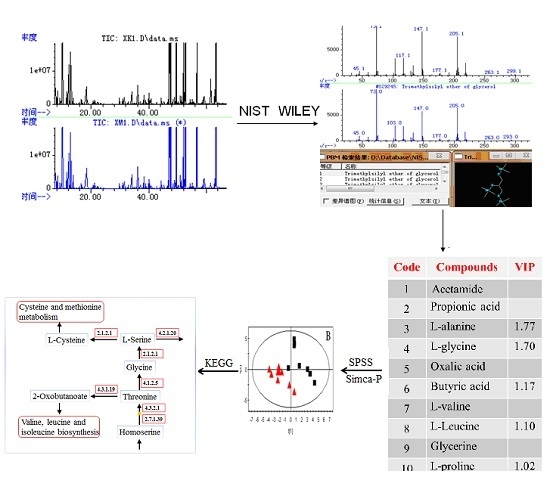
A GC-MS Based Metabonomics Study of Rheumatoid Arthritis and the Interventional Effects of the Simiaowan in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Appearance and Histopathological Observation
2.2. GC-MS Analysis of Plasma Samples
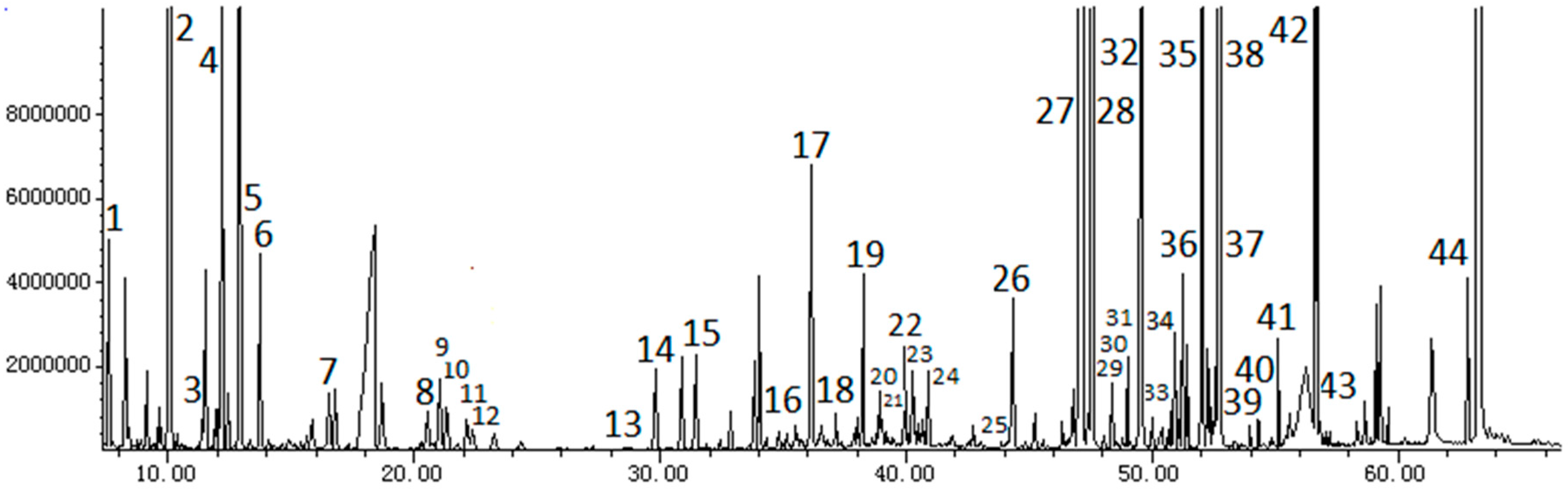
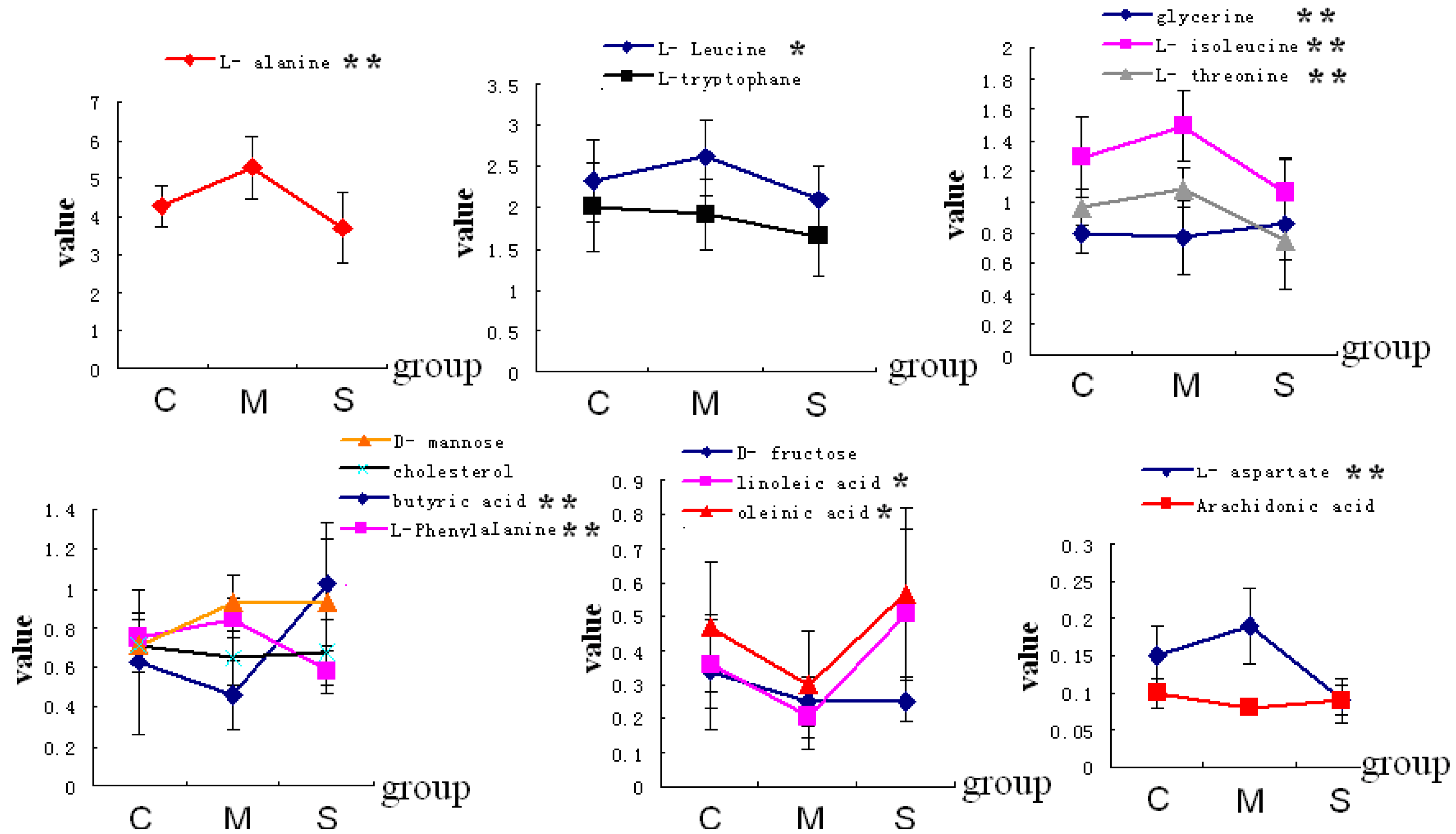
| Code | RT (min) | Compounds | VIP | Mean ± SD | ||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| 1 | 7.566 | Acetamide | 0.90 | 3.83 ± 0.18 | 3.83 ± 0.15 | 3.94 ± 0.13 |
| 2 | 10.038 | Propionic acid | 0.84 | 6.82 ± 1.56 | 7.64 ± 2.07 | 4.91 ± 0.45 |
| 3 | 11.508 | l-alanine | 1.77 | 4.27 ± 0.52 | 5.29 ± 0.81 * | 3.70 ± 0.92 # |
| 4 | 12.081 | l-glycine | 1.70 | 2.85 ± 0.21 | 3.48 ± 0.60 * | 1.97 ± 0.40 # |
| 5 | 12.901 | Oxalic acid | 0.98 | 4.28 ± 0.83 | 3.88 ± 0.71 | 4.35 ± 1.36 |
| 6 | 13.724 | Butyric acid | 1.17 | 0.63 ± 0.37 | 0.46 ± 0.18 * | 1.02 ± 0.31 # |
| 7 | 16.520 | l-valine | 0.88 | 2.06 ± 0.37 | 2.27 ± 0.44 | 1.72 ± 0.34 |
| 8 | 20.549 | l-Leucine | 1.10 | 2.32 ± 0.50 | 2.61 ± 0.47 * | 2.11 ± 0.39 # |
| 9 | 21.048 | Phosphoric Acid | 0.97 | 3.21 ± 0.32 | 3.11 ± 0.44 | 2.62 ± 0.32 |
| 10 | 21.325 | glycerine | 0.94 | 0.80 ± 0.14 | 0.77 ± 0.24 | 0.86 ± 0.43 |
| 11 | 22.158 | l-proline | 0.98 | 8.58 ± 1.01 | 8.91 ± 0.70 | 7.98 ± 0.72 |
| 12 | 22.391 | l-isoleucine | 1.20 | 1.29 ± 0.26 | 1.49 ± 0.23 * | 1.06 ± 0.22 # |
| 13 | 28.615 | 2,3-dihydroxy butyric acid | 0.85 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| 14 | 29.813 | l-serine | 0.95 | 1.93 ± 0.36 | 2.05 ± 0.25 | 1.46 ± 0.37 |
| 15 | 31.448 | l-threonine | 1.37 | 0.96 ± 0.12 | 1.09 ± 0.13 * | 0.75 ± 0.12 # |
| 16 | 35.589 | Malic acid | 0.86 | 0.08 ± 0.02 | 0.09 ± 0.04 | 0.05 ± 0.02 |
| 17 | 36.482 | l-aspartate | 1.46 | 0.15 ± 0.04 | 0.19 ± 0.05 * | 0.09 ± 0.02 # |
| 18 | 37.153 | Creatinine | 0.92 | 0.81 ± 0.44 | 0.76 ± 0.49 | 0.49 ± 0.42 |
| 19 | 38.994 | l-Phenyl Alanine | 1.41 | 0.75 ± 0.12 | 0.85 ± 0.10 * | 0.58 ± 0.11 # |
| 20 | 39.007 | Glutaminate | 0.84 | 0.52 ± 0.12 | 0.49 ± 0.10 | 0.40 ± 0.08 |
| 21 | 39.120 | Arbinofuranose | 0.89 | 0.06 ± 0.02 | 0.06 ± 0.03 | 0.05 ± 0.01 |
| 22 | 40.142 | l-Asparagine | 0.92 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.05 ± 0.01 |
| 23 | 40.678 | d-fructose | 1.41 | 0.34 ± 0.17 | 0.25 ± 0.07 * | 0.25 ± 0.06 |
| 24 | 40.899 | l-lysine | 0.95 | 0.72 ± 0.20 | 0.89 ± 0.52 | 0.53 ± 0.16 |
| 25 | 43.900 | l-ornithine | 0.82 | 0.09 ± 0.09 | 0.09 ± 0.07 | 0.08 ± 0.03 |
| 26 | 44.329 | Tetradecoic acid | 0.84 | 1.12 ± 0.20 | 1.09 ± 0.14 | 1.04 ± 0.20 |
| 27 | 45.565 | d-mannose | 2.16 | 0.71 ± 0.07 | 0.93 ± 0.14 * | 0.93 ± 0.32 |
| 28 | 47.545 | d-glucose | 0.90 | 55.04 ± 7.82 | 54.08 ± 9.30 | 50.88 ± 4.87 |
| 29 | 48.365 | d-galactose | 0.93 | 14.7 ± 3.42 | 13.55 ± 3.36 | 11.59 ± 1.86 |
| 30 | 48.995 | Glucitol | 0.87 | 0.56 ± 0.11 | 0.53 ± 0.11 | 0.43 ± 0.09 |
| 31 | 49.550 | Hexadecanoic Acid | 0.92 | 14.47 ± 4.60 | 13.75 ± 3.20 | 13.65 ± 3.81 |
| 32 | 49.991 | β-d-glucopyranose | 0.83 | 0.31 ± 0.11 | 0.32 ± 0.12 | 0.28 ± 0.06 |
| 33 | 50.925 | Inositol | 0.85 | 0.59 ± 0.11 | 0.66 ± 0.17 | 0.40 ± 0.05 |
| 34 | 51.240 | heptadecanoic acid | 0.91 | 0.81 ± 0.21 | 0.77 ± 0.11 | 0.75 ± 0.18 |
| 35 | 52.262 | Linoleic acid | 1.69 | 0.36 ± 0.13 | 0.21 ± 0.10 * | 0.51 ± 0.25 # |
| 36 | 52.433 | Oleinic acid | 1.45 | 0.47 ± 0.19 | 0.30 ± 0.16 * | 0.57 ± 0.25 # |
| 37 | 52.607 | l-tryptophane | 1.26 | 2.01 ± 0.55 | 1.91 ± 0.43 * | 1.64 ± 0.46 |
| 38 | 52.741 | Stearic acid | 0.89 | 27.01 ± 4.75 | 27.42 ± 3.49 | 26.54 ± 4.60 |
| 39 | 53.964 | Nonadecanoic acid | 0.87 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| 40 | 54.311 | Arachidonic acid | 1.75 | 0.10 ± 0.02 | 0.08 ± 0.01 * | 0.09 ± 0.03 |
| 41 | 55.124 | Eicosanoic acid | 0.93 | 0.33 ± 0.08 | 0.32 ± 0.05 | 0.30 ± 0.07 |
| 42 | 56.814 | 2,3-Dihydroxy Hexadecanoic acid | 0.92 | 0.18 ± 0.04 | 0.20 ± 0.02 | 0.19 ± 0.03 |
| 43 | 57.193 | Docosanoic acid | 0.85 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| 44 | 62.851 | Cholesterol | 1.18 | 0.71 ± 0.14 | 0.65 ± 0.14 * | 0.68 ± 0.17 |
2.3. Identification of Potential Biomarkers
| Retention Time | Metabolites | RA Group Compared with Control Group | Metabolic Pathway |
|---|---|---|---|
| 11.508 | l-alanine | ↑ | Alanine, aspartate and glutamate metabolism |
| 12.081 | l-glycine | ↑ | Glycine, serine and threonine metabolism |
| 13.724 | Butyric acid | ↓ | |
| 20.549 | l-leucine | ↑ | Valine, leucine and isoleucine metabolism |
| 22.391 | l-isoleucine | ↑ | Valine, leucine and isoleucine metabolism |
| 31.448 | l-threonine | ↑ | Glycine, serine and threonine metabolism |
| 36.482 | l-aspartate | ↑ | Alanine, aspartate and glutamate metabolism |
| 38.944 | l-phenylalanine | ↑ | Phenylalanine metabolism |
| 40.678 | d-fructose | ↓ | Fructose and mannose metabolism |
| 45.565 | d-mannose | ↑ | Fructose and mannose metabolism |
| 52.262 | Linoleic acid | ↓ | Lipid metabolism |
| 52.433 | Oleinic acid | ↓ | Lipid metabolism |
| 52.607 | l-tryptophan | ↓ | Tryptophan metabolism |
| 54.311 | Arachidonic Acid | ↓ | Arachidonic acid metabolism Lipid metabolism |
| 62.851 | Cholesterol | ↓ | Lipid metabolism |
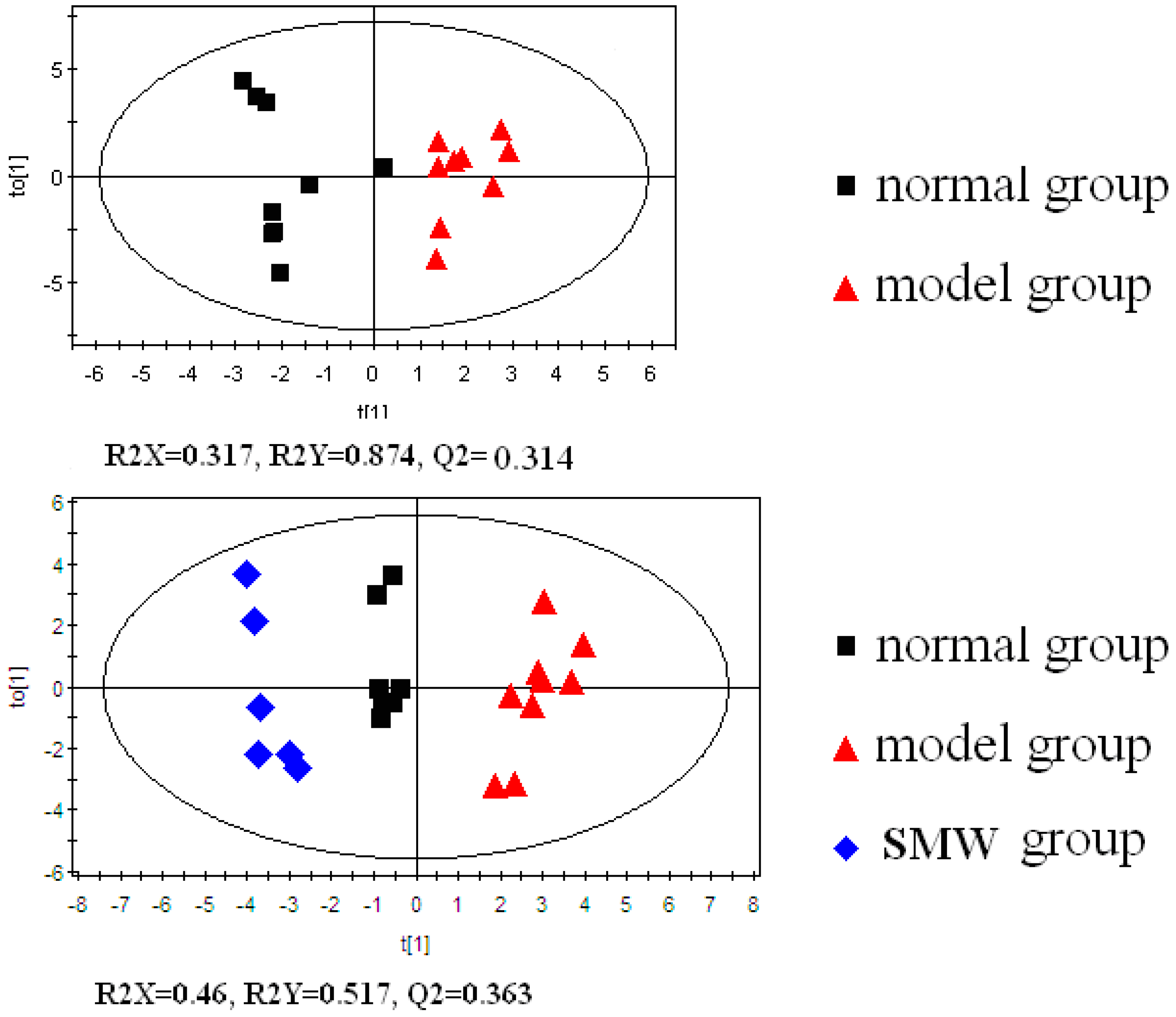
2.4. Biological Interpretation
2.4.1. Amino Acid Metabolism
2.4.2. Lipid Metabolism
2.4.3. Energy Metabolism
2.5. Mechanism Supposition of SMW
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of SMW
3.3. RA Model and Drug Administration
3.4. Preprocessing of Samples
3.5. GC–MS Analysis
3.6. Data Processing and Pattern Recognition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sweeney, S.E.; Firestein, G.S. Rheumatoid arthritis: Regulation of synovial inflammation. Int. J. Biochem. Cell Biol. 2004, 36, 372–378. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Han, Y.; Yu, X.W.; Qin, L. Traditional Chinese medicine in the treatment of rheumatoid arthritis: A general review. Rheumatol. Int. 2010, 30, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.K.; Peng, C.; Lv, X.; Li, Y.; Fang, W.; Han, C. Effects of Yishen Juanbi (YJB) Pill on Experimental Rheumatoid Arthritis. Chin. J. Nat. Med. 2010, 8, 57–61. [Google Scholar] [CrossRef]
- Editorial Committee of Pharmacopoeia of Ministry of Health P.R. China. The Pharmacopoeia of People’s Republic of China, year 2010 ed.; China Chemical Industry Press: Beijing, China, 2010; p. 646. [Google Scholar]
- Shi, X.D.; Li, G.C.; Qian, Z.X.; Jin, Z.Q.; Song, Y. Randomized and controlled clinical study of modified prescriptions of Simiao Pill in the treatment of acute gouty arthritis. Chin. J. Nat. Med. 2008, 14, 17–22. [Google Scholar]
- Hu, Q.H.; Jiao, R.Q.; Wang, X.; Lv, Y.Z.; Kong, L.D. Simiao pill ameliorates urate under excretion and renal dysfunction in hyperuricemic mice. J. Ethnopharmacol. 2010, 128, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Connelly, J.; Lindon, J.C.; Holmes, E. Metabonomics: A platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002, 1, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Weljie, A.M.; Dowlatabadi, R.; Miller, B.J.; Vogel, H.J.; Jirik, F.R. An inflammatory arthritis-associated metabolite biomarker pattern revealed by 1H NMR spectroscopy. J. Proteome Res. 2007, 6, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Li, X.Z.; Liu, S.M.; Lu, F. Metabonomic Study of the Effects of Acanthopanax senticosus on Peripheral System of Rats. Planta Med. 2015, 9, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Chen, H.; Tian, T.; Chen, D.Q.; Bai, X.; Wei, F. A pharmaco-metabonomic study on chronic kidney disease and therapeutic effect of ergone by UPLC-QTOF/HDMS. PLoS ONE 2014, 12, e115467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Hua, Y.L.; Zhang, M.; Ji, P.; Li, J.X.; Zhang, L.; Li, P.L.; Wei, Y.M. Metabonomic analysis of the anti-inflammatory effects of volatile oils of Angelica sinensis on rat model of acute inflammation. Biomed. Chromatogr. 2015, 29, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lum, J.H.; Ng, C.K.; McKay, J.; Butt, Y.K.; Wong, M.S.; Lo, S.C. Pain Controlling and Cytokine-regulating Effects of Lyprinol, a Lipid Extract of Perna Canaliculus, in a Rat Adjuvant-induced Arthritis Model. Evid. Based Complement. Altern. Med. 2009, 6, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, X.L.; Zhang, L.; Li, Y.B. Effects and mechanisms of Simiao pill on adjuvant arthritis rats model. China J. Chin. Mater. Med. 2010, 21, 2889–2892. [Google Scholar]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Costa Rosa, L.F. The effect of BCAA supplementation upon the immune response of triathletes. Med. Sci. Sports Exerc. 2000, 32, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Martins, E.J.; Santos, R.V.; Caperuto, E.C.; Rogeri, P.; Costa Rosa, L.F. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 2002, 18, 376–379. [Google Scholar] [CrossRef]
- Kokkonen, H.; Söderström, I.; Rocklöv, J.; Hallmans, G.; Lejon, K.; Rantapää Dahlqvist, S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheumatol. 2010, 62, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.M.; McInnes, I.B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Investig. 2008, 118, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.P.; Qu, L.P.; Wu, Y.T.; Fan, G.R. A plasma metabonomic investigation into the intervention of volatile oil of Magnolia biondii Pamp on rat model of acute inflammation. J. Ethnopharmacol. 2011, 137, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Sivakumar, B. Hypoxia and angiogenesis in rheumatoid arthritis. Curr. Opin. Rheumatol. 2005, 17, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Saulot, V.; Vittecoq, O.; Charlionet, R.; Fardellone, P.; Lange, C.; Marvin, L.; Machour, N.; Le Loët, X.; Gilbert, D.; Tron, F. Presence of autoantibodies to the glycolytic enzyme alpha-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheumatol. 2002, 46, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Bendele, A.M. Animal models of osteoarthritis. J. Musculoskelet. Neuron. Interact. 2011, 1, 363–376. [Google Scholar]
- Panosian, N.; Heyner, S.; Capetola, R.J.; Orzechowski, R.F. Humoral and cellular immunologic aspects of adjuvant and collagen arthritis in rats. J. Immunopharmacol. 1986, 8, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shao, L.; Gong, Y.F.; Mao, Y.; Liu, C.X.; Qu, H.B.; Cheng, Y.Y. A metabonomic characterization of CCl4-induced acute liver failure using partial least square regression based on the GC/MS metabolic profiles of plasma in mice. J. Chromatogr. B 2008, 870, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, G.; Ji-ye, A.; Wu, D.; Zhu, L.L.; Ma, B.; Du, Y. Application of GC/MS-based metabonomic profiling in studying the lipid-regulating effects of Ginkgo biloba extract on diet-induced hyperlipidemia in rats. Acta Pharmacol. Sin. 2009, 30, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Phua, L.C.; Koh, P.K.; Cheah, P.Y.; Ho, H.K.; Chan, E.C. Global gas chromatography/time-of-flight mass spectrometry (GC/TOFMS)-based metabonomic profiling of lyophilized human feces. J. Chromatogr. B 2013, 937, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds l-leucine, l-isoleucine, l-serine, l-threonine, Arachidonic acid and d-glucose are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Guo, X.; Xie, J.; Hou, Z.; Li, Y. A GC-MS Based Metabonomics Study of Rheumatoid Arthritis and the Interventional Effects of the Simiaowan in Rats. Molecules 2015, 20, 21364-21372. https://doi.org/10.3390/molecules201219776
Wang Y, Guo X, Xie J, Hou Z, Li Y. A GC-MS Based Metabonomics Study of Rheumatoid Arthritis and the Interventional Effects of the Simiaowan in Rats. Molecules. 2015; 20(12):21364-21372. https://doi.org/10.3390/molecules201219776
Chicago/Turabian StyleWang, Yuming, Xuejun Guo, Jiabin Xie, Zhiguo Hou, and Yubo Li. 2015. "A GC-MS Based Metabonomics Study of Rheumatoid Arthritis and the Interventional Effects of the Simiaowan in Rats" Molecules 20, no. 12: 21364-21372. https://doi.org/10.3390/molecules201219776