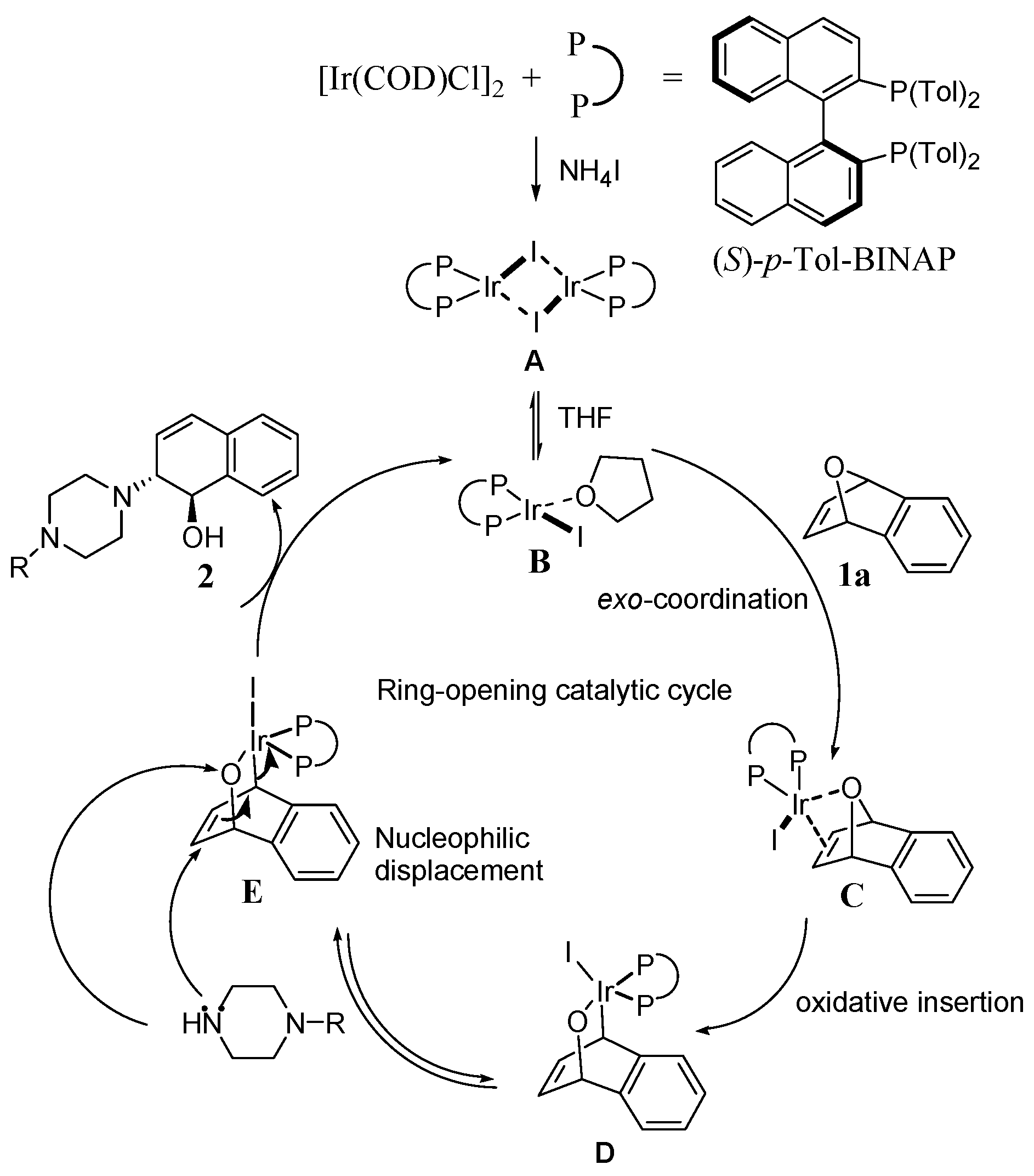
3.1.4. General Procedure for the Asymmetric Ring-Opening of 1a with N-Substituted Piperazines
A 5.0 mL round-bottomed flask was equipped with a reflux condenser, 2.5 mol % chloro(1,5-cyclooctadiene)iridium (I) dimer [Ir(COD)Cl]2 and 5.0 mol % (S)-p-Tol-BINAP were added and followed by addition of anhydrous tetrahydrofuran (2.0 mL). After they were stirred for 10 min to produce a yellow solution. 1,4-Dihydro-1,4-epoxynaphthalene 1a (50 mg, 0.3468 mmol) was added; then 10 min later, additive of ammonium iodide (1.0 equiv. to 1a) was added and heated to reflux. At the first sign of reflux, N-substituted piperazine nucleophiles (2.0 equiv. to 1a) were added. The reaction mixture was stirred at reflux and monitored by TLC until completion (typically 6–12 h). The solvent was removed in vacuo and the crude mixture was purified by column chromatography on silica gel to afford the desired products.
(1S,2S)-2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2a). Prepared according to general procedure. 2a was obtained as a white solid (111.9 mg, 99%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.21 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 54% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 19.0 min (major) and 20.9 min (minor). m.p.: 125–126 °C; = +83.2° (c = 68.9 mg, CHCl3); IR (thin film, cm−1) 3510 (br), 3054 (w), 2977 (s), 2934 (s), 2862 (s), 1490 (s), 1445 (s), 1383 (s), 1351 (s), 1077 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.60 (1H, d, J = 7.2 Hz), 7.31–7.23 (2H, m), 7.11–6.93 (5H, m), 6.58 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.17 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.93 (1H, 11.6), 3.53 (1H, dt, J = 11.6 Hz, J = 2.4 Hz), 3.22 (1H, br), 3.21–3.05 (4H, m), 3.02–2.98 (2H, m), 2.78–2.73 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 157.1, 154.7, 140.2, 137.2, 131.9, 129.6, 128.1, 127.6, 126.4, 124.8, 124.7, 122.8, 119.2, 116.4, 67.9, 67.7, 51.3, 49.2. MS (ESI): calcd m/z for C20H21FN2O (M+) 324.16, found: 325.12 [M + H]+. Anal. Calcd for C20H21FN2O: C, 74.05; H, 6.53; N, 8.64. Found: C, 74.29; H, 6.74; N, 8.56.
(1S,2S)-2-(4-Phenyl-piperazin-1-yl)-1,2-dihydro-naphthalen-1-ol (2b). Prepared according to general procedure. 2b was obtained as a white solid (93 mg, 87%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.42 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 36% using HPLC analysis on a CHIRALCEL OD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 2% 2-propanol in hexanes were 31.3 min (minor) and 35.9 min (major). m.p.: 162–163 °C; = + 62.8° (c = 39.5 mg, CHCl3); IR (thin film, cm−1) 3345 (br), 3016 (w), 2928 (s), 2824 (s), 1597 (m), 1492 (s), 1452 (s), 1369 (m), 1226 (s), 1169 (s), 1133 (m), 1045 (m), 763 (s); 1H-NMR (400 MHz, CDCl3) δ 7.56 (1H, d, J = 7.2 Hz), 7.26–7.19 (4H, m), 7.05 (1H, dd, J = 4.2 Hz, J = 2.8 Hz), 6.91–6.82 (3H, m), 6.52 (1H, dd, J = 2.8 Hz, J = 2.8 Hz), 6.08 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.89 (1H, J = 7.6 Hz), 3.50 (1H, J = 2.8 Hz), 3.37 (1H, br), 3.23–3.13 (4H, m), 2.95–2.89 (2H, m), 2.70–2.65 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 151.4, 137.2, 131.9, 129.6, 129.3, 128.1, 127.6, 126.4, 125.0, 124.5, 120.1, 116.4, 67.9, 67.6, 49.9, 49.1. MS (ESI): calcd m/z for C20H22N2O (M+) 306.17, found: 307.16 [M + H]+. Anal. Calcd for C20H22N2O: C, 78.40; H, 7.24; N, 9.14. Found: C, 78.21; H, 7.31; N, 9.28.
(1S,2S)-2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2c). Prepared according to general procedure. 2c was obtained as a white solid (112 mg, 86%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.37 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 67% using HPLC analysis on a CHIRALCEL. AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 33.9 min (major) and 36.9 min (minor). m.p.: 142–143 °C; = +100.4° (c = 45.6 mg, CHCl3); IR (thin film, cm−1) 3456 (br), 3027 (w), 2936 (m), 2836 (s), 1594 (s), 1552 (m), 1483 (s), 1452 (m), 1237 (s), 1139 (m), 1044 (s), 782 (s); 1H-NMR (400 MHz, CDCl3) δ 7.51 (1H, d, J = 7.2 Hz), 7.30–7.20 (3H, m), 7.05 (1H, d, J = 6.8 Hz), 6.91 (1H, d, J = 2.8 Hz), 6.72 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 6.46 (1H, dd, J = 2.0 Hz, J = 1.6 Hz), 6.02 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.83 (1H, d, J = 11.6 Hz), 3.42 (1H, d, J = 10.8 Hz), 3.22–3.05 (5H, m), 2.98–2.85 (2H, m), 2.70–2.61 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 150.7, 137.0, 132.9, 131.8, 130.6, 129.7, 128.1, 127.7, 126.4, 125.1, 124.3, 122.4, 117.5, 115.6, 67.9, 67.5, 49.4, 48.8. MS (ESI): calcd m/z for C20H20Cl2N2O (M+) 374.10, found: 375.05 [M + H]+. Anal. Calcd for C20H20Cl2N2O: C, 64.01; H, 5.37; N, 7.46. Found: C, 63.82; H, 5.69; N, 7.47.
(1S,2S)-1-{4-(1-Hydroxy-1,2-dihydro-naphthalen-2-yl)-piperazin-1-yl]-phen-yl}-ethanone (2d). Prepared according to general procedure. 2d was obtained as a white solid (105 mg, 87%) by flash chromatography (ethyl acetate: petroleum ether = 2:1, v/v). Rf = 0.40 on silica gel (ethyl acetate: petroleum ether = 2:1, v/v). The ee was determined to be 38% using HPLC analysis on a CHIRALCEL OD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 2% 2-propanol in hexanes were 30.3 min (minor) and 34.9 min (major). m.p.: 202–203 °C; = −3.0° (c = 26.3 mg, CHCl3); IR (thin film, cm−1) 3396 (br), 3018 (w), 2923 (s), 2841 (s), 1739 (m), 1643 (s), 1597 (s), 1388 (s), 1245 (s), 1086 (s), 817 (s); 1H-NMR (400 MHz, CDCl3) δ 7.88 (2H, d, J = 8.8 Hz), 7.58 (1H, d, J = 6.8 Hz), 7.27 (2H, td, J = 7.6 Hz, J = 7.2 Hz), 7.10 (1H, d, J = 6.8 Hz), 6.88 (2H, d, J = 7.6 Hz), 6.57 (1H, d, J = 6.0 Hz), 6.08 (1H, d, J = 9.6 Hz), 4.93 (1H, d, J = 11.2 Hz), 3.54 (1H, d, J = 2.0 Hz), 3.41–3.35 (4H, m), 3.19 (1H, br), 2.97–2.94 (2H, m), 2.73–2.71 (2H, m), 2.52 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 196.7, 154.3, 137.0, 131.9, 130.6, 129.8, 128.2, 128.0, 127.8, 126.5, 125.2, 124.3, 113.7, 68.0, 67.6, 48.9, 48.1, 26.3. MS (ESI): calcd m/z for C22H24N2O2 (M+) 348.18, found: 349.15 [M + H]+. Anal. Calcd for C22H24N2O2: C, 75.83; H, 6.94; N, 8.04. Found: C, 75.65; H, 7.34; N, 8.01.
(1S,2S)-2-(4-Benzhydryl-piperazin-1-yl)-1,2-dihydro-naphthalen-1-ol (2e). Prepared according to general procedure. 2e was obtained as a white solid (135 mg, 98%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.22 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 49% using HPLC analysis on a CHIRALCEL OD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 2% 2-propanol in hexanes were 18.2 min (major) and 19.8 min (minor). m.p.: 160–161 °C; = +150.2° (c = 33.3 mg, CHCl3); IR (thin film, cm−1) 3429 (br), 3027 (w), 2923 (s), 2807 (m), 1594 (s), 1487 (m), 1451 (s), 1383 (m), 1133 (s), 1040 (s), 741 (s), 697 (s); 1H-NMR (400 MHz, CDCl3) δ 7.66 (1H, d, J = 7.2 Hz), 7.52 (4H, d, J = 7.6 Hz), 7.39–7.25 (8H, m), 7.15 (1H, d, J = 7.2 Hz), 6.62 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.26 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.94 (1H, J = 11.6 Hz), 4.34 (1H, s), 3.53 (1H, dt, J = 11.6 Hz, J = 2.4 Hz), 3.45 (1H, br), 2.98–2.84 (2H, m), 2.69–2.61 (2H, m), 2.49–2.42 (4H, m); 13C-NMR (100 MHz, CDCl3) δ 142.8, 137.3, 132.0, 129.3, 128.7, 128.5, 128.1, 127.9, 127.5, 127.1, 126.3, 125.0, 124.9, 67.8, 67.3, 52.5, 49.2. MS (ESI): calcd m/z for C27H28N2O (M+) 396.22, found: 397.19 [M + H]+. Anal. Calcd for C27H28N2O: C, 81.87; H, 7.12; N, 7.06. Found: C, 81.52; H, 7.43; N, 7.02.
(1S,2S)-2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2f). Prepared according to general procedure. 2f was obtained as a white solid (95 mg, 81%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.17 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v); The ee was determined to be 33% using HPLC analysis on a CHIRALCEL OD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 2% 2-propanol in hexanes were 27.6 min (minor) and 29.3 min (major); m.p.: 148–149 °C; = +53.8° (c = 69.9 mg, CHCl3); IR (thin film, cm−1) 3519 (br), 3089 (w), 2978 (s), 2934 (s), 2863 (s), 2805 (s), 1490 (s), 1445 (s), 1415 (s), 1383 (s), 1351 (s), 1297 (m), 1076 (s), 1044 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.61 (1H, d, J = 7.2 Hz), 7.29–7.25 (2H, m), 7.10 (1H, d, J = 7.2 Hz), 7.02 (1H, dt, J = 8.0 Hz, J = 2.4 Hz), 6.98–6.95 (2H, m), 6.88 (1H, d, J = 8.0 Hz), 6.57 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.20 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.95 (1H, d, J = 11.6 Hz), 3.88 (3H, s), 3.54 (1H, J = 6.4 Hz), 3.41 (1H, br), 3.18–3.01 (4H, m), 3.01–2.98 (2H, m), 2.79–2.70 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 152.4, 141.3, 137.3, 132.0, 129.4, 128.0, 127.6, 126.3, 125.0, 124.9, 123.2, 121.1, 118.4, 111.2, 67.8, 67.7, 55.5, 51.4, 49.3. MS (ESI): calcd m/z for C21H24N2O3 (M+) 336.18, found: 337.10 [M + H]+. Anal. Calcd for C21H24N2O3: C, 74.97; H, 7.19; N, 8.33. Found: C, 74.89; H, 7.44; N, 8.56.
(1S,2S)-2-[4-(2-Chloro-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2g). Prepared according to general procedure. 2g was obtained as a white solid (105 mg, 89%) by flash chromatography (ethyl acetate: petroleum ether = 1:3, v/v). Rf = 0.25 on silica gel (ethyl acetate: petroleum ether = 1:3, v/v). The ee was determined to be 50% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 19.4 min (major) and 20.3 min (minor). m.p.: 122–123 °C; = +91.6° (c = 29.7 mg, CHCl3); IR (thin film, cm−1) 3468 (br), 3060 (w), 2927 (s), 2828 (s), 1588 (s), 1480 (s), 1453 (s), 1377 (m), 1230 (s), 1123 (s), 1040 (s), 781 (s), 749 (s); 1H-NMR (400 MHz, CDCl3) δ 7.61 (1H, d, J = 7.2 Hz), 7.37 (1H, d, J = 7.2 Hz), 7.31–7.22 (3H, m), 7.11–6.99 (2H, m), 6.97 (1H, d, J = 8.4 Hz), 6.58 (1H, dd, J = 2.8 Hz, J = 2.8 Hz), 6.21 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 4.95 (1H, 11.6), 3.53 (1H, dt, J = 11.6 Hz, J = 2.4 Hz), 3.34 (1H, br), 3.15–3.09 (4H, m), 3.05–2.95 (2H, m), 2.79–2.74 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 149.3, 137.3, 132.0, 130.9, 129.6, 129.0, 128.1, 127.8, 127.6, 126.4, 124.9, 124.0, 120.6, 67.9, 67.8, 51.9, 49.4. MS (ESI): calcd m/z for C20H21ClN2O (M+) 340.13, found: 341.11 [M + H]+. Anal.Calcd for C20H21ClN2O: C, 70.48; H, 6.21; N, 8.22. Found: C, 70.20; H, 6.49; N, 8.49.
(1S,2S)-2-[4-o-Toyl-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2h). Prepared according to general procedure. 2h was obtained as a white solid (97 mg, 87%) by flash chromatography (ethyl acetate: petroleum ether = 1:3, v/v). Rf = 0.2 on silica gel (ethyl acetate: petroleum ether = 1:3, v/v). The ee was determined to be 54% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 14.7 min (major) and 16.4 min (minor). m.p.: 115–116 °C; = +95.9° (c = 49.1 mg, CHCl3); IR (thin film, cm−1) 3461 (br), 3019 (m), 2949 (s), 2878 (s), 2816 (w), 1596 (w), 1490 (s), 1453 (s), 1256 (m), 1224 (s), 1195 (m), 1132 (m), 1049 (s), 781 (s), 768 (s); 1H-NMR (400 MHz, CDCl3) δ 7.69 (1H, d, J = 7.2 Hz), 7.38–7.25 (4H, m), 7.19–7.07 (3H, m), 6.66 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.29 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 5.03 (1H, J = 12.0 Hz), 3.60 (1H, dt, J = 12.0 Hz, J = 2.8 Hz), 3.39 (1H, br), 3.11–3.02 (6H, m), 2.83–2.79 (2H, m), 2.42 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 151.6, 137.4, 132.8, 132.0, 131.3, 129.5, 128.1, 127.6, 126.3, 125.0, 124.9, 123.5, 119.2, 67.9, 67.8, 52.5, 49.6, 18.1. MS (ESI): calcd m/z for C21H24N2O (M+) 320.19, found: 321.16 [M + H]+. Anal. Calcd for C21H24N2O: C, 78.71; H, 7.55; N, 8.74. Found: C, 78.49; H, 7.82; N, 8.69.
(1S,2S)-2-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2i). Prepared according to general procedure. 2i was obtained as a white solid (101 mg, 91%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.34 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 45% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 26.9 min (major) and 29.4 min (minor). m.p.: 167–168 °C; = +133.2° (c = 48.2 mg, CHCl3); IR (thin film, cm−1) 3592 (br), 3214 (w), 2978 (s), 2934 (s), 2872 (s), 2806 (s), 1627 (w), 1489 (s), 1445 (s), 1415 (s), 1298 (s), 1067 (s), 1044 (s), 935 (m), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.59 (1H, d, J = 7.2 Hz), 7.29–7.25 (2H, m), 7.09 (1H, d, J = 7.2 Hz), 7.00–6.96 (2H, m), 6.91–6.87 (2H, m), 6.57 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 6.13 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.93 (1H, d, J = 11.6 Hz), 3.53 (1H, dt, J = 11.6 Hz, J = 2.8 Hz), 3.27 (1H, br), 3.18–3.13 (4H, m), 3.00–2.95 (2H, m), 2.75–2.71 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 158.7, 156.3, 148.1, 137.2, 131.9, 129.7, 128.1, 127.7, 126.4, 125.0, 125.0, 118.2, 115.9, 67.9, 67.6, 51.0, 49.1. MS (ESI): calcd m/z for C20H21FN2O (M+) 324.16, found: 325.15 [M + H]+. Anal. Calcd for C20H21FN2O: C, 74.05; H, 6.53; N, 8.64. Found: C, 74.19; H, 6.74; N, 8.66.
(1S,2S)-2-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2j). Prepared according to general procedure. 2j was obtained as a white solid (99 mg, 85%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.23 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 54% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL / min; Retention times in 10% 2-propanol in hexanes were 32.5 min (major) and 36.4 min (minor). m.p.: 178–179 °C; = +102.5° (c = 59.9 mg, CHCl3); IR (thin film, cm−1) 3566 (br), 3219 (w), 2978 (s), 2934 (s), 2862 (s), 2805 (s), 1491 (s), 1445 (s), 1416 (m), 1383 (s), 1351 (s), 1297 (m), 1077 (s), 1044 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.60 (1H, d, J = 7.2 Hz), 7.31–7.23 (2H, m), 7.10 (1H, d, J = 8.4 Hz), 6.95–6.84 (4H, m), 6.57 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.15 (1H, dd, J = 2.0 Hz, J = 2.4 Hz), 4.93 (1H, J = 11.6 Hz), 3.73 (3H, s), 3.51 (1H, dt, J = 11.6 Hz, J = 2.4 Hz), 3.32 (1H, br), 3.19–3.08 (4H, m), 3.01–2.97 (2H, m), 2.76–2.71 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 154.2, 145.8, 137.3, 132.0, 129.6, 128.1, 127.7, 126.5, 125.0, 124.7, 118.6, 114.7, 68.0, 67.7, 55.8, 51.5, 49.3. MS (ESI): calcd m/z for C21H24N2O2 (M+) 336.18, found: 337.10 [M + H]+. Anal. Calcd for C21H24N2O2: C, 74.97; H, 7.19; N, 8.33. Found: C, 74.77; H, 7.40; N, 8.25.
(1S,2S)-2-[4-(2,5-Dimethyl-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2k). Prepared according to general procedure. 2k was obtained as a white solid (119 mg, 98%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.32 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 36% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 13.8 min (major) and 14.3 min (minor). m.p.: 128–130 °C; = +100.0° (c = 44.7 mg, CHCl3); IR (thin film, cm−1) 3509 (br), 3216 (w), 2977 (s), 2934 (s), 2862 (s), 2806 (s), 1490 (s), 1445 (s), 1415 (s), 1383 (s), 1351 (s), 1298 (m), 1127 (s), 1077 (s), 1044 (m), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.52 (1H, d, J = 7.2 Hz), 7.21–7.13 (2H, m), 6.98 (2H, t, J = 6.8 Hz), 6.73 (2H, t, J = 11.6 Hz, J = 7.6 Hz), 6.47 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 6.10 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.84 (1H, 12.0), 3.42 (1H, dt, J = 12.0 Hz, J = 2.4 Hz), 3.37 (1H, br), 2.90–2.82 (6H, m), 2.64–2.58 (2H, m), 2.23 (3H, s), 2.19 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 151.4, 137.3, 136.3, 132.0, 131.1, 129.4, 129.4, 128.0, 127.5, 126.3, 125.0, 124.8, 124.0, 119.9, 67.9, 67.7, 52.4, 49.3, 21.4, 17.7. MS (ESI): calcd m/z for C22H26N2O (M+) 334.20, found: 335.23 [M + H]+. Anal. Calcd for C22H26N2O: C, 79.00; H, 7.84; N, 8.38. Found: C, 78.83; H, 8.12; N, 8.32.
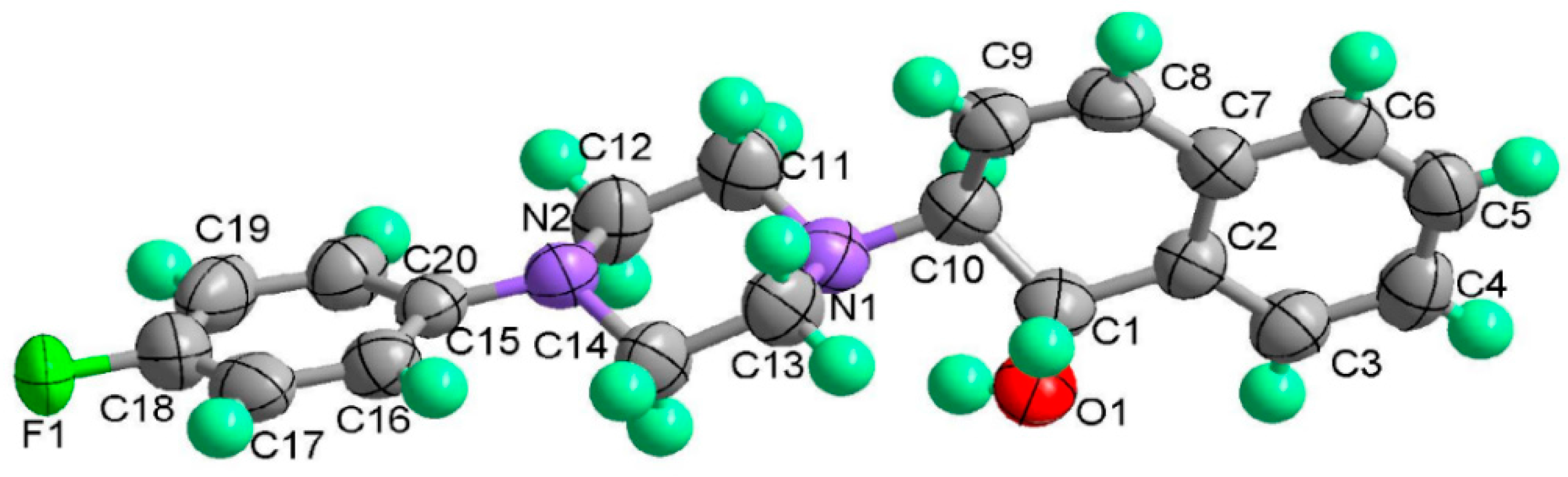
(1S,2S)-2-[4-(2,5-Difluoro-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2l). Prepared according to general procedure. 2l was obtained as a white solid (115 mg, 97%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). The absolute stereochemistry was determined by X-ray crystallography. Rf = 0.14 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 43% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 20.2 min (major) and 23.2 min (minor). m.p.: 122–124 °C; = +150.7° (c = 26.8 mg, CHCl3); IR (thin film, cm−1) 3507 (br), 3203 (w), 2988 (s), 2943 (s), 2872 (s), 1509 (s), 1445 (s), 1383 (s), 1297 (m), 1131 (s), 935 (s), 846 (s), 793 (s); 1H-NMR (400 MHz, CDCl3) δ 7.59 (1H, d, J = 7.2 Hz), 7.31–7.23 (2H, m), 7.09 (1H, d, J = 6.0 Hz), 6.95–6.78 (3H, m), 6.57 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.16 (1H, dd, J = 2.8 Hz, J = 2.8 Hz), 4.93 (1H, 11.6), 3.53 (1H, dt, J = 11.6 Hz, J = 2.8 Hz), 3.31 (1H, br), 3.13–2.96 (4H, m), 2.96–2.92 (2H, m), 2.75–2.70 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 159.4, 157.0, 154.6, 137.2, 136.9, 131.9, 129.6, 128.1, 127.7, 126.4, 125.0, 119.7, 111.0, 110.8, 105.2, 104.7, 67.9, 67.7, 51.7, 49.2. MS (ESI): calcd m/z for C20H20F2N2O (M+) 342.15, found: 343.20 [M + H]+. Anal. Calcd for C20H20F2N2O: C, 70.16; H, 5.89; N, 8.18. Found: C, 70.19; H, 5.94; N, 8.26.
(1S,2S)-2-[4-(2,3-Dimethyl-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2m). Prepared according to general procedure. 2m was obtained as a white solid (102 mg, 88%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.29 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 47% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 13.5 min (major) and 14.4 min (minor). m.p.: 163–164 °C; = +82.7° (c = 99.7 mg, CHCl3); IR (thin film, cm−1); 3590 (br), 3228 (w), 2974 (s), 2934 (s), 2873 (s), 2806 (s), 1490 (s), 1445 (s), 1415 (m), 1383 (s), 1351 (s), 1297 (m), 1133 (s), 1077 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.60 (1H, d, J = 7.2 Hz), 7.27–7.23 (2H, m), 7.08 (2H, t, J = 7.2 Hz), 6.91 (2H, dd, J = 4.8 Hz, J = 4.4 Hz), 6.55 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 6.19 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.92 (1H, J = 14.6 Hz), 3.50 (1H, dt, J = 11.6 Hz, J = 2.8 Hz), 3.41 (1H, br), 2.97–2.88 (6H, m), 2.69 (2H, t, J = 2.4 Hz), 2.27 (3H, s), 2.24 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 151.7, 138.3, 137.5, 132.1, 131.5, 129.6, 128.2, 127.7, 126.5, 126.2, 125.4, 125.2, 125.0, 116.9, 68.0, 67.8, 53.0, 49.7, 21.0, 14.3. MS (ESI): calcd m/z for C22H26N2O (M+) 334.20, found: 335.28 [M + H]+. Anal. Calcd for C22H26N2O: C, 79.00; H, 7.84; N, 8.38. Found: C, 78.84; H, 8.17; N, 8.27.
(1S,2S)-2-[4-(1-Hydroxy1,2-dihydro-naphthalen-2-yl-piperazin-1-yl]–benzo-nitrile (2n). Prepared according to general procedure. 2n was obtained as a white solid (109 mg, 95%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.20 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 54% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 43.6 min (major) and 53.4 min (minor). m.p.: 126–127 °C; = +111.4° (c = 64.9 mg, CHCl3); IR (thin film, cm−1); 3692 (br), 3210 (w), 2934 (s), 2874 (s), 2805 (s), 2272 (w), 1627 (w), 1597 (w), 1491 (s), 1445 (s), 1383 (s), 1298 (s), 1118 (s), 1077 (s), 935 (s), 846 (s), 795 (m); 1H-NMR (400 MHz, CDCl3) δ 7.61–7.57 (2H, m), 7.51 (1H, td, J = 7.6 Hz, J = 1.6 Hz), 7.31–7.23 (2H, m), 7.10 (1H, d, J = 5.6 Hz), 7.05–7.02 (2H, m), 6.58 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.18 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 6.15 (1H, dd, J = 2.0 Hz, J = 2.4 Hz), 4.94 (1H, J = 11.2 Hz), 3.53 (1H, dt, J = 11.6 Hz, J = 2.4 Hz), 3.33–3.23 (4H, m), 3.07–3.01 (2H, m), 2.82–2.76 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 155.8, 137.1, 134.5, 134.0, 132.5, 131.9, 128.1, 127.7, 126.4, 124.9, 122.1, 118.6, 106.3, 67.9, 67.7, 52.3, 49.2. MS (ESI): calcd m/z for C21H21N3O (M+) 331.17, found: 332.22 [M + H]+. Anal. Calcd for C21H21N3O: C, 76.11; H, 6.39; N, 12.68. Found: C, 76.01; H, 6.69; N, 12.51.
(1S,2S)-2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2o). Prepared according to general procedure. 2o was obtained as a white solid (111 mg, 96%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.20 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 58% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 22.6 min (minor) and 23.8 min (major). m.p.: 125–126 °C; = +109.7° (c = 59.5 mg, CHCl3); IR (thin film, cm−1); 3512 (br), 3211 (w), 2974 (s), 2806 (s), 1615 (s), 1490 (s), 1445 (s), 1416 (s), 1383 (m), 1298 (s), 1142 (s), 1044 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.59 (1H, d, J = 7.2 Hz), 7.31–7.23 (2H, m), 7.07 (1H, d, J = 7.2 Hz), 7.04 (1H, d, J = 7.2 Hz), 6.78 (1H, d, J = 2.4 Hz), 6.71 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.56 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 6.15 (1H, dd, J = 2.0 Hz, J = 2.4 Hz), 4.93 (1H, 12.0), 3.53 (1H, dt, J = 11.6 Hz, J = 2.8 Hz), 3.37 (1H, br), 3.24–3.14 (4H, m), 3.01–2.96 (2H, m), 2.75–2.70 (2H, m), 2.25 (3H, s), 2.20 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 149.8, 137.3, 137.2, 131.9, 130.4, 129.6, 128.5, 128.1, 127.6, 126.4, 124.9, 124.7, 118.5, 114.2, 67.9, 67.7, 50.6, 49.2, 20.4, 19.0. MS (ESI): calcd m/z for C22H26N2O (M+) 334.20, found: 335.28 [M + H]+. Anal. Calcd for C22H26N2O: C, 79.00; H, 7.84; N, 8.38. Found: C, 78.72; H, 8.31; N, 8.48.
(1S,2S)-2-[4-p-Tolyl-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2p). Prepared according to general procedure. 2p was obtained as a white solid (94 mg, 85%) by flash chromatography (ethyl acetate: petroleum ether = 1:3 , v/v). Rf = 0.28 on silica gel (ethyl acetate: petroleum ether = 1:3, v/v). The ee was determined to be 27% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 23.0 min (major) and 23.6 min (minor). m.p.: 193–194 °C; = +108.9° (c = 57.1 mg, CHCl3); IR (thin film, cm−1) 3422 (br), 3022 (w), 2918 (w), 2833 (m), 1649 (s), 1515 (s), 1499 (s), 1382 (s), 1241 (s), 1140 (s), 1046 (s), 781 (s), 746 (s); 1H-NMR (400 MHz, CDCl3) δ 7.60 (1H, d, J = 6.8 Hz), 7.31–7.23 (2H, m), 7.09 (3H, d, J = 7.2 Hz), 6.86 (2H, d, J = 7.2 Hz), 6.57 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.15 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.94 (1H, d, J = 11.6 Hz), 3.52 (1H, dt, J = 11.2 Hz, J = 2.8 Hz), 3.36 (1H, br), 3.24–3.14 (4H, m), 3.01–2.96 (2H, m), 2.75–2.70 (2H, m), 2.29 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 149.4, 137.2, 131.9, 129.9, 129.7, 129.6, 128.1, 127.6, 126.4, 124.9, 124.6, 116.8, 67.9, 67.7, 50.6, 49.2, 20.6. MS (ESI): calcd m/z for C21H24N2O (M+) 320.19, found: 321.25 [M + H]+. Anal. Calcd for C21H24N2O: C, 78.71; H, 7.55; N, 8.74. Found: C, 78.52; H, 7.81; N, 8.66.
(1S,2S)-2-(4-Benzo[1,3] dioxol-5-yl-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2q). Prepared according to general procedure. 2q was obtained as a white solid (108 mg, 86%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.27 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 57% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 20.5 min (minor) and 25.5 min (major). m.p.: 112–113 °C; = +81.8° (c = 99.3 mg, CHCl3); IR (thin film, cm−1) 3514 (br), 3211 (w), 2925 (s), 2868 (s), 1627 (w), 1488 (s), 1446 (s), 1415 (s), 1298 (s), 1044 (s), 935 (s), 846 (s), 795 (s); 1H-NMR (400 MHz, CDCl3) δ 7.59 (1H, d, J = 6.8 Hz), 7.29–7.25 (2H, m), 7.09 (1H, d, J = 8.4 Hz), 6.89 (1H, s), 6.78 (2H, s), 6.55 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 6.45 (1H, dd, J = 2.0, J = 2.4 Hz), 5.97 (2H, s), 4.90 (1H, d, J = 11.2 Hz), 3.49–3.46 (4H, m), 2.89–2.84 (2H, m), 2.63–2.53 (6H, m); 13C-NMR (100 MHz, CDCl3) δ 147.8, 146.8, 137.3, 132.0, 132.0, 129.3, 128.0, 127.6, 126.3, 125.0, 124.8, 122.5, 109.7, 108.1, 67.9, 67.5, 62.9, 53.6, 49.0. MS (ESI): calcd m/z for C22H24N2O3 (M+) 364.18. Found 365.26 [M + H]+. Anal. Calcd for C22H24N2O3: C, 72.50; H, 6.64; N, 7.69. Found: C, 72.29; H, 6.44; N, 7.76.
(1S,2S)-2-[4-(2,4-Dimethyl-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2r). Prepared according to general procedure. 2r was obtained as a white solid (103 mg, 89%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.26 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 59% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 13.1 min (major) and 14.7 min (minor). m.p.: 113–114 °C; = +67.6° (c = 65.1 mg, CHCl3); IR (thin film, cm−1) 3589 (br), 3209 (w), 2990 (s), 2806 (s), 2778 (s), 1627 (w), 1491 (s), 1445 (s), 1415 (s), 1383 (s), 1298 (m), 1141 (s), 1076 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.62 (1H, d, J = 7.6 Hz), 7.31–7.23 (2H, m), 7.09 (1H, d, J = 7.2 Hz, J = 2.4 Hz), 7.02–6.95 (3H, m), 6.57 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.21 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.94 (1H, d, J = 11.6 Hz), 3.51 (1H, dt, J = 12 Hz, J = 2.4 Hz), 3.38 (1H, s), 2.99–2.90 (6H, m), 3.72–2.69 (2H, m), 2.31 (6H, s); 13C-NMR (100 MHz, CDCl3) δ 149.2, 137.4, 132.8, 132.6, 132.0, 132.0, 129.4, 128.0, 127.6, 127.2, 126.3, 125.1, 124.9, 119.1, 67.9, 67.7, 52.6, 49.6, 20.9, 17.9. MS (ESI): calcd m/z for C22H26N2O (M+) 334.20, found: 335.25 [M + H]+. Anal. Calcd for C22H26N2O: C, 79.00; H, 7.84; N, 8.38. Found: C, 79.03; H, 8.13; N, 8.27.
(1S,2S)-2-[4-m-Tolyl-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2s). Prepared according to general procedure. 2s was obtained as a white solid (100 mg, 90%) by flash chromatography (ethyl acetate: petroleum ether = 1:4, v/v). Rf = 0.26 on silica gel (ethyl acetate: petroleum ether = 1:4, v/v). The ee was determined to be 59% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 22.2 min (minor) and 22.7 min (major). m.p.: 94–96 °C; = +107.4° (c = 46.2 mg, CHCl3); IR (thin film, cm−1) 3507 (br), 3158 (w), 2978 (s), 2934 (s), 2861 (s), 2806 (m), 1491 (s), 1445 (s), 1416 (s), 1383 (s), 1351 (s), 1298 (m), 1154 (s), 1077 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.69 (1H, d, J = 7.2 Hz), 7.36 (2H, td, J = 2.4 Hz, J = 7.2 Hz), 7.27 (1H, t, J = 7.6 Hz), 7.19 (1H, d, J = 7.2 Hz), 6.84 (3H, dd, J = 4.8 Hz, J = 7.6 Hz), 6.66 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.23 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 5.03 (1H, d, J = 11.6 Hz), 3.62 (1H, dt, J = 11.6 Hz, J = 2.0 Hz), 3.43 (1H, br), 3.36–3.27 (4H, m), 3.09–3.04 (2H, m), 2.83–2.78 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 151.5, 139.0, 137.2, 131.9, 129.7, 129.1, 128.1, 127.6, 126.4, 124.6, 121.0, 117.3, 113.6, 67.9, 67.7, 50.1, 49.2, 21.9. MS (ESI): calcd m/z for C21H24N2O (M+) 320.19, found: 321.25 [M + H]+. Anal. Calcd for C21H24N2O: C, 78.71; H, 7.55; N, 8.74. Found: C, 78.69; H, 7.94; N, 8.56.
(1S,2S)-2-(4-Phenethyl-cyclohexyl)-1,2-dihydro-naphthalen-1-ol (2t). Prepared according to general procedure. 2t was obtained as a white solid (90 mg, 78%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.20 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 50% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 10.1 min (major) and 11.4 min (minor). m.p.: 112–113 °C; = +87.9° (c = 36.5 mg, CHCl3); IR (thin film, cm−1) 3519 (br), 3089 (w), 2978 (s), 2934 (s), 2863 (s), 2805 (s), 1490 (s), 1445 (s), 1415 (s), 1383 (s), 1351 (s), 1297 (m), 1076 (s), 1044 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.60 (1H, d, J = 7.2 Hz), 7.34–7.21 (7H, m), 7.09 (1H, d, J = 1.2 Hz), 6.56 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.15 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.91 (1H, d, J = 11.6 Hz), 3.48 (2H, ddd, J = 2.4 Hz, J = 2.4 Hz, J = 6.8 Hz), 2.85–2.96 (4H, m), 2.65 (8H, dd, J = 4.8 Hz, J = 6.4 Hz); 13C-NMR (100 MHz, CDCl3) δ 140.5, 137.4, 132.1, 129.5, 128.9, 128.8, 128.7, 128.6, 128.1, 127.6, 126.3, 125.1, 67.7, 67.6, 60.7, 53.9, 49.1, 33.9. MS (ESI): calcd m/z for C22H26N2O (M+) 334.20, found: 335.18 [M + H]+. Anal. Calcd for C22H26N2O: C, 79.00; H, 7.84; N, 8.38. Found: C, 79.89; H, 8.05; N, 8.26.
(1S,2S)-4-(1-Hydroxy-1,2-dihydro-naphthalen-2-yl)-piperazine-1-carboxy-lic acid ethyl ester (2u). Prepared according to general procedure. 2u was obtained as a white solid (89 mg, 85%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.17 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 51% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 16.0 min (minor) and 21.7 min (major). m.p.: 147–148 °C; = +93.2° (c = 65.3 mg, CHCl3); IR (thin film, cm−1) 3517 (br), 3087 (w), 2977 (s), 2934 (s), 2867 (s), 2806 (s), 1710 (m), 1490 (s), 1445 (s), 1415 (m), 1383 (s), 1351 (s), 1298 (m), 1077 (s), 1044 (m), 935 (m), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.57 (1H, d, J = 7.6 Hz), 7.31–7.23 (2H, m), 7.09 (1H, d, J = 6.8 Hz), 6.56 (1H, dd, J = 2.0 Hz, J = 2.0 Hz), 6.03 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 4.90 (1H, d, J = 10.8 Hz), 4.16 (2H, dd, J = 14.4 Hz, J = 6.8 Hz), 3.56–3.47 (5H, m), 3.38 (1H, br), 2.74 (2H, t, J = 6.8 Hz), 2.54 (2H, t, J = 6.0 Hz), 1.29 (3H, t, J = 7.2 Hz); 13C-NMR (100 MHz, CDCl3) δ 155.5, 137.0, 131.8, 129.5, 128.0, 127.6, 126.3, 125.2, 124.5, 67.8, 67.6, 61.4, 48.9, 44.2, 14.7. MS (ESI): calcd m/z for C17H22N2O3 (M+) 302.16, found: 303.08 [M + H]+. Anal. Calcd for C17H22N2O3: C, 67.53; H, 7.33; N, 9.26. Found: C, 67.39; H, 7.68; N, 9.15.
(1S,2S)-2-[4-(3-Trifluoromethyl-phenyl)-piperazin-1-yl]-1,2-dihydro–naphthalen-1-ol (2v). Prepared according to general procedure. 2v was obtained as a white solid (107 mg, 83%) by flash chromatography (ethyl acetate: petroleum ether = 1:3, v/v). Rf = 0.26 on silica gel (ethyl acetate: petroleum ether = 1:3, v/v). The ee was determined to be 54% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 2% 2-propanol in hexanes were 35.2 min (minor) and 36.3 min (major). m.p.: 120–121 °C; = +87.6° (c = 46.8 mg, CHCl3); IR (thin film, cm−1) 3517 (br), 3092 (w), 2978 (s), 2869 (s) 1628 (w), 1491 (m), 1444 (s), 1383 (s), 1351 (s), 1297 (m), 1133 (s), 1077 (s), 934 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.59 (1H, d, J = 7.2 Hz), 7.34 (1H, t, J = 8.0 Hz), 7.29–7.23 (2H, m), 7.13–7.06 (4H, m), 6.57 (1H, d, J = 10.0 Hz), 6.11 (1H, dd, J = 2.0 Hz, J = 2.0 Hz), 4.94 (1H, d, J = 12.0 Hz), 3.53 (1H, dd, J = 2.4 Hz, J = 2.0 Hz), 3.30–3.22 (5H, m), 2.97 (2H, dd, J = 3.2 Hz, J = 5.2 Hz), 2.72 (2H, t, J = 11.2 Hz); 13C-NMR(100 MHz, CDCl3) δ 151.5, 137.1, 131.9, 129.8, 129.7, 128.2, 127.8, 126.5, 125.1, 124.7, 119.1, 116.2, 116.2, 112.5, 112.5, 68.0, 67.6, 49.5, 49.0. MS (ESI): calcd m/z for C21H21F3N2O (M+) 374.16, found: 375.12 [M + H]+. Anal. Calcd for C21H21F3N2O: C, 67.37; H, 5.65; N, 7.48. Found: C, 67.16; H, 5.89; N, 7.36.
(1S,2S)-2-[4-(4-Trifluoromethyl-phenyl)-piperazin-1-yl]-1,2-dihydro-naphth-alen-1-ol (2w). Prepared according to general procedure. 2w was obtained as a white solid (98 mg, 76%) by flash chromatography (ethyl acetate: petroleum ether = 1:3, v/v). Rf = 0.20 on silica gel (ethyl acetate: petroleum ether = 1:3, v/v). The ee was determined to be 56% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 13.7 min (major) and 15.5 min (minor). m.p.: 215–216 °C; = +102.8° (c = 52.7 mg, CHCl3); IR (thin film, cm−1) 3591 (br), 3210 (w), 2978 (s), 2873 (s), 2805 (s) 1617 (w), 1490 (s), 1445 (s), 1416 (s), 1389 (s), 1297 (s), 1131 (s), 1077 (s), 935 (s), 845 (s); 1H-NMR (400 MHz, CDCl3) δ 7.51 (1H, d, J = 7.2 Hz), 7.42 (2H, d, J = 8.8 Hz), 7.18 (2H, m), 7.02 (1H, d, J = 7.2 Hz), 6.86 (2H, d, J = 8.8 Hz), 6.50 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 6.03 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 4.86 (1H, d, J = 11.2 Hz), 3.47 (1H, d, J = 11.2 Hz), 3.30–3.20 (5H, m), 2.93–2.88 (2H, m), 2.68–2.63 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 153.5, 137.1, 131.9, 129.9, 128.3, 127.8, 126.7, 126.7, 126.7, 126.6, 126.5, 125.1, 124.2, 114.9, 68.1, 67.8, 49.0, 48.9. MS (ESI): calcd m/z for C21H21F3N2O (M+) 374.16, found: 375.25 [M + H]+. Anal. Calcd for C21H21F3N2O: C, 67.37; H, 5.65; N, 7.48. Found: C, 67.13; H, 5.86; N, 7.36.
(1S,2S)-2-[4-(4-Chloro-phenyl)-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (2x). Prepared according to general procedure. 2x was obtained as a white solid (97 mg, 82%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.3 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 39% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 15.2 min (major) and 16.9 min (minor). m.p.: 203–204 °C; = +100.1° (c = 65.1 mg, CHCl3); IR (thin film, cm−1) 3585 (br), 3212 (w), 2978 (s), 2935 (s), 2806 (s) 1634 (w), 1490 (s), 1445 (s), 1415 (s), 1351 (s), 1298 (s), 1134 (s), 1076 (s), 935 (s), 845 (s); 1H-NMR (400 MHz, CDCl3) δ 7.60 (d, 1H, J = 7.2 Hz), 7.30–7.22 (m, 4H), 7.1 (d, 1H, J = 7.2 Hz), 6.86 (d, 2H, J = 8.8 Hz), 6.58 (dd, 1H, J = 2.0 Hz, J = 2.4 Hz), 6.13 (dd, 1H, J = 2.4 Hz, J = 2.4 Hz), 4.94 (d, 1H, J = 11.6 Hz), 3.54 (d, 1H, J = 11.6 Hz), 3.24–3.19 (m, 5H), 3.00–2.96 (m, 2H), 2.76–2.71 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ 150.1, 137.2, 131.9, 129.8, 129.2, 128.2, 127.8, 126.5, 125.1, 125.0, 124.4, 117.6, 68.0, 67.7, 50.0, 49.1. MS (ESI): calcd m/z for C20H21ClN2O (M+) 340.13, found: 341.20 [M + H]+. Anal. Calcd for C20H21ClN2O: C, 70.48; H, 6.21; N, 8.22. Found: C, 70.29; H, 6.44; N, 8.16.
(1S,2S)-2-[4-(1-Hydroxy-6,7-dimethoxy-1,2-dihydro-naphthalen-2-yl)-piper-azin-1-yl]-benzonitrile (3a). Prepared according to general procedure. 3a was obtained as a white solid (74 mg, 77%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.16 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 37% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 73.4 min (major) and 86.0 min (minor). m.p.: 192–193 °C; = +65.5° (c = 37.8 mg, CHCl3); IR (thin film, cm−1) 3512 (br), 3018 (w), 2927 (s), 2809 (s), 2271 (w), 1635 (w), 1490 (s), 1445 (s), 1351 (s), 1297 (s), 1135 (s), 1078 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.57 (1H, dd, J = 1.2 Hz, J = 1.2 Hz), 7.48 (1H, t, J = 7.2 Hz), 7.13 (1H, s), 7.03–6.99 (2H, m), 6.45 (1H, s), 6.47 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.07 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.86 (1H, d, J = 11.2 Hz), 3.93 (3H, s), 3.87 (3H, s), 3.45 (1H, d, J = 7.2 Hz), 3.28–3.22 (4H, m), 3.15 (1H, br), 2.99–2.98 (2H, m), 2.78–2.77 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 155.7, 148.8, 148.2, 134.5, 134.0, 130.0, 129.1, 124.8, 122.1, 118.8, 110.2, 108.8, 106.2, 68.0, 67.8, 56.2, 56.2, 52.2, 49.2. MS (ESI): calcd m/z for C23H25N3O3 (M+) 391.19, found: 392.10 [M + H]+. Anal. Calcd for C23H25N3O3: C, 70.57; H, 6.44; N, 10.73. Found: C, 70.46; H, 6.67; N, 10.55.
(1S,2S)-2-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-6,7-dimethoxy-1,2-dihydro-Naphthalen-1-ol (3b). Prepared according to general procedure. 3b was obtained as a white solid (69 mg, 73%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.20 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 49% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 40.5 min (major) and 42.9 min (minor). m.p.: 200–201 °C; = +79.1° (c = 41.2 mg, CHCl3); IR (thin film, cm−1) 3516 (br), 3022 (w), 2972 (s), 2935 (s), 2806 (s), 1635 (w), 1490 (s), 1445 (s), 1351 (s), 1298 (s), 1135 (s), 1078 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.15 (1H, s), 6.97 (2H, d, J = 8.4 Hz), 6.91–6.87 (2H, m), 6.66 (1H, s), 6.49 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.03 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 4.87 (1H, d, J = 11.2 Hz), 3.94 (3H, s), 3.88 (3H, s), 3.49 (1H, dt, J = 11.2 Hz, J = 2.4 Hz), 3.25 (1H, br), 3.18–3.13 (4H, m), 2.96–2.94 (2H, m), 2.74–2.73 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 158.7, 156.3, 148.9, 148.3, 148.1, 130.0, 129.2, 124.8, 122.5, 118.2, 115.8, 115.6, 110.3, 108.9, 67.9, 67.8, 56.3, 56.3, 51.0, 49.2. MS (ESI): calcd m/z for C22H25FN2O3 (M+) 384.18, found: 385.07 [M + H]+. Anal. Calcd for C22H25FN2O3: C, 68.73; H, 6.55; N, 7.29. Found: C, 68.56; H, 6.78; N, 7.19.
(1S,2S)-2-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-6,7-dimethoxy-1,2-dihydro-naphthalen-1-ol (3c). Prepared according to general procedure. 3c was obtained as a white solid (74 mg, 79%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.29 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 38% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL/min; Retention times in 10% 2-propanol in hexanes were 55.6 min (minor) and 60.3 min (major). m.p.: 160–161 °C; = 70.9° (c = 35.9 mg, CHCl3); IR (thin film, cm−1) 3507 (br), 3188 (w), 2968 (s), 2890 (s), 2811 (s), 1610 (w), 1458 (s), 1379 (s), 1281 (s), 1183 (s), 1099 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.14 (1H, s), 7.06–6.93 (4H, m), 6.65 (1H, s), 6.47 (1H, dd, J = 2.4 Hz, J = 2.0 Hz), 6.05 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.86 (1H, d, J = 11.6 Hz), 3.93 (3H, s), 3.87 (3H, s), 3.46 (1H, d, J = 11.2 Hz), 3.22 (1H, br), 3.15–3.11 (4H, m), 2.98–2.95 (2H, m), 2.76–2.72 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 148.8, 148.2, 130.0, 129.1, 124.8, 124.6, 122.7, 119.1, 119.1, 116.4, 116.2, 110.1, 108.8, 67.9, 67.8, 56.2, 56.2, 51.2, 51.2, 49.2. MS (ESI): calcd m/z for C22H25FN2O3 (M+) 384.18, found: 385.10 [M + H]+. Anal. Calcd for C22H25FN2O3: C, 68.73; H, 6.55; N, 7.29. Found: C, 68.62; H, 6.73; N, 7.18.
(1S,2S)-6,7-Dimethoxy-2-(4-(4-methoxy-phenyl)-piperazin-1-yl]-1,2-dihydro–naphthalene-1-ol (3d). Prepared according to general procedure. 3d was obtained as a white solid (60 mg, 62%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.20 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 59% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 0.5 mL / min; Retention times in 5% 2-propanol in hexanes were 92.2 min (major) and 110.1 min (minor). m.p.: 174–175 °C; = +77.4° (c = 26.5 mg, CHCl3); IR (thin film, cm−1) 3696 (br), 3036 (w), 2924 (s), 2877 (s), 1682 (s), 1512 (s), 1452 (s), 1383 (w), 1244 (s), 1111 (s), 1013 (s), 935 (w), 815 (s); 1H-NMR (400 MHz, CDCl3) δ 7.15 (1H, s), 6.92 (2H, d, J = 2.0 Hz), 6.85 (2H, d, J = 2.0 Hz), 6.66 (1H, s), 6.48 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 6.05 (1H, dd, J = 2.4 Hz, J = 2.8 Hz), 4.86 (1H, d, J = 11.2 Hz), 3.94 (3H, s), 3.88 (3H, s), 3.77 (3H, s), 3.48 (1H, dt, J = 11.2 Hz, J = 2.4 Hz), 3.25 (1H, br), 3.15–3.10 (4H, m), 2.98–2.93 (2H, m), 2.75–2.70 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 154.2, 148.9, 148.3, 145.9, 130.1, 129.1, 124.8, 122.8, 118.6, 114.7, 110.3, 108.9, 68.0, 67.9, 56.3, 56.3, 51.5, 49.3. MS (ESI): calcd m/z for C23H28N2O4 (M+) 396.20, found: 397.14 [M + H] +. Anal. Calcd for C23H28N2O4: C, 69.67; H, 7.12; N, 7.07. Found: C, 74.89; H, 7.44; N, 8.56.
(1S,2S)-2-[4-(3,4-Dichloro-phenyl)-piperazin-1-yl]-6,7-dimethoxy-1,2-dihydronaphthalen-1-ol (3e). Prepared according to general procedure. 3e was obtained as a white solid (50 mg, 47%) by flash chromatography (ethyl acetate: petroleum ether = 2:3, v/v). Rf = 0.20 on silica gel (ethyl acetate: petroleum ether = 2:3, v/v). The ee was determined to be 16% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 10% 2-propanol in hexanes were 44.9 min (major) and 49.4 min (minor). m.p.: 187–188 °C; = +25.2° (c = 11.9 mg, CHCl3); IR (thin film, cm−1) 3591 (br), 3210 (w), 2973 (s), 2933 (s), 2874 (s), 2806 (s), 1676 (s), 1490 (s), 1445 (s), 1415 (s), 1383 (s), 1297 (m), 1077 (s), 1044 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.28 (1H, dd, J = 2.8 Hz, J = 2.8 Hz), 7.15 (1H, s), 6.97 (1H, t, J = 2.8 Hz), 6.75 (1H, dt, J = 8.8 Hz, J = 2.7 Hz), 6.66 (1H, d, J = 2.4 Hz), 6.50 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 6.01 (1H, dd, J = 2.8 Hz, J = 2.4 Hz), 4.86 (1H, d, J = 11.6 Hz), 3.95 (3H, d, J = 2.4 Hz), 3.89 (3H, J = 2.4 Hz), 3.49 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 3.24–3.16 (5H, m), 2.95–2.91 (2H, m), 2.73–2.69 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 150.8, 148.9, 148.3, 133.0, 130.6, 129.9, 129.3, 124.7, 122.5, 122.2, 117.5, 115.6, 110.3, 109.0, 68.0, 67.8, 56.2, 56.2, 49.4, 48.9. MS (ESI): calcd m/z for C22H24Cl2N2O3 (M+) 434.12, found: 435.04 [M + H]+. Anal. Calcd for C22H24Cl2N2O3: C, 60.70; H, 5.56; N, 6.43. Found: C, 60.57; H, 5.84; N, 6.36.
(1S,2S)-2-[4-(2-Chloro-phenyl)-piperazin-1-yl]-6,7-dimethoxy-1,2-dihydro-naphthalen-1-ol (3f). Prepared according to general procedure. 3f was obtained as a white solid (50 mg, 51%) by flash chromatography (ethyl acetate: petroleum ether = 1:1, v/v). Rf = 0.22 on silica gel (ethyl acetate: petroleum ether = 1:1, v/v). The ee was determined to be 43% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 2% 2-propanol in hexanes were 114.4 min (minor) and 125.3 min (major). m.p.: 140–141 °C; = +75.3° (c = 38.3 mg, CHCl3); IR (thin film, cm−1) 3591 (br), 3211 (w), 2975 (s), 2934 (s), 2875 (s), 2806 (s), 1678 (w), 1490 (s), 1445 (s), 1378 (s), 1297 (m), 1108 (s), 1077 (s), 918 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.49 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 7.39–7.33 (1H, m), 7.27 (1H, s), 7.18 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.95 (1H, t, J = 7.2 Hz), 6.77 (1H, s), 6.60 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.21 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.98 (1H, d, J = 11.6 Hz), 4.07 (3H, s), 3.99 (3H, s), 3.61 (1H, d, J = 11.6 Hz), 3.24 (4H, br), 3.15–2.95 (2H, m), 2.83–2.64 (2H, m); 13C-NMR (100 MHz, CDCl3) δ 149.4, 148.9, 148.3, 130.9, 130.1, 139.1, 129.0, 127.8, 124.9, 124.0, 122.9, 120.6, 110.3, 108.9, 68.0, 56.3, 56.3, 52.0, 49.4, 29.9. MS (ESI): calcd m/z for C22H25ClN2O3 (M+) 400.16, found: 401.12 [M + H]+. Anal. Calcd for C22H25ClN2O3: C, 65.91; H, 6.29; N, 6.99. Found: C, 65.79; H, 6.48; N, 6.76.
(1S,2S)-6,7-Dimethoxy-2-(4-o-tolyl-piperazin-1-yl]-1,2-dihydro-naphthalen-1-ol (3g). Prepared according to general procedure. 3g was obtained as a white solid (71 mg, 76%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.18 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 38% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 2% 2-propanol in hexanes were 79.8 min (minor) and 85.1 min (major). m.p.: 120–121 °C; = +40.1° (c = 38.9 mg, CHCl3); IR (thin film, cm−1) 3693 (br), 3218 (w), 2980 (s), 2869 (s), 1681 (w), 1498 (s), 1445 (s), 1381 (s), 1351 (s), 1297 (m), 1142 (s), 935 (s), 846 (s);. 1H-NMR (400 MHz, CDCl3) δ 7.21–7.18 (3H, m), 7.06–6.99 (2H, m), 6.67 (1H, s), 6.50 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.12 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.88 (1H, d, J = 11.6 Hz), 3.96 (3H, s), 3.89 (3H, s), 3.49 (1H, dt, J = 6.8 Hz, J = 2.4 Hz), 3.27 (1H, br), 3.02–2.96 (6H, m), 2.75–2.71 (2H, m), 2.34 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 151.6, 148.8, 148.2, 132.7, 131.3, 130.2, 128.9, 126.8, 124.8, 123.4, 123.0, 119.2, 110.2, 108.8, 68.0, 68.0, 56.3, 56.2, 52.4, 49.7, 18.1. MS (ESI): calcd m/z for C23H28N2O3 (M+) 380.21, found: 381.15 [M + H]+. Anal. Calcd for C23H28N2O3: C, 72.60; H, 7.42; N, 7.36. Found: C, 72.30; H, 7.64; N, 7.18.
(1S,2S)-2-[4-(2,5-Dimethyl-phenyl)-piperazin-1-yl]-6,7-dimethoxy-1,2-di-hydro-naphthalen-1-ol (3h). Prepared according to general procedure. 3h was obtained as a white solid (83 mg, 86%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.29 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 35% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 2% 2-propanol in hexanes were 63.6 min (minor) and 65.1 min (major). m.p.: 138–139 °C; = +73.2° (c = 68.5 mg, CHCl3); IR (thin film, cm−1) 3592 (br), 3211 (w), 2975 (s), 2934 (s), 2863 (s), 2805 (s), 1677 (w), 1490 (s), 1444 (s), 1381 (s), 1297 (m), 1119 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.19 (1H, s), 7.08 (1H, d, J = 7.6 Hz), 6.87 (1H, s), 6.83 (1H, d, J = 7.6 Hz), 6.67 (1H, s), 6.50 (1H, dd, J = 2.0 Hz, J = 2.0 Hz), 6.12 (1H, dd, J = 2.0 Hz, J = 2.0 Hz), 4.89 (1H, d, J = 11.6 Hz), 3.96 (3H, s), 3.90 (3H, s), 3.49 (1H, d, J = 12.0 Hz), 3.37 (1H, br), 3.04–2.95 (6H, m), 2.72 (2H, t, J = 6.8 Hz), 2.33 (3H, s), 2.29 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 151.4, 148.8, 148.2, 136.3, 131.1, 130.2, 129.4, 129.0, 124.8, 124.0, 123.0, 119.9, 110.2, 108.8, 68.0, 67.9, 56.2, 56.2, 52.4, 49.7, 21.3, 17.6. MS (ESI): calcd m/z for C24H30N2O3 (M+) 394.23, found: 395.15 [M + H]+. Anal. Calcd for C24H30N2O3: C, 73.07; H, 7.66; N, 7.10. Found: C, 73.16; H, 7.91; N, 6.95.
(1S,2S)-2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-yl]-6,7-dimethoxy-1,2-di-hydro-naphthalen-1-ol (3i). Prepared according to general procedure. 3i was obtained as a white solid (78 mg, 81%) by flash chromatography (ethyl acetate: petroleum ether = 1:2, v/v). Rf = 0.16 on silica gel (ethyl acetate: petroleum ether = 1:2, v/v). The ee was determined to be 45% using HPLC analysis on a CHIRALCEL AD column, λ = 254 nm. Flow rate = 1.0 mL/min; Retention times in 2% 2-propanol in hexanes were 21.6 min (major) and 22.6 min (minor). m.p.: 145–146 °C; = +95.6° (c = 29.3 mg, CHCl3); IR (thin film, cm−1) 3695 (br), 3219 (w), 2978 (s), 2936 (s), 2867 (s), 1699 (w), 1490 (w), 1445 (s), 1383 (s), 1351 (s), 1297 (m), 1124 (s), 1077 (s), 935 (s), 846 (s); 1H-NMR (400 MHz, CDCl3) δ 7.16 (1H, s), 7.04 (1H, d, J = 8.4 Hz), 6.78 (1H, d, J = 2.4 Hz), 6.77 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 6.66 (1H, s), 6.48 (1H, dd, J = 2.4 Hz, J = 2.4 Hz), 4.88 (1H, d, J = 6.8 Hz), 3.95 (3H, s), 3.89 (3H, s), 3.48 (1H, dt, J = 6.8 Hz, J = 2.4 Hz), 3.20 (1H, br), 3.19–3.16 (4H, m), 2.95–2.87 (2H, m), 2.75–2.69 (2H, m), 2.25 (3H, s), 2.21 (3H, s); 13C-NMR (100 MHz, CDCl3) δ 149.7, 148.7, 148.1, 137.2, 130.3, 130.0, 129.0, 128.4, 124.7, 122.6, 118.4, 114.1, 110.1, 108.8, 67.8, 67.8, 56.2, 56.2, 50.6, 49.2, 20.3, 18.9. MS (ESI): calcd m/z for C24H30N2O3 (M+) 394.23, found: 395.15 [M + H]+. Anal. Calcd for C24H30N2O3: C, 73.07; H, 7.66; N, 7.10. Found: C, 73.30; H, 8.16; N, 6.74.