Synthesis and Fungicidal Activities of (Z/E)-3,7-Dimethyl-2,6-octadienamide and Its 6,7-Epoxy Analogues
Abstract
:1. Introduction

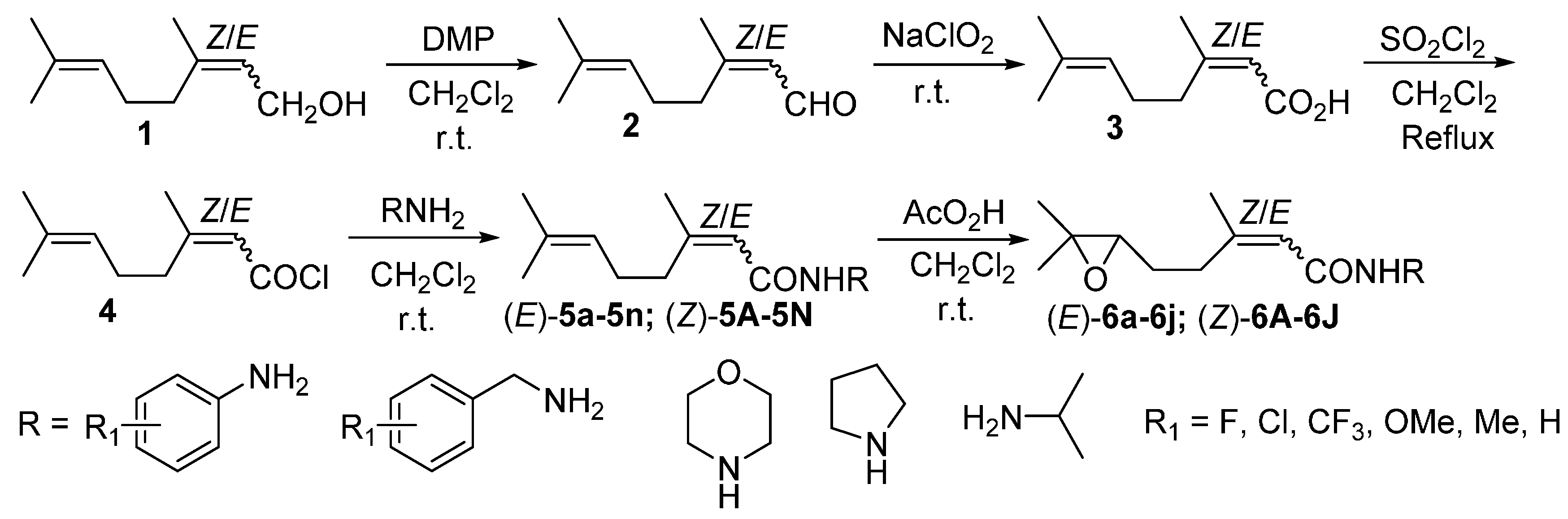
2. Results and Discussion
| Compd. | R | F. G | R. S | A. S | S. S | B. C | Compd. | R | F. G | R. S | A. S | S. S | B. C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | Ph | 68.1 | 88.9 | 86.4 | 62.9 | 60.2 | 5A | Ph | 63.7 | 79.9 | 85.0 | 72.3 | 66.7 |
| 5b | 2,4-Cl2Ph | 17.7 | 59.5 | 77.6 | 36.2 | 22.9 | 5B | 2,4-Cl2Ph | 47.2 | 55.4 | 51.3 | 56.5 | 4.6 |
| 5c | 2-ClPh | 51.9 | 85.3 | 86.0 | 57.4 | 44.4 | 5C | 2-ClPh | 52.2 | 94.0 | 81.3 | 63.4 | 44.5 |
| 5d | 2-FPh | 74.3 | 84.3 | 88.9 | 47.9 | 48.0 | 5D | 2-FPh | 55.2 | 82.3 | 82.4 | 45.3 | 43.5 |
| 5e | 4-CF3Ph | 73.4 | 65.6 | 63.9 | 23.4 | 26.8 | 5E | 4-CF3Ph | 55.0 | 83.8 | 70.5 | 52.5 | 31.2 |
| 5f | 4-CH3Ph | 77.0 | 86.0 | 77.1 | 53.6 | 26.5 | 5F | 4-CH3Ph | 46.7 | 71.4 | 74.4 | 70.4 | 42.7 |
| 5g | Ph, CH3 | 50.6 | 86.7 | 88.7 | 77.1 | 83.2 | 5G | Ph, CH3 | 71.3 | 86.4 | 85.1 | 69.2 | 65.4 |
| 5h | PhCH2 | 55.5 | 86.7 | 72.4 | 34.6 | 51.0 | 5H | PhCH2 | 53.6 | 80.7 | 80.2 | 57.0 | 34.6 |
| 5i | 4-FPhCH2 | 30.7 | 54.4 | 82.0 | 24.7 | 31.6 | 5I | 4-FPhCH2 | 75.2 | 93.4 | 89.5 | 76.9 | 47.4 |
| 5j | 4-OCH3PhCH2 | 45.3 | 83.0 | 78.9 | 46.6 | 64.0 | 5J | 4-OCH3PhCH2 | 52.5 | 77.6 | 81.6 | 46.9 | 19.1 |
| 5k | 2-ClPhCH2 | 44.5 | 79.9 | 81.1 | 69.5 | 64.6 | 5K | 2-ClPhCH2 | 56.6 | 89.3 | 83.3 | 69.7 | 51.0 |
| 5l | morpholino | 16.4 | 44.8 | 50.9 | 40.7 | 17.8 | 5L | morpholino | 30.1 | 44.9 | 62.2 | 14.2 | 7.3 |
| 5m | pyrrolidin-1-yl | 39.7 | 60.0 | 79.8 | 28.1 | 29.2 | 5M | pyrrolidin-1-yl | 63.4 | 78.8 | 85.5 | 43.1 | 35.6 |
| 5n | isopropyl | 15.6 | 48.7 | 58.2 | 12.8 | 14.3 | 5N | isopropyl | 46.2 | 50.3 | 43.2 | 43.1 | 26.0 |
| 6a | Ph | 39.1 | 23.2 | 38.0 | 19.2 | 16.9 | 6A | Ph | 32.8 | 30.3 | 69.3 | 36.4 | 22.8 |
| 6b | 2,4-Cl2Ph | 63.0 | 91.5 | 73.6 | 72.7 | 30.4 | 6B | 2,4-Cl2Ph | 46.9 | 82.6 | 81.1 | 43.7 | 39.1 |
| 6c | 2-ClPh | 39.8 | 35.6 | 59.1 | 33.3 | 13.5 | 6C | 2-ClPh | 33.2 | 30.4 | 15.4 | 33.9 | 31.6 |
| 6d | 4-CH3Ph | 74.0 | 79.2 | 58.4 | 18.4 | 32.9 | 6D | 4-CH3Ph | 46.7 | 43.9 | 73.4 | 3.72 | 2.50 |
| 6e | Ph, CH3 | 68.5 | 59.1 | 66.5 | 46.2 | 10.7 | 6E | Ph, CH3 | 36.2 | 62.6 | 54.2 | 0.0 | 1.6 |
| 6f | PhCH2 | 65.0 | 57.7 | 71.3 | 0.0 | 9.7 | 6F | PhCH2 | 54.3 | 76.8 | 57.8 | 31.7 | 19.8 |
| 6g | 4-FPhCH2 | 17.7 | 68.4 | 29.1 | 0.0 | 15.1 | 6G | 4-FPhCH2 | 40.4 | 38.9 | 55.7 | 28.7 | 10.9 |
| 6h | morpholino | 68.6 | 52.5 | 43.3 | 38.7 | 33.2 | 6H | morpholino | 33.8 | 73.3 | 52.9 | 0.0 | 17.6 |
| 6i | pyrrolidin-1-yl | 9.2 | 37.8 | 66.3 | 19.9 | 0.0 | 6I | pyrrolidin-1-yl | 59.3 | 48.1 | 76.3 | 0.0 | 12.1 |
| 6j | isopropyl | 22.6 | 18.0 | 65.4 | 8.4 | 15.2 | 6J | isopropyl | 36.3 | 53.0 | 36.8 | 10.6 | 38.4 |
| Carbendazim | 100 | 100 | 100 | 81.0 | 4.2 | Chlorothalonil | 95.8 | 99.9 | 100 | 100 | 100 | ||
| Compd. | F. graminearum | R. solani | A. solani | S. sclerotiorum | B. cinerea |
|---|---|---|---|---|---|
| 5a | - | 26.1 (20.3–33.6) | 19.8 (14.5–27.1) | - | - |
| 5b | - | - | 51.0 (45.3–57.4) | - | - |
| 5c | - | 33.9 (26.0–44.0) | 28.6 (25.8–31.7) | - | - |
| 5d | - | 19.2 (12.7–25.6) | 39.8 (28.6–48.6) | 202.7 (155.0–263.1) | - |
| 5e | 11.9 (7.6–16.9) | - | - | - | - |
| 5f | 4.3 (3.2–5.8) | 9.7 (25.1–56.2) | 37.6 (45.6–59.5) | - | - |
| 5g | - | 19.1 (13.9–26.1) | 27.2 (21.9–33.7) | 61.2 (44.5–101.2) | 56.2 (38.2–82.6) |
| 5h | - | 30.4 (24.5–37.6) | 27.6 (21.3-35.7) | - | - |
| 5i | - | - | 32.8 (25.5–42.1) | - | - |
| 5j | - | 35.5 (25.2-44.4) | 27.8 (23.3–29.6) | - | - |
| 5k | - | 29.8 (24.1-32.9) | 41.3 (32.0–47.5) | - | - |
| 5m | - | - | 50.3 (33.3–75.7) | - | - |
| 6b | - | 62.3 (44.9-86.6) | 43.4 (31.1–60.5) | 21.6 (13.8–33.5) | - |
| 6d | 57.4 (52.7–62.3) | 130.7 (92.1–185.1) | - | - | - |
| 6f | - | - | 189.4 (155.4–230.4) | - | - |
| 5A | - | 97.9 (88.3–108.4) | 14.0 (11.5–17.1) | 92.9 (67.9–126.9) | - |
| 5C | - | 80.1 (56.4–113.4) | 62.6 (41.8–93.6) | - | - |
| 5D | - | 54.5 (35.6–73.5) | 26.0 (19.7–30.2) | - | - |
| 5E | - | 51.2 (42.5–55.5) | 22.9 (16.9–27.9) | - | - |
| 5F | - | 39.0 (29.5–51.4) | 42.6 (30.6–59.0) | 23.2 (16.8–31.9) | - |
| 5G | - | 13.4 (11.6–15.5) | 35.9 (27.0–47.6) | 125.2 (96.3–161.3) | - |
| 5H | - | 41.4 (26.8–63.6) | 21.2 (16.3–27.4) | - | - |
| 5I | 38.8 (32.5–46.3) | 18.7 (12.6–27.6) | 17.1 (12.6–23.2) | 40.7 (35.2–47.0) | - |
| 5J | - | 57.9 (38.9–76.7) | 31.1 (23.5–36.6) | - | - |
| 5K | - | 18.6 (14.9–20.8) | 32.0 (25.3–36.1) | - | - |
| 5M | - | 284.1(207.7–387.9) | 63.8 (42.6–95.3) | - | - |
| 6B | - | 31.6 (20.3–49.2) | 43.4 (28.8–65.1) | - | - |
| 6D | - | - | 667.3 (462.3–999.5) | - | - |
| 6F | - | >1000 | - | - | - |
| 6H | - | 677.8 (622.7–736.6) | - | - | - |
| 6I | - | - | 83.8 (56.9–123.1) | - | - |
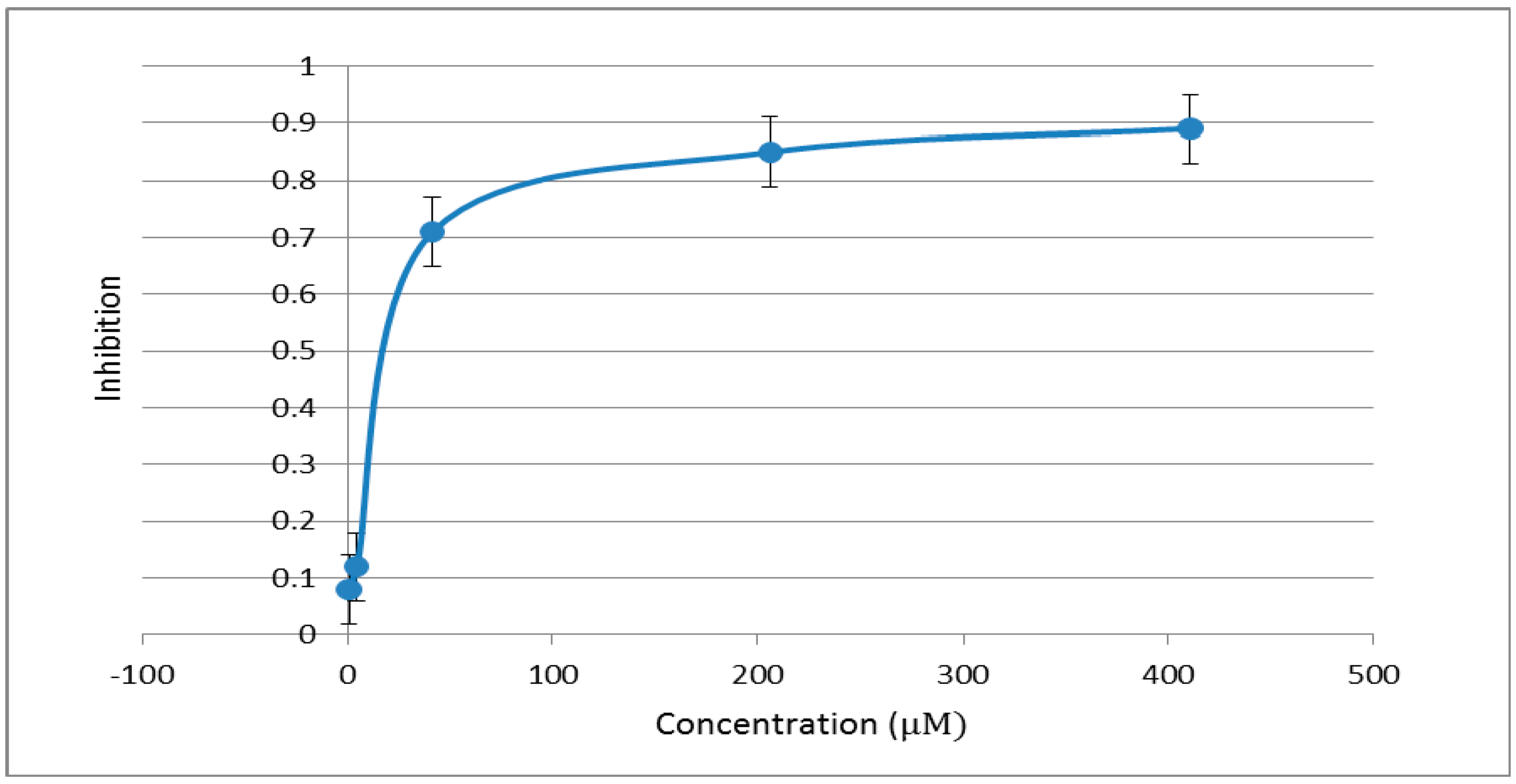
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of (Z/E)-3,7-Dimethyl-2,6-octadienal (2, Neral and Geranial)
3.2.2. Synthesis of (Z/E)-3,7-Dimethyl-2,6-octadienoic Acid (3, Geranic Acid and Nerolic Acid)
3.2.3. General Procedure for the Synthesis of Compounds 5
3.2.4. General Procedure for the Synthesis of Compounds 6
3.3. Bioassay of Fungicidal Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antoszczak, M.; Maj, E.; Stefanska, J.; Wietrzyk, J.; Janczak, J.; Brzezinski, B.; Huczynski, A. Synthesis, antiproliferative and antibacterial activity of new amides of salinomycin. Bioorg. Med. Chem. 2014, 24, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Xu, J.; Xin, M.H.; Lu, S.M.; Zhang, S.Q. Design, synthesis and antiproliferative activity evaluation of m-(4-morpholinyl-1,3,5-triazin-2-yl)benzamides in vitro. Bioorg. Med. Chem. Lett. 2015, 25, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Plescia, F.; Cascioferro, S.; Raimondi, M.V.; Cancemi, G.; D’Anneo, A.; Lauricella, M.; Cusimano, M.G.; Bai, R. Synthesis, antiproliferative activity and possible mechanism of action of novel 2-acetamidobenzamides bearing the 2-phenoxy functionality. Bioorg. Med. Chem. 2015, 23, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M.; Lieutaud, A.; Lome, V.; Pagès, J.-M.; Bolla, J.-M. Polyamino geranic derivatives as new chemosensitizers to combat antibiotic resistant Gram-negative bacteria. Bioorg. Med. Chem. 2013, 21, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Soni, J.N.; Soman, S.S. Synthesis and antimicrobial evaluation of amide derivatives of benzodifuran-2-carboxylic acid. Eur. J. Med. Chem. 2014, 75, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Cusimano, M.G.; Schillaci, D. Antiadhesion agents against Gram-positive pathogens. Future Microbiol. 2014, 9, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Benoit, A.R.; Schiaffo, C.; Salomon, C.E.; Goodell, J.R.; Hiasa, H.; Ferguson, D.M. Synthesis and evaluation of N-alkyl-9-aminoacridines with antibacterial activity. Bioorg. Med. Chem. 2014, 24, 3014–3017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.S.; Lu, X.H.; Chen, L.; Liu, X.L. Research advances in carboxylic acid amide fungicides. J. Pestic. Sci. 2010, 12, 1–12. [Google Scholar]
- Wang, Z.J.; Gao, Y.; Hou, Y.L.; Yu, S.J.; Bian, Q.; Li, Z.M.; Zhao, W.G. Design, synthesis, and fungicidal evaluation of a series of novel 5-methyl-1H-1,2,3-trizole-4-carboxyl amide and ester analogues. Eur. J. Med. Chem. 2014, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Bian, Q.; Wang, H.X.; Yu, S.J.; Kou, J.J.; Wang, Z.P.; Li, Z.M.; Zhao, W.G. Design, synthesis, and fungicidal activity of novel carboxylic acid amides represented by N-benzhydryl valinamide carbamates. Org. Biomol. Chem. 2014, 12, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Liu, B.; Li, Y.H.; Zhao, W.G. Synthesis and bioactivity of novel 1-substituted-1H-1,2,3-triazole-4-carboxamide. Chin. J. Org. Chem. 2011, 31, 317–323. [Google Scholar]
- Xie, F.; Liu, T.T.; Yang, G.; Yuan, J.; Kong, X.L.; Xu, T.M.; Tan, C.X. Synthesis and acaricidal activity of new 1H-pyrazole-4-carboxamide derivatives. Chin. J. Org. Chem. 2013, 33, 2596–2601. [Google Scholar] [CrossRef]
- Ma, Q.S.; Liu, X.H.; Weng, J.Q.; Li, Y.S.; Zhang, M.; Zhang, X.Y.; Tan, C.X. Synthesis and herbicidal activity of new pyrazine derivative. Chin. J. Org. Chem. 2013, 33, 1749–1754. [Google Scholar] [CrossRef]
- Liu, T.T.; Ni, Y.; Zhong, L.K.; Huang, H.Y.; Hu, W.Q.; Xu, T.M.; Tan, C.X. Synthesis and fungicidal activity of difluoromethyl substituted carboxamide derivatives. Chin. J. Org. Chem. 2015, 35, 422–427. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, Y.; Chen, N.Q.; Lv, K.Z.; Zhou, C.; Xiong, X.H.; Li, F.S. Synthesis and fungicidal activity of novel chloro-containing 1-aryl-3-oxypyrazoles with an oximino ester or oximino amide moiety. Molecules 2014, 19, 8140–8150. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.N.; Liang, W.Q.; Sun, M.Y.; Shi, L.; Zhou, L.B.; Ouyang, G.P. Synthesis, fungicidal, and insecticide activity of novel substituted-N-(5-cyanopyridine-4-yl) benzamides. J. Chem. Pharm. Res. 2014, 6, 284–287. [Google Scholar]
- Yang, Y.; Jiang, J.Z.; Qimei, L.B.; Yan, X.J.; Zhao, J.X.; Yuan, H.Z.; Qin, Z.H.; Wang, M.A. The fungicidal terpenoids and essential oil from Litsea cubeba in Tibet. Molecules 2010, 15, 7075–7082. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, G.L.; Pena, N.I.; Cabrera, G.M. Neric acid derivatives produced by the honey bee fungal entomopathogen Ascosphaera apis. Phytochem. Lett. 2008, 1, 155–158. [Google Scholar] [CrossRef]
- Dong, H.B.; Yang, M.Y.; Jiang, J.Z.; Wang, M.A. Total synthesis of 3,7-dimethyl-7-hydroxy-2-octen-1,6-olide and 3,7-dimethyl-2,6-octadien-1,6-olide. J. Asian Nat. Prod. Res. 2013, 15, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, H.B.; Yang, M.Y.; Du, J.; Jiang, J.Z.; Wang, M.A. Synthesis and fungicidal activity of 7-methyl-7-hydroxy-2,3-benzo[c]octa-1,6-olide. J. Asian Nat. Prod. Res. 2014, 16, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.B.; Yang, M.Y.; Tang, B.; Wang, M.A. Total Synthesis of 3,7-dimethyl-7-hydroxy-2-octen-1,6-olide and (E)-6,7-dihydroxy-3,7-dimethyl-2-octenic acid. Chin. J. Org. Chem. 2014, 34, 2350–2353. [Google Scholar] [CrossRef]
- Wilson, M.S.; Woo, J.C.S.; Dake, G.R. A synthetic approach toward nitiol: Construction of two 1,22-dihydroxynitianes. J. Org. Chem. 2006, 71, 4237–4245. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, E.; Shin, D.-S.; Kang, H.; Oh, K.-B. Evaluation of morphogenic regulatory activity of farnesoic acid and its derivatives against Candida albicans Dimorphism. Bioorg. Med. Chem. Lett. 2002, 12, 895–898. [Google Scholar] [CrossRef]
- Thota, N.; Koul, S.; Reddy, M.V.; Sangwan, P.L.; Khan, I.A.; Kumar, A.; Raja, A.F.; Andotra, S.S.; Qazi, G.N. Citral derived amides as potent bacterial NorA efflux pump inhibitors. Bioorg. Med. Chem. 2008, 16, 6535–6543. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, D.; Maity, S.; Chandrasekaran, S. Regio- and stereoselective synthesis of aziridino epoxides from cyclic dienes. J. Org. Chem. 2006, 71, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Picquet, M.; Fernandez, A.; Bruneau, C.; Dixneuf, P.H. Efficient ruthenium-catalysed synthesis of 3-hydroxy-1-propen-1-yl benzoates: En route to an improved isomerization of 2-propyn-1-ols into α,β-unsaturated aldehydes. Eur. J. Org. Chem. 2000, 13, 2361–2366. [Google Scholar] [CrossRef]
- Velusamy, S.; Ahamed, M.; Punniyamurthy, T. Selective synthesis of α,β-unsaturated ketones by dibutyltin dimethoxide-catalyzed condensation of aldehydes with alkenyl trichloroacetates. Org. Lett. 2004, 6, 4281–4283. [Google Scholar]
- Reed, M.A.; Weaver, D.; Sun, S.; McLellan, A.; Lu, E. Terpenoid Analogues and Uses Thereof for Treating Neurological Conditions. Patent WO 2012034232 A1, 22 March 2012. [Google Scholar]
- Zweifel, T.; Naubron, J.-V.; Grutzmacher, H. Catalyzed dehydrogenative coupling of primary alcohols with water, methanol, or amines. Angew. Chem. Int. Ed. 2009, 48, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Oakleaf, J.A.; Thomas, M.T.; Wu, A.; Snieckus, V. Alkylation of α,β-Unsaturated amides via metalated and dimetalated intermediates. Tetrahedron Lett. 1978, 19, 1645–1648. [Google Scholar] [CrossRef]
- Lo, J.C.; Yabe, Y.; Baran, P.S. A practical and catalytic olefin coupling. J. Am. Chem. Soc. 2014, 136, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Chekroun, A.; Jarid, A.; Benharref, A.; Boutalib, A. Regio- and stereoselectivity of β-himachalene epoxidation by m-CPBA. A theoretical study. J. Org. Chem. 2000, 65, 4431–4434. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yang, M.Y.; Zhao, Y.; Kong, L.Q.; Wang, W.W.; Wang, M.A. Discovery of 5-(5,5-dimethylbutenolide-3-ethylidene)-2-amino-imidazolinone derivatives as fungicidal agents. Molecules 2015, 20, 13740–13752. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, J.T.; Tang, B.; Wang, M.A. Synthesis and fungicidal activity of D-ribose and D-xylose with hydantion. Chin. J. Org. Chem. 2014, 34, 1442–1446. [Google Scholar] [CrossRef]
- Han, J.T.; Dong, H.B.; Xu, Z.H.; Wang, J.M.; Wang, M.A. Synthesis and activity of novel acylthiourea with hydantoin. Int. J. Mol. Sci. 2013, 14, 19526–19539. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Dong, H.B.; Xu, Z.H.; Lei, J.P.; Wang, M.A. Facile synthesis of 5-arylidene thiohydantoin by sequential sulfonylation/desulfination reaction. Int. J. Mol. Sci. 2013, 14, 12484–12495. [Google Scholar] [CrossRef]
- Lei, J.P.; Han, J.T.; Xu, Z.H.; Dong, H.B.; Wang, M.A. Synthesis and fungicidal activity of 5-cyclohexylidene-2-aminoimidazolin-4-one derivatives. Chin. J. Org. Chem. 2012, 32, 1993–1998. [Google Scholar] [CrossRef]
- Chen, N.C. The Bioassay Technologies for Pesticides; Beijing Agricultural University Press: Beijing, China, 1991; pp. 161–162. [Google Scholar]
- Berkson, J. A statistically precise and relatively simple method of estimating the bioassay with quantal response, based on the logistic function. J. Am. Stat. Assoc. 1953, 48, 565–599. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5a–5N and 6a–6J are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Dong, H.; Jiang, J.; Wang, M. Synthesis and Fungicidal Activities of (Z/E)-3,7-Dimethyl-2,6-octadienamide and Its 6,7-Epoxy Analogues. Molecules 2015, 20, 21023-21036. https://doi.org/10.3390/molecules201219743
Yang M, Dong H, Jiang J, Wang M. Synthesis and Fungicidal Activities of (Z/E)-3,7-Dimethyl-2,6-octadienamide and Its 6,7-Epoxy Analogues. Molecules. 2015; 20(12):21023-21036. https://doi.org/10.3390/molecules201219743
Chicago/Turabian StyleYang, Mingyan, Hongbo Dong, Jiazhen Jiang, and Mingan Wang. 2015. "Synthesis and Fungicidal Activities of (Z/E)-3,7-Dimethyl-2,6-octadienamide and Its 6,7-Epoxy Analogues" Molecules 20, no. 12: 21023-21036. https://doi.org/10.3390/molecules201219743






