40–45. [Google Scholar] [CrossRef]
- Gallo, L.; Llabot, J.M.; Allemandi, D.; Bucalá, V.; Piña, J. Influence of spray-drying operating conditions on Rhamnus purshiana (Cáscara sagrada) extract powder physical properties. Powder Technol. 2011, 208, 204–214. [Google Scholar]
- Taylor, M.K.; Ginsburh, J.; Hickey, A.J.; Gheyas, F. Composite method to quantify powder flow as a screening method in early tablet or capsule formulation development. AAPS PharmSciTech 2000, 1. article 18. [Google Scholar]
- Adbullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar]
- Faulhammer, E.; Llusa, M.; Radeke, C.; Scheibelhofer, O.; Lawrence, S.; Biserni, S.; Calzolari, V.; Khinast, J.G. The effects of material attributes on capsule fill weight and weight variability in dosator nozzle machines. Int. J. Pharm. 2014, 471, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Staniforth, J.J. Powder Flow. In Pharmaceutics: The Science of Dosage Form Design, 2nd ed.; Aulton, M.E., Ed.; Churchill Livingstone: Edinburgh, Scotland, UK, 2002; pp. 197–210. [Google Scholar]
- Chang, C.K.; Alvarez-Nunez, F.A.; Rinella, J.V., Jr.; Magnusson, L.; Sueda, K. Roller compactation, granulation and capsule product dissolution of drug formulations containing a lactose or mannitol filler, starch and talc. AAPS PharmSciTech 2008, 9, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Flores, D.; García-Patiño, M.; Serna-Guerrero, D.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Characterization and quantification of saponins and flavonoids in sprouts, seed coats and cotyledons of germinated black beans. Food Chem. 2012, 134, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- United Sates Pharmacopeial Convention (USP). The United States Pharmacopeial Convention USP 32-NF 27; USP: Rockville, MD, USA, 2007; p. 4134. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guajardo-Flores, D.; Rempel, C.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Influence of Excipients and Spray Drying on the Physical and Chemical Properties of Nutraceutical Capsules Containing Phytochemicals from Black Bean Extract. Molecules 2015, 20, 21626-21635. https://doi.org/10.3390/molecules201219792
Guajardo-Flores D, Rempel C, Gutiérrez-Uribe JA, Serna-Saldívar SO. Influence of Excipients and Spray Drying on the Physical and Chemical Properties of Nutraceutical Capsules Containing Phytochemicals from Black Bean Extract. Molecules. 2015; 20(12):21626-21635. https://doi.org/10.3390/molecules201219792
Chicago/Turabian StyleGuajardo-Flores, Daniel, Curtis Rempel, Janet A. Gutiérrez-Uribe, and Sergio O. Serna-Saldívar. 2015. "Influence of Excipients and Spray Drying on the Physical and Chemical Properties of Nutraceutical Capsules Containing Phytochemicals from Black Bean Extract" Molecules 20, no. 12: 21626-21635. https://doi.org/10.3390/molecules201219792






