Soluble Epoxide Hydrolase Inhibitory Activity of Selaginellin Derivatives from Selaginella tamariscina
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation
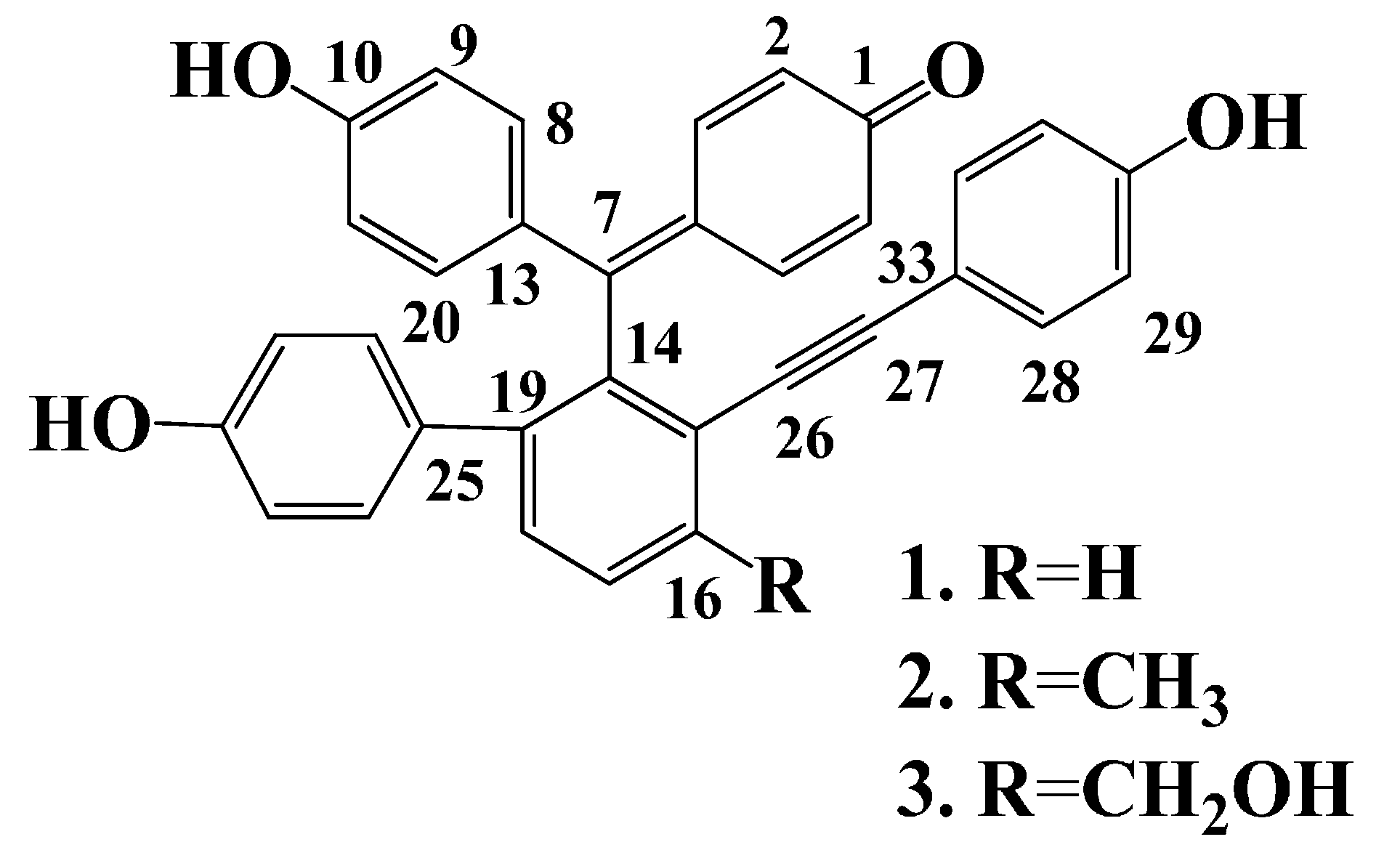
2.2. Enzyme Inhibition Activity
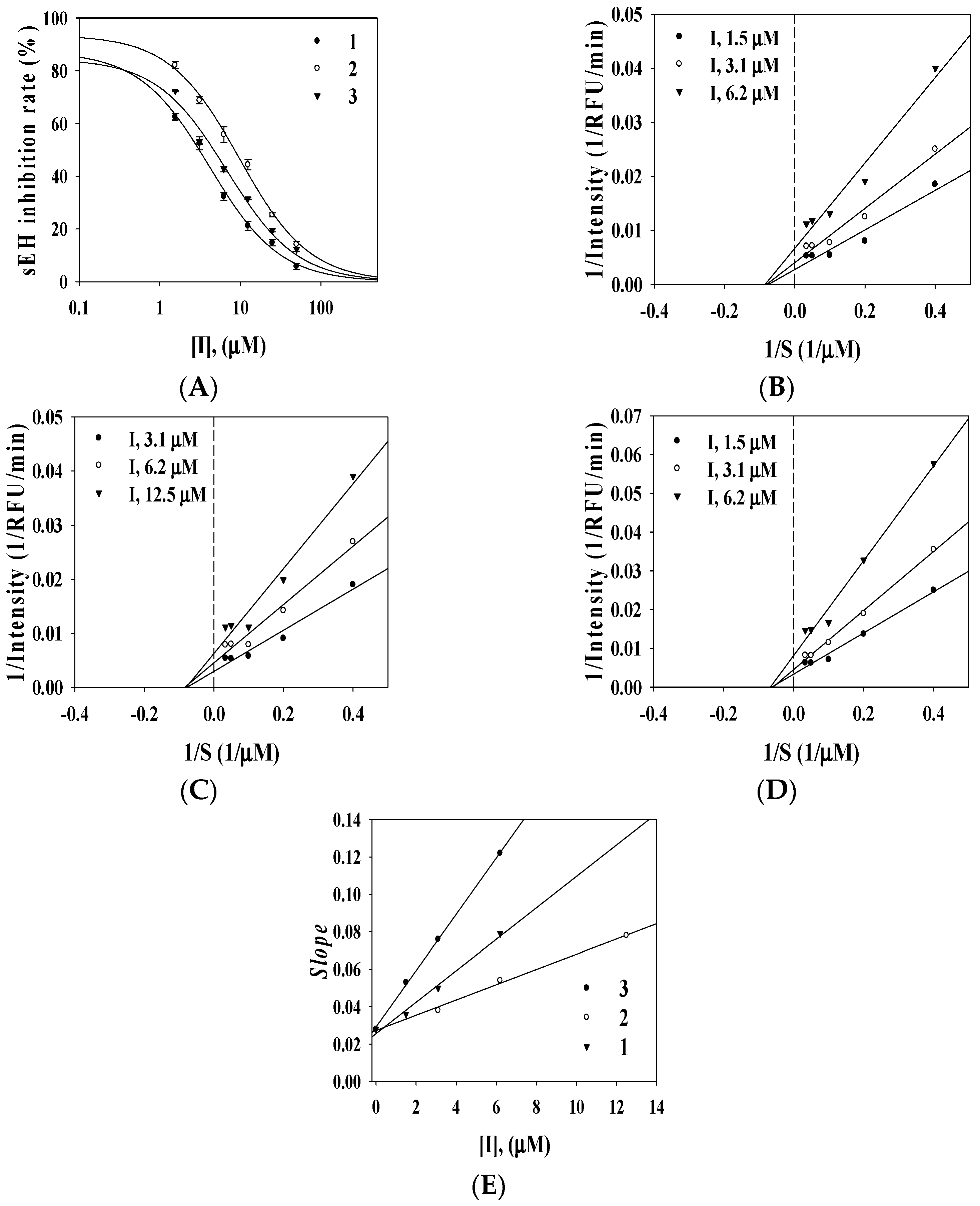
| Compound | Inhibitory Rate at 100 μM (%) a | sEH a | |||
|---|---|---|---|---|---|
| AChE | Tyrosinase | sEH | IC50 (μM) | Type (Ki, μM) | |
| 1 | 32.8 ± 2.1 | 36.2 ± 2.1 | 92.5 ± 0.8 | 3.1 ± 0.1 | Noncompetitive (2.9 ± 1.2) |
| 2 | 19.6 ± 1.0 | 32.5 ± 1.5 | 89.3 ± 2.1 | 8.2 ± 2.2 | Noncompetitive (6.8 ± 0.5) |
| 3 | 12.2 ± 0.8 | 40.1 ± 3.2 | 94.5 ± 5.0 | 4.2 ± 0.2 | Noncompetitive (1.8 ± 1.5) |
| Positive control b | 1.4 ± 3.5 μM | 25.2 ± 1.2 μM | 8.2 ± 1.3 nM | ||
2.3. Docking Calculation
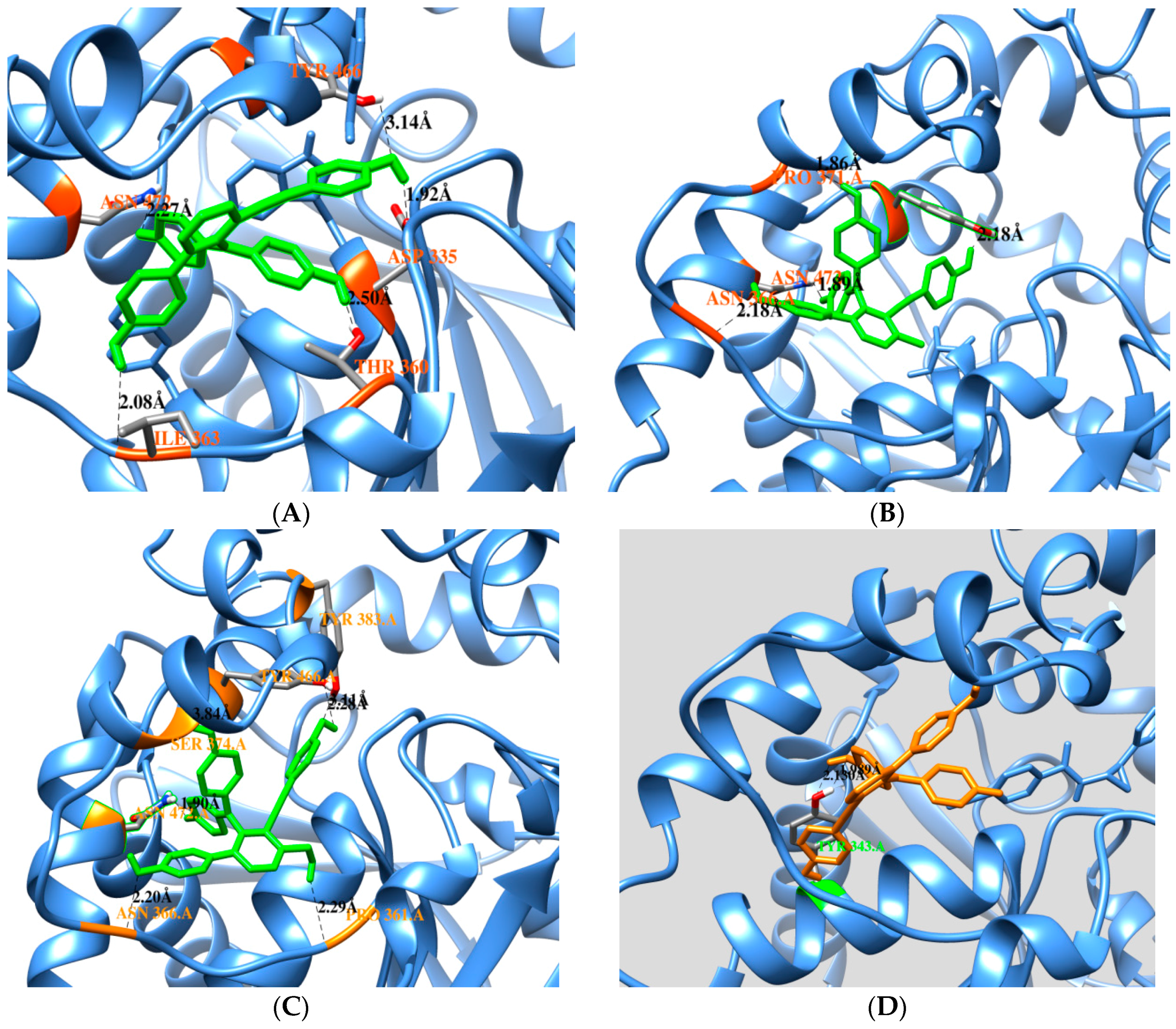
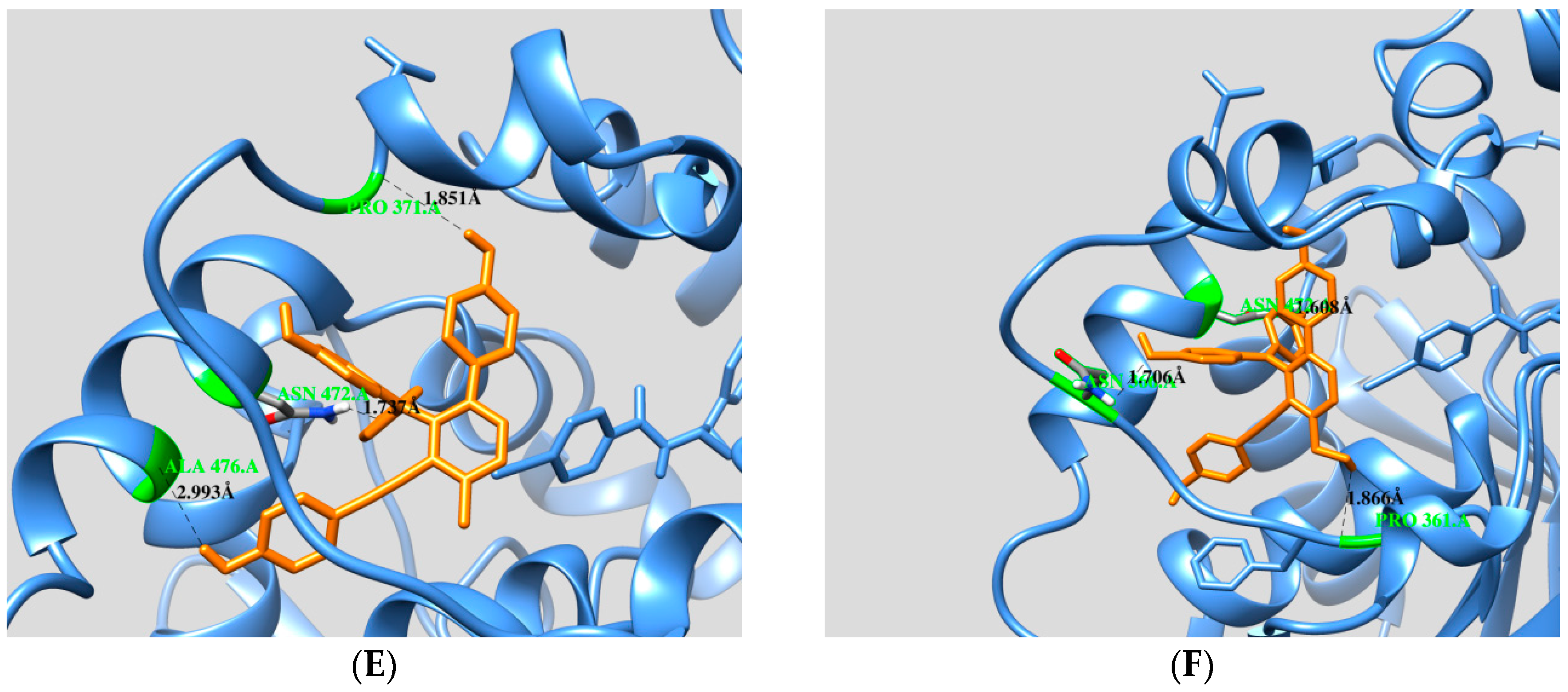
| Free Enzyme | Affinity (kcal/mol) | Residues in Close Contact | Hydrogen Bond (Å) |
| Selaginelin A | −10.81 | Asp335, Trp336, Met339, Thr360, Ile363, Phe381, Tyr383, Gln384, Tyr466,Met469, Asn472, Val498, Leu499 | Asn472(2.2), Ile363(2.0), Thr360(2.5), Asp335(1.9), Tyr466(3.1) |
| Selaginelin B | −10.76 | Trp336, Met339, Tyr343, Thr360, Pro361, Ile363, Asn366, Pro371, Asn472, Ser374, Tyr466, Met469, Leu499, Met503 | Asn366(2.1), Asn372(1.8), Pro371(1.8), Typ466(2.1) |
| selaginelin | −10.24 | Trp336, Met339, Thr360, Pro361, Ile363, Asn366, Met369, Pro371, Ser374, Ile375, Tyr383, Gln384, Tyr466, Met469, Asn472, Trp473, Leu499, Met503 | Asn366(2.2), Asn472(1.8), Tyr383(2.2), Pro361(2.2), Tyr466 (2.4,3.1) |
| Enzyme-Substrate Complex | Affinity (kcal/mol) | Residues in Close Contact | Hydrogen Bond (Å) |
| Selaginelin A | −9.45 | Met339, Tyr343, Phe362, Ile363, Asn472, Ser374, Phe381, Ala476, Met503 | Tyr343(2.1,1.9) |
| Selaginelin B | −11.8 | Trp336, Met339, Tyr343, Leu346, Pro361, Phe362, Ile363, Ala365, Asn366, Met369, Pro371Ile375, Gln384, Met469, Asn472, Tpr473, Ala476, | Ala476(2.9), Asn 472(1.7), Pro371(1.8) |
| selaginelin | −10.87 | Trp336, Met339, Tyr343, Leu346, Pro361, Phe362, Ile363, Asn366, Met369, Pro371, Ser374, Ile375, Gln384, Met469, Asn472, Trp473, Ala476 | Asn366(1.7), Asn452(1.6), Pro361(1.8) |
2.4. HPLC Analysis
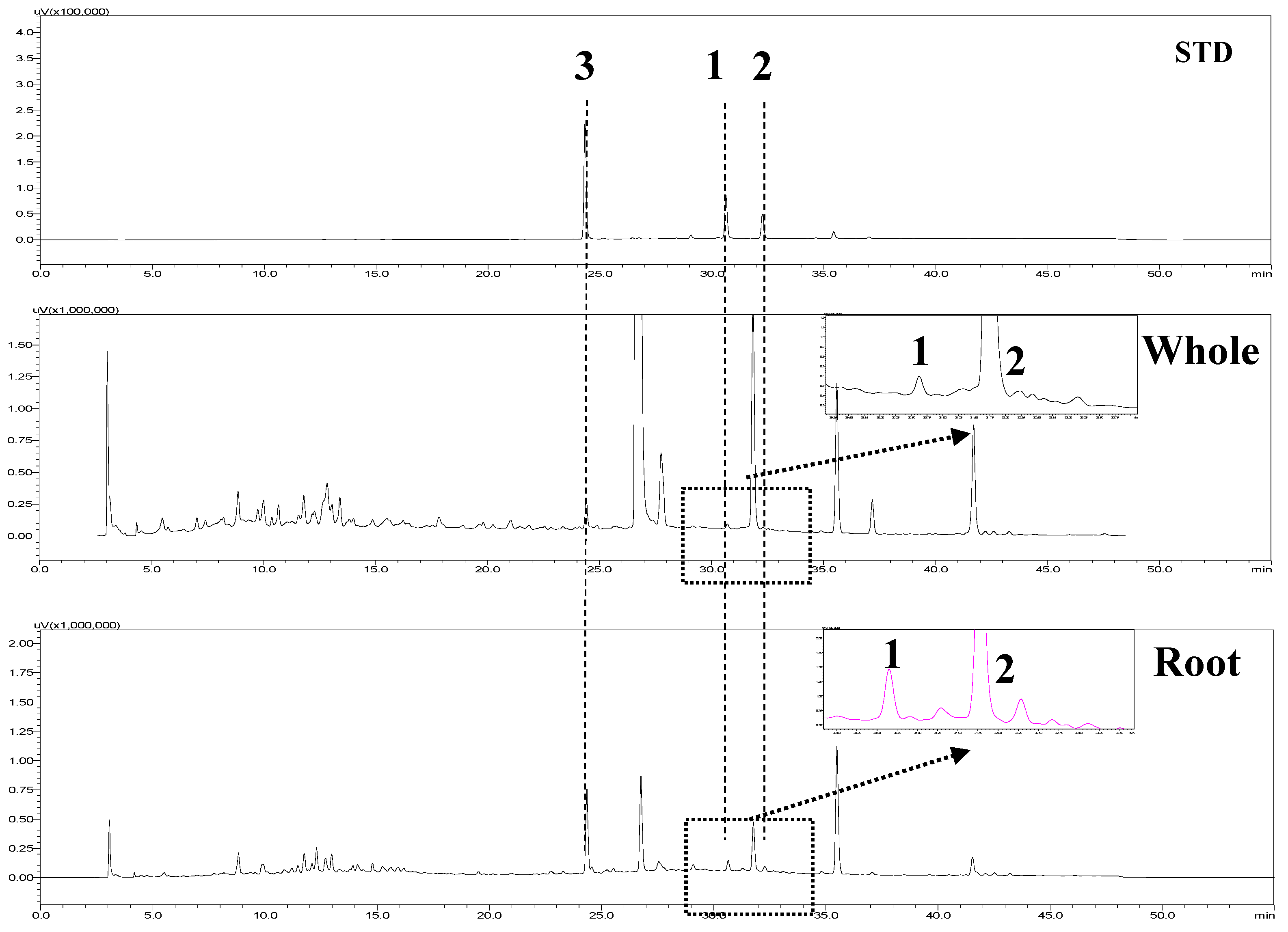
| Parts | Content (μg/g, Dried Material, n = 3) | ||
|---|---|---|---|
| Selaginellin | Selaginellin A | Selaginellin B | |
| Whole | 61.2 ± 0.3 a | 22.9 ± 0.1 | 10.2 ± 0.3 |
| Root | 189.3 ± 0.0 | 56.0 ± 0.1 | 42.5 ± 0.1 |
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Enzymatic Assay
3.5. sEH Kinetic Analysis
3.6. Docking Calculation
3.7. HPLC Analysis Condition
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, C.; Shao, Y.; Li, K.; Xia, W. Bioactive selaginellins from Selaginella tamariscina (Beauv.). J. Org. Chem. 2012, 8, 1884–1889. [Google Scholar]
- Zhang, L.P.; Liang, Y.M.; Wei, X.C.; Cheng, D.L. A new unusual natural pigment form Selaginella sinensis and its noticeable physicochemical properties. J. Org. Chem. 2007, 72, 3921–3924. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-P.; Wang, Y.-Z.; Zheng, X.-K.; Feng, W.-S.; Tu, P.-F. Chemotaxonomic significance and biosynthesis of selaginellin from Selaginella tamariscina. Biochem. Syst. Ecol. 2012, 45, 151–154. [Google Scholar] [CrossRef]
- Wang, C.J.; Hu, C.P.; Xu, K.P.; Yuan, Q.; Li, F.S.; Zou, H.; Tan, G.S.; Li, Y.J. Protective effect of selaginellin on glutamate-induced cytotoxicity and apoptosis in differentiated PC12 cells. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Hammock, B.D. Soluble epoxide hydrolase as a therapeutic target for cardiocascular disease. Nat. Rev. Drug Discov. 2009, 8, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Epoxides and soluble epoxide hydrolases in cardiovascular physiology. Physiol. Rev. 2012, 92, 101–130. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc. Drug Rev. 2006, 24, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Dufot, T.; Roche, C.; Lamoureux, F.; Guerrot, D.; Bellien, J. Design and discovery of soluble epoxide hydrolase inhibitors for the treatment of cardiovascular diseases. Expert Opin. Drug Discov. 2014, 9, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, D.C.; DuBois, R.N.; Falck, J.R.; Capdevila, J.H. Moleular cloning, experssion and characterization of an endogenous hyman cytochrome P450 arachidonic acid epoxygenase isoform. Arch. Biochem. Biophys. 1995, 322, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.R.; Hammock, B.D. Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene 2013, 526, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Park, S.H.; Morisseau, C.; Hwang, S.H.; Hammock, B.D.; Weissm, R.H. Sorafenib has sEH inhibitory activity which contributes to its effect profile in vivo. Mol. Cancer Ther. 2009, 8, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Oh, S.J.; Lee, S.Y.; Lee, J.Y.; Ma, J.Y.; Kim, Y.H.; Kim, S.K. Discovery of soluble epoxide hydrolase inhibitors from natural products. Food Chem. Toxicol. 2014, 64, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.L.; Ma, S.C.; Yu, J.D.; Yang, S.Y.; Xiao, X.Y.; Hu, J.Y.; Lu, Y.; Shaw, P.C.; But, P.P.; Lin, R.C. Selaginellin A and B, two novel natural pigments isolated from Selaginella tamariscina. Chem. Pharm. Bull. 2008, 56, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.G.; Jing, Y.; Zhang, H.M.; Ma, E.L.; Guan, J.; Xue, F.N.; Liu, H.X.; Sun, X.Y. Isolation and cytotoxic activity of selaginellin derivatives and bioflavonoids from Selaginella tamariscina. Planta Med. 2012, 78, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Morgan, A.M.; Tai, B.H.; Van, D.T.; Cuong, N.M.; Kim, Y.H. Inhibition of soluble epoxide hydrolase activity by compounds isolated from the aerial parts of Glycosmis stenocarpa. J. Enzym. Inhib. Med. Chem. 2015, 7, 1–5. [Google Scholar]
- Lee, G.Y.; Kim, J.H.; Choi, S.K.; Kim, Y.H. Constituents of the seeds of Cassia tora with inhibitory activity on soluble epoxide hydrolase. Bioorg. Med. Chem. Lett. 2015, 25, 5097–5101. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ryu, Y.B.; Lee, W.S.; Kim, Y.H. Neuraminidase inhibitory activies of quaterary isoquinoline alkaloids from Corydalis turtschaninovii rhizome. Bioorganic Med. Chem. 2014, 22, 6047–6052. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, Y.; Zhou, X.; Qian, F.; Fan, H.; Wang, Q. Simultaneous determination of selabinellins and biflavones in Selaginella tamariscina and S. pulvinata by HPLC. Zhongguo Zhong Yao Za Zhi 2012, 37, 1254–1258. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Cho, C.W.; Tai, B.H.; Yang, S.Y.; Choi, G.-s.; Kang, J.S.; Kim, Y.H. Soluble Epoxide Hydrolase Inhibitory Activity of Selaginellin Derivatives from Selaginella tamariscina. Molecules 2015, 20, 21405-21414. https://doi.org/10.3390/molecules201219774
Kim JH, Cho CW, Tai BH, Yang SY, Choi G-s, Kang JS, Kim YH. Soluble Epoxide Hydrolase Inhibitory Activity of Selaginellin Derivatives from Selaginella tamariscina. Molecules. 2015; 20(12):21405-21414. https://doi.org/10.3390/molecules201219774
Chicago/Turabian StyleKim, Jang Hoon, Chong Woon Cho, Bui Huu Tai, Seo Young Yang, Gug-seoun Choi, Jong Seong Kang, and Young Ho Kim. 2015. "Soluble Epoxide Hydrolase Inhibitory Activity of Selaginellin Derivatives from Selaginella tamariscina" Molecules 20, no. 12: 21405-21414. https://doi.org/10.3390/molecules201219774