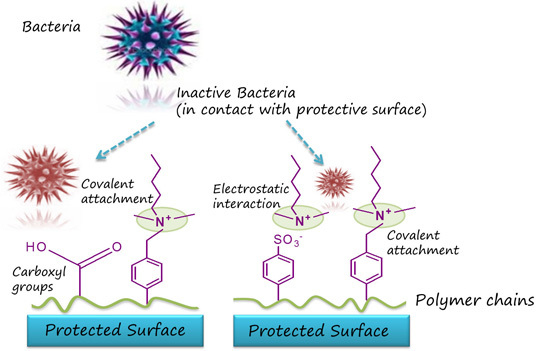
Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds
Abstract
:1. Introduction
2. Results and Discussion
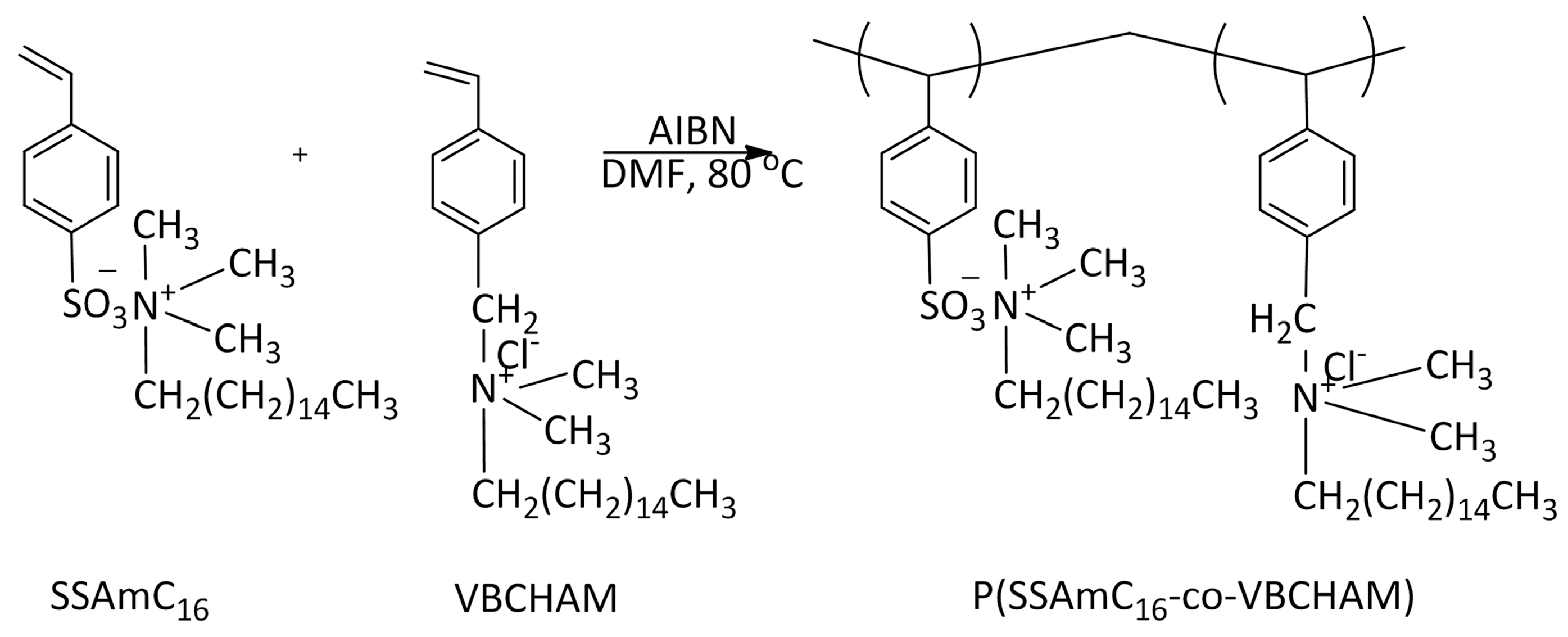
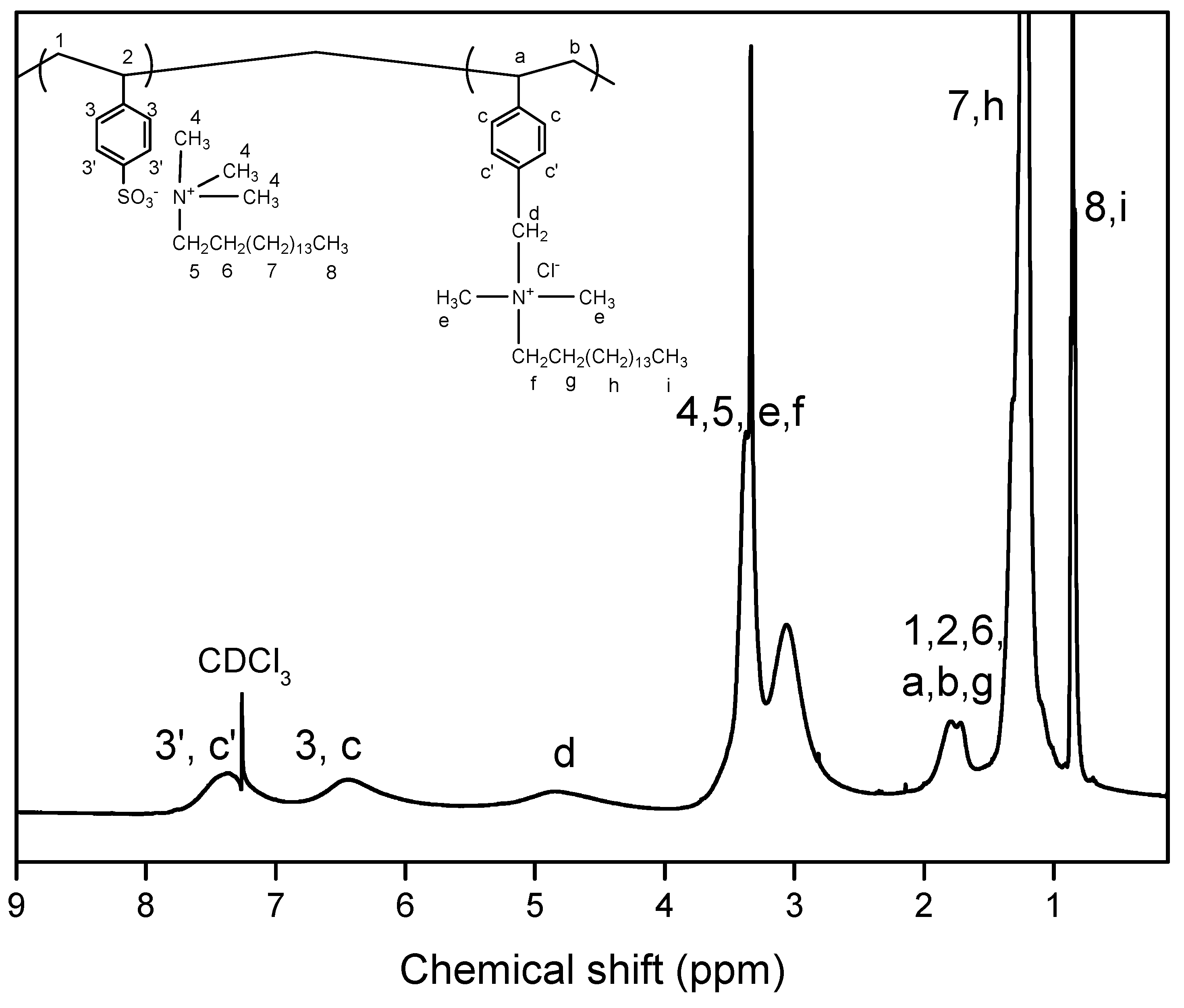
2.1. Synthesis and Characterization of Polymers
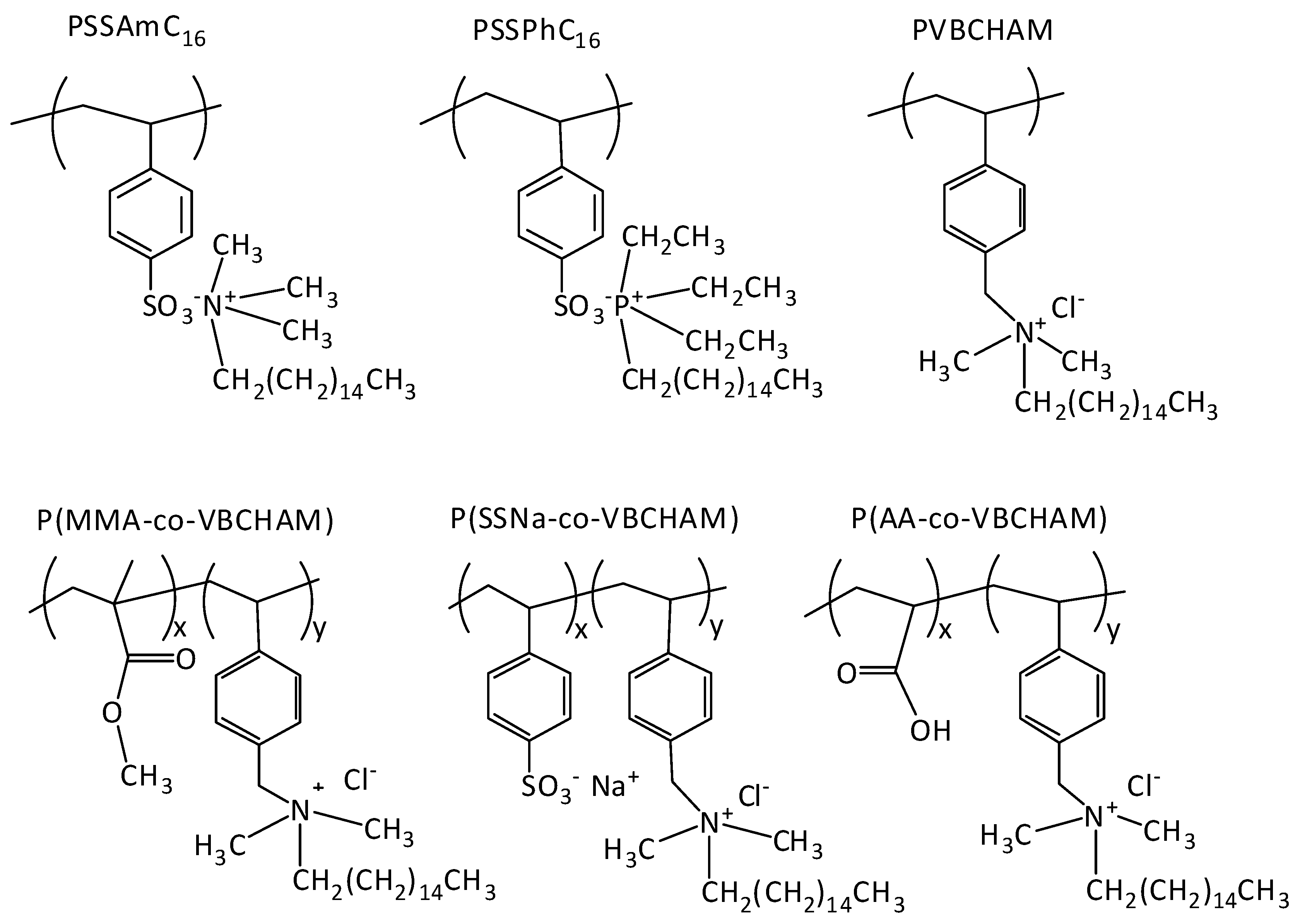
| Polymer Sample | Code | Polymer Type | % Composition of Bacteriostatic Group | References |
|---|---|---|---|---|
| PSSAmC16 | D1 | Homopolymer of cetyltrimethylammonium 4-styrene sulfonate | 100% QAC electrostatically attached | (Oikonomou et al., 2012) [18] |
| PVBCHAM | D2 | Homopolymer of vinyl benzyl dimethylhexadecylammonium chloride | 100% QAC covalently attached | (Koromilas et al., 2014) [22] |
| P(SSAmC16-co-VBCHAM65) | D3 | Copolymer of cetyltrimethylammonium 4-styrene sulfonate and vinyl benzyl dimethylhexadecylammonium chloride | 35% QAC electrostatically attached-65% QAC covalently attached | Present study |
| P(SSAmC16-co-VBCHAM25) | D3a | Copolymer of cetyltrimethylammonium 4-styrene sulfonate and vinyl benzyl dimethylhexadecylammonium chloride | 75% QAC electrostatically attached-25% QAC covalently attached | Present study |
| PSSPhC16 | D4 | Homopolymer of hexadecyltributylphosphonium 4-styrene sulfonate | 100% QPC electrostatically attached | (Oikonomou et al., 2012) [18] |
| P(MMA-co-VBCHAM47) | D5 | Copolymer of methyl methacrylate and vinyl benzyl dimethylhexadecylammonium chloride | 47% QAC covalently attached | (Koromilas et al., 2014) [22] |
| P(SSNa-co-VBCHAM20) | D6 | Copolymer of sodium 4-styrene sulfonate and vinyl benzyl dimethylhexadecylammonium chloride | 20% QAC covalently attached | (Koromilas et al., 2014) [22] |
| P(SSNa-co-VBCHAM85) | D6a | Copolymer of sodium 4-styrene sulfonate and vinyl benzyl dimethylhexadecylammonium chloride | 85% QAC covalently attached | (Koromilas et al., 2014) [22] |
| P(AA-co-VBCHAM88) | D7 | Copolymer of acrylic acid and vinyl benzyl dimethylhexadecylammonium chloride | 88% QAC covalently attached | (Koromilas et al., 2014) [26] |
| P(AA-co-VBCHAM20) | D7a | Copolymer of acrylic acid and vinyl benzyl dimethylhexadecylammonium chloride | 20% QAC covalently attached | (Koromilas et al., 2014) [26] |
2.2. Antibacterial Activity Studies
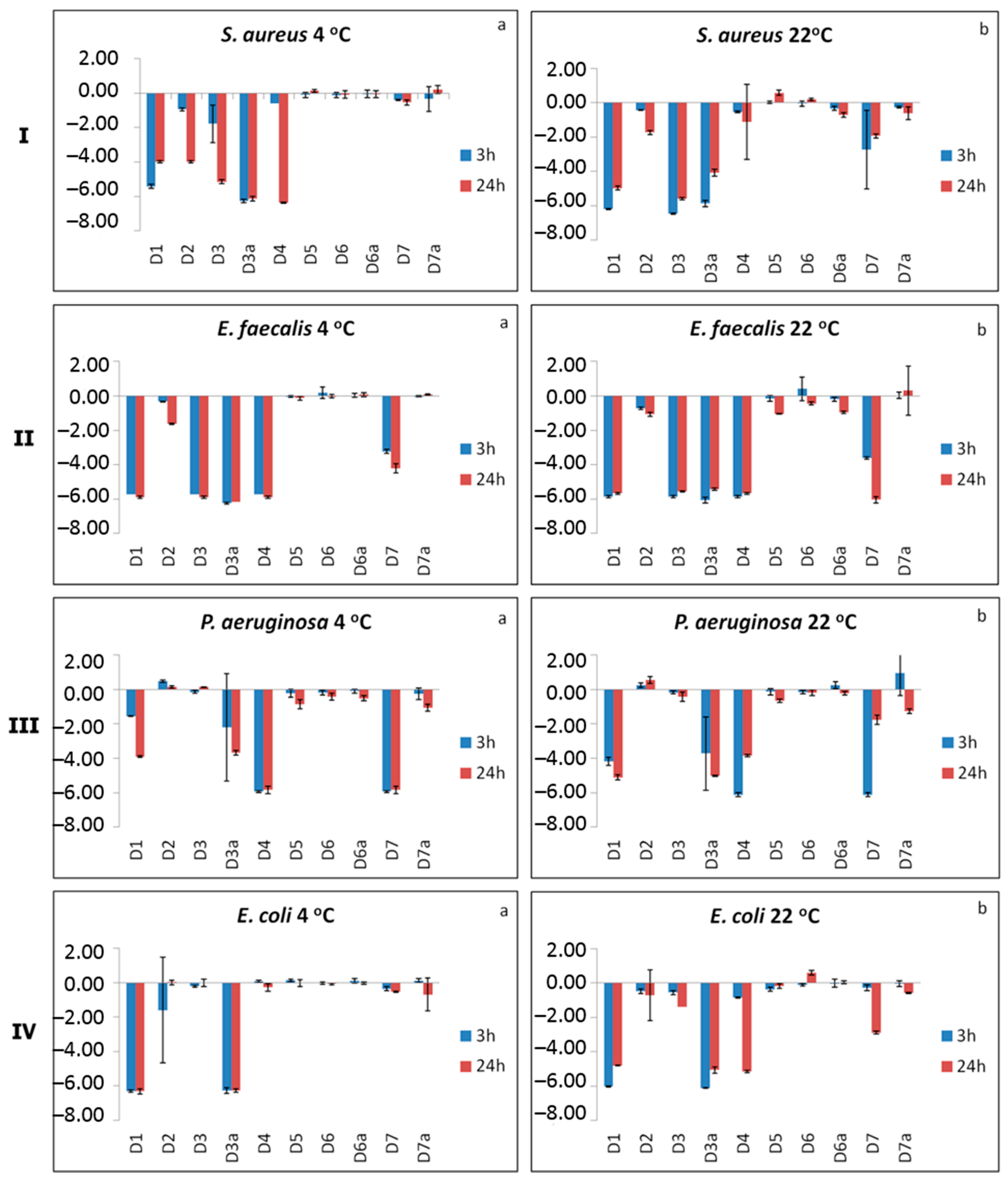
2.3. Antimicrobial Activity of Bacteriostatic Polysulfone Membranes
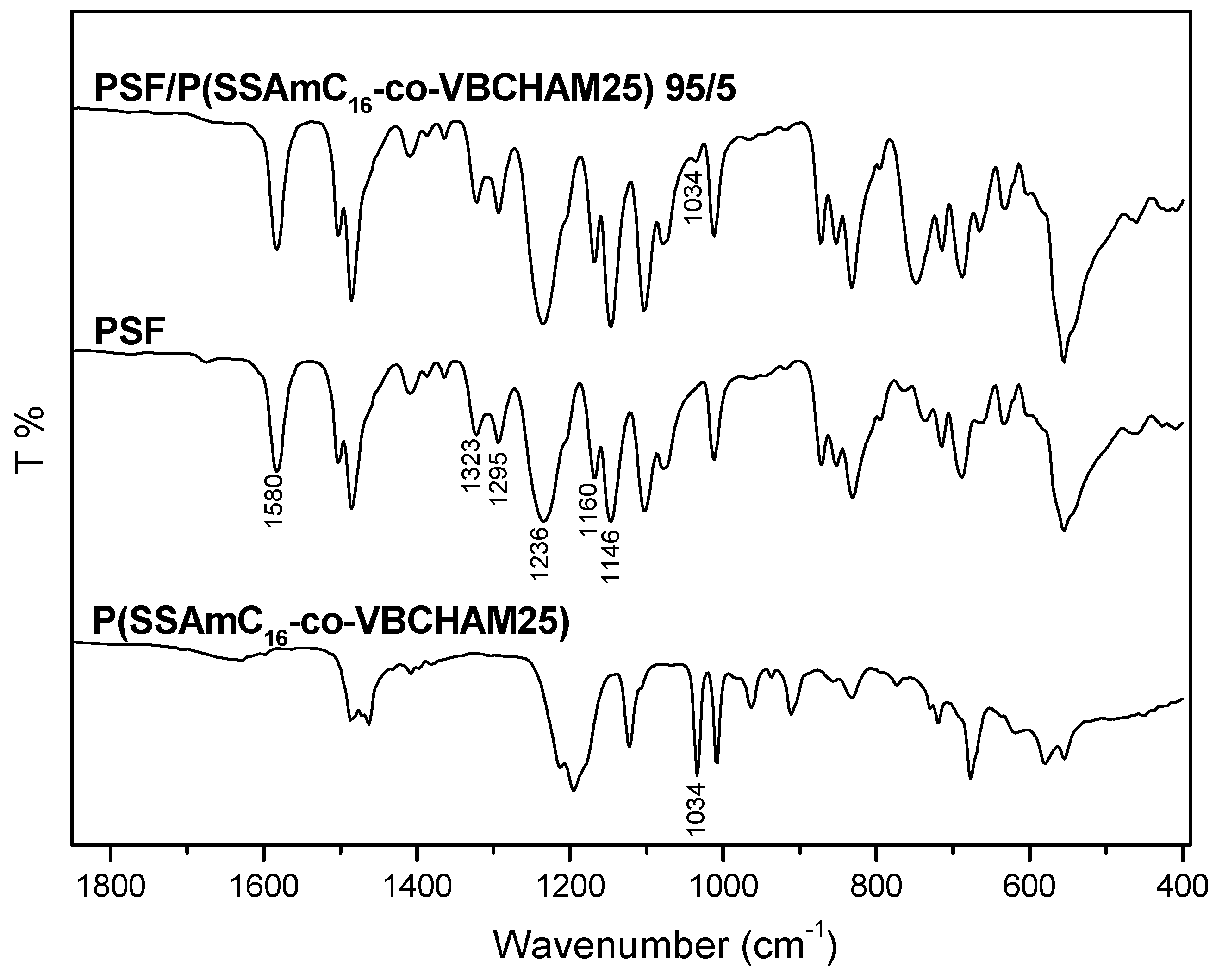
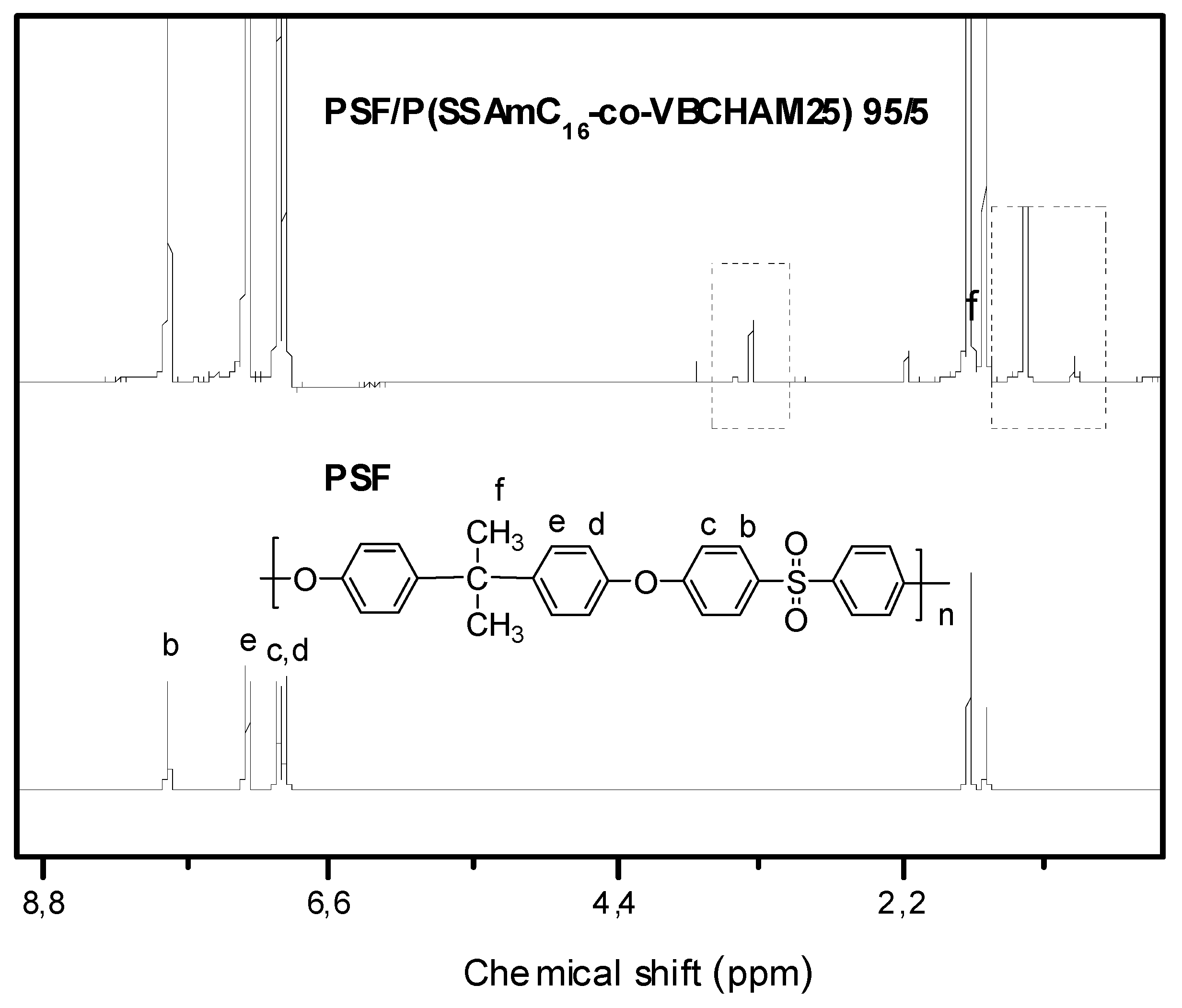

3. Experimental Section
3.1. Materials
3.2. Polymers Containing Quaternary Ammonium or Phosphonium Groups
3.2.1. Bacteriostatic Functionalization of the Homopolymers
3.2.2. Synthesis of Pre- or Post-Polymerization Quaternization Materials
3.3. Membrane Preparation
3.4. Bacterial Culture Preparation
3.5. Preparation of Polymeric Samples for Bacteriostatic Testing
3.6. Bacterial Reduction on Polymer Coupons
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, G.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloid Surf. A 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Popelka, A.; Novák, I.; Lehocký, M.; Chodák, I.; Sedliačik, J.; Gajtanska, M.; Sedliačiková, M.; Vesel, A.; Junkar, I.; Kleinová, A.; et al. Anti-bacterial Treatment of Polyethylene by Cold Plasma for Medical Purposes. Molecules 2012, 17, 762–785. [Google Scholar] [CrossRef] [PubMed]
- Jaballah, N.B.; Bouziri, A.; Mnif, K.; Hamdi, A.; Khaldi, A.; Kchaou, W. Epidemiology of hospital-acquired bloodstream infections in a Tunisian pediatric intensive care unit: A 2-year prospective study. Am. J. Infect. Control 2007, 35, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Skippen, I.; Shemko, M.; Turton, J.; Kaufmann, M.E.; Palmer, C.; Shetty, N. Epidemiology of infections caused by extended-spectrum b-lactamase-producing Escherichia coli and Klebsiella spp.: A nested case-control study from a tertiary hospital in London. J. Hosp. Infect. 2006, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Talon, D. The role of the hospital environment in the epidemiology of multi-resistant bacteria. J. Hosp. Infect. 1999, 43, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Liu, J.; Li, M.; Huang, J.; Sun, J.; Qu, H. Epidemiology and antimicrobial resistance among commonly encountered bacteria associated with infections and colonization in intensive care units in a university affiliated hospital in Shanghai. J. Microbiol. Immunol. 2014, 47, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Blythe, D.; Keenlyside, D.; Dawson, S.J.; Galloway, A. Environmental contamination due to methicillin-resistant Staphylococcus aureus (MRSA). J. Hosp. Infect. 1998, 38, 67–69. [Google Scholar] [CrossRef]
- Boyce, J.M.; Opal, S.M.; Chow, J.W.; Zervos, M.J.; Potter–Bynoe, G.; Sherman, C.B.; Romulo, R.L.; Fortna, S.; Medeiros, A.A. Outbreak of multidrug—Resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J. Clin. Microbiol. 1994, 32, 1148–1153. [Google Scholar] [PubMed]
- Thomassin, J.M.; Lenoir, S.; Riga, J.; Jérôme, R.; Detrembleur, C. Grafting of poly[2-(tert-butylamino)ethyl methacrylate] onto polypropylene by reactive blending and antibacterial activity of the copolymer. Biomacromolecules 2007, 8, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Charnley, M.; Textor, M.; Acikgoz, C. Designed polymer structures with antifouling-antimicrobial properties. React. Funct. Polym. 2011, 71, 329–334. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State of the Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Dębek, C.; Olędzka, E.; Kozłowski, R. Polymeric Systems of Antimicrobial Peptides-Strategies and Potential Applications. Molecules 2013, 18, 14122–14137. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Abdel-Hay, F.I.; El-Shanshoury, A-E.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly (glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.J.; Anwar, J.; Munawar, M.A. A novel application of quaternary ammonium compounds as antibacterial hybrid coating on glass surfaces. Langmuir 2009, 25, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Yudovin-Farber, I.; Beyth, N.; Nyska, A.; Weiss, E.I.; Golenser, J.; Domb, A.J. Surface characterization and biocompatibility of restorative resin containing nanoparticles. Biomacromolecules 2008, 9, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Iatridi, Z.; Moschakou, M.; Damigos, P.; Bokias, G.; Kallitsis, J.K. Development of Cu2+- and/or phosphonium-based polymeric biocidal materials and their potential application in antifouling paints. Prog. Org. Coat. 2012, 75, 190–199. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, J.C.; Tu, Q.; Nie, N.; Sha, J.; Liu, W.M.; Liu, R.; Zhanga, Y.; Wanga, J. Surface modification of PDMS by surface-initiated atom transfer radical polymerization of water-soluble dendronized PEG methacrylate. Colloids Surf. B Biointerfaces 2011, 88, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, M.; Zhou, L.; Tan, H.; Li, J.; Xiao, H.; Li, J.; Snow, J. Synthesis and antibacterial characterization of gemini surfactant monomers and copolymers. Polym. Chem. 2012, 3, 907–913. [Google Scholar] [CrossRef]
- Xiao, H.; Qian, L. Water-soluble antimicrobial polymers for functional cellulose fibres and hygiene paper products. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications, 1st ed.; Muñoz-Bonilla, A., Cerrada, M.L., Fernández-García, M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 75–96. [Google Scholar]
- Koromilas, N.D.; Lainioti, G.C.; Oikonomou, E.K.; Bokias, G.; Kallitsis, J.K. Synthesis and self-organization in dilute aqueous solution of hydrophobically modified polycations and polyampholytes based on 4-vinyl benzyl chloride. Eur. Polym. J. 2014, 54, 39–51. [Google Scholar] [CrossRef]
- Guo, A.; Wang, F.; Lin, W.; Xu, X.; Tang, T.; Shen, Y.; Guo, S. Evaluation of antibacterial activity of N-phosphonium chitosan as a novel polymeric antibacterial agent. Int. J. Biol. Macromol. 2014, 67, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Wynne, K.J.; Kurt, P.; Brunson, K.; Chakravorty, A.; Gupta, M.; Zhang, W.; Wood, L.; Ohman, D.E. Health and Safety via Surface Modification of Polyurethanes. In Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications; Scholz, C., Kressler, J., Eds.; Oxford University Press Inc.: Cary, NC, USA, 2013; Volume 1135, p. 303. [Google Scholar]
- Simoncic, B.; Tomsic, B. Structures of novel antimicrobial agents for textiles—A review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Koromilas, N.D.; Lainioti, G.C.; Gialeli, C.; Barbouri, D.; Kouravelou, K.B.; Karamanos, N.K.; Voyiatzis, G.A.; Kallitsis, J.K. Preparation and toxicological assessment of functionalized carbon nanotube-polymer hybrids. PLoS ONE 2014, 9, e107029. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. X. Antibacterial activity of filters incorporating phosphonium biocides. J. Appl. Polym. Sci. 1994, 54, 1305–1310. [Google Scholar] [CrossRef]
- Xue, Y.; Pan, Y.; Xiao, H.; Zhao, Y. Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv. 2014, 4, 46887–46895. [Google Scholar] [CrossRef]
- Ma, C.; Yang, H.; Zhou, X.; Wu, B.; Zhang, G. Polymeric material for anti-biofouling. Colloid Surf. B 2012, 100, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, Z.; Wang, J.; Wang, S. A novel method of surface modification to polysulfone ultrafiltration membrane by preadsorption of citric acid or sodium bisulfate. Membr. Water Treat. 2012, 3, 35–49. [Google Scholar] [CrossRef]
- Kumar, R.; Isloor, A.M.; Ismail, A.F.; Rashid, S.A.; Matsuura, T. Polysulfone–Chitosan blend ultrafiltration membranes: Preparation, characterization, permeation and antifouling properties. RSC Adv. 2013, 3, 7855–7861. [Google Scholar] [CrossRef]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Elguindi, Z.; Rensing, C.; Ravishankar, S. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012, 30, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Nada, S. Test Method for Efficacy of Copper Alloy Surfaces as a Sanitizer. In ATS Labs. Protocol. # CSC02032905.CUST.1; 2005. Available online: http://www2.epa.gov/nscep (accessed on 1 October 2015). [Google Scholar]
- Bekiari, V.; Nikolaou, K.; Koromilas, N.; Lainioti, G.; Avramidis, P.; Hotos, G.; Kallitsis, J.K.; Bokias, G. Release of polymeric biocides from synthetic matrices for marine biofouling applications. Agric. Agric. Sci. Procedia 2015, 4, 445–450. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds described in this publication may be available under agreement.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.C.; Koromilas, N.D.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J.K. Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds. Molecules 2015, 20, 21313-21327. https://doi.org/10.3390/molecules201219768
Kougia E, Tselepi M, Vasilopoulos G, Lainioti GC, Koromilas ND, Druvari D, Bokias G, Vantarakis A, Kallitsis JK. Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds. Molecules. 2015; 20(12):21313-21327. https://doi.org/10.3390/molecules201219768
Chicago/Turabian StyleKougia, Efstathia, Maria Tselepi, Gavriil Vasilopoulos, Georgia Ch. Lainioti, Nikos D. Koromilas, Denisa Druvari, Georgios Bokias, Apostolos Vantarakis, and Joannis K. Kallitsis. 2015. "Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds" Molecules 20, no. 12: 21313-21327. https://doi.org/10.3390/molecules201219768