3.1. Chemistry
All melting points were measured on a Gallenkamp melting point apparatus (Weiss Gallenkamp, London, UK). The infrared spectra were recorded in potassium bromide disks on a PyeUnicam SP 3300 and Shimadzu FT IR 8101 PC infrared spectrophotometers (PyeUnicam Ltd. Cambridge, UK and Shimadzu, Tokyo, Japan, respectively). The NMR spectra were recorded on a Varian Mercury VX-300 NMR spectrometer (Varian, Palo Alto, CA, USA).
1H spectra were run at 300 MHz and
13C spectra were run at 75.46 MHz in deuterated chloroform (CDCl
3) or dimethyl sulfoxide (DMSO-
d6). (Sigma-Aldrich, St. Louis, MO, USA). Chemical shifts are given in parts per million and were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX mass spectrometer (Shimadzu, Tokyo, Japan) at 70 eV. Elemental analyses were carried out at the Micro-analytical Centre of Cairo University, Giza, Egypt and recorded on Elementar-Vario EL (ELTRA GmbH, Haan, Germany) automatic analyzer. Compounds
1b,
14a–
c and
18a–
c were prepared by following the reported procedures in the literature [
19,
20,
21,
22]. The
in vitro antimicrobial screening was performed by Chemistry of Natural and Microbial Products Dept., National Research Centre, Cairo-12622, Cairo, Egypt.
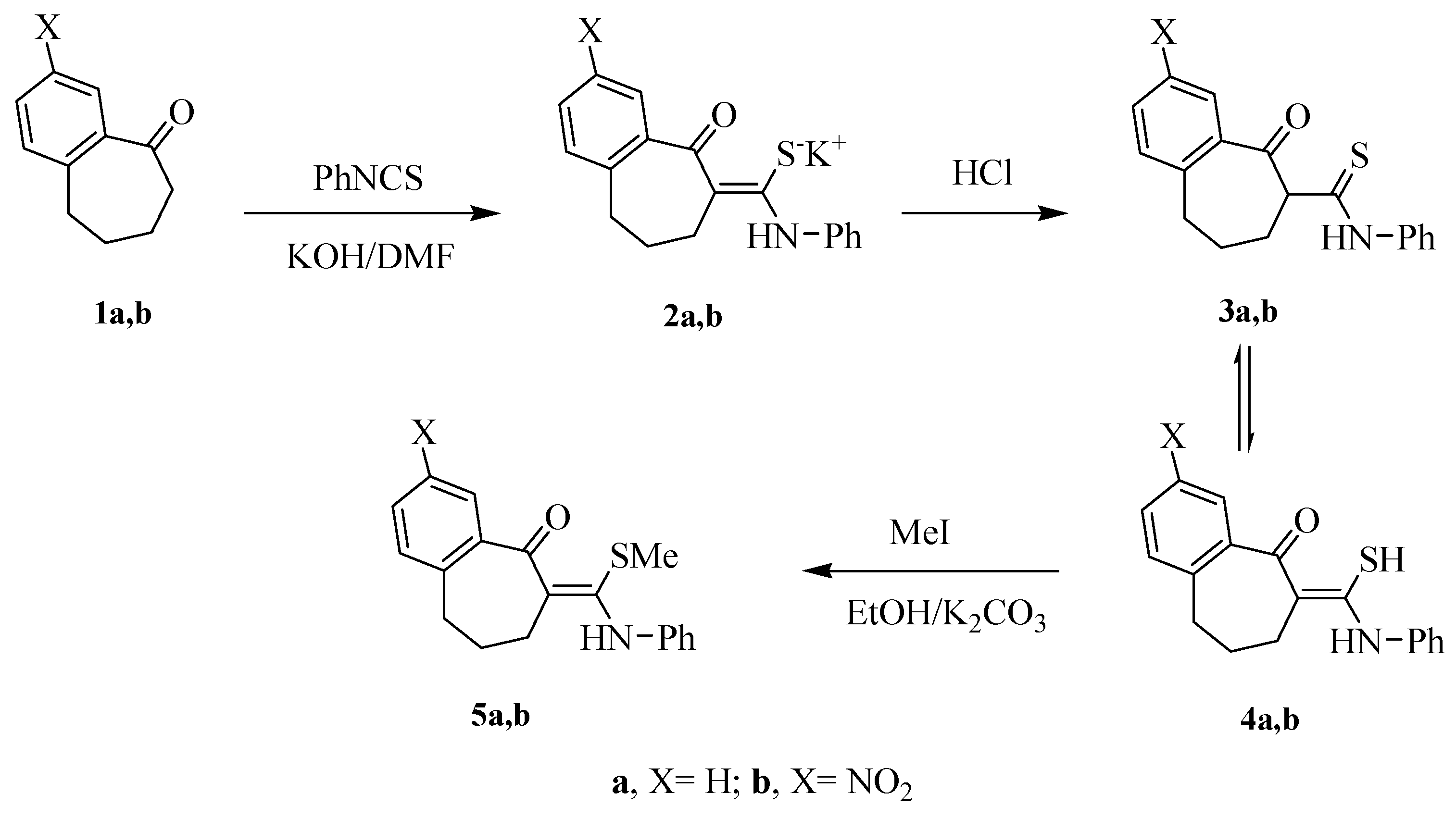
3.1.1. Preparation of the Thioamide Derivatives 4a,b
A solution of finely ground KOH (0.12 g, 2 mmol) and benzosuberone derivatives 1a,b (2 mmol) in DMF (10 mL), was stirred for 2 h. Phenyl isothiocyanate (0.27 g, 10.0 mmol) was then added drop-wise and the mixture was stirred for 10–12 h. The mixture was poured onto cold water acidified with 1N HCl. the solid product obtained was filtered off, washed with water, dried, and finally recrystallized with the prober solvent to afford the thioamidederivatives 4a,b.
6,7,8,9-Tetrahydro-6-(mercapto(phenylamino)methylene)benzo[7]annulen-5-one 4a. Yield: (0.24 g, 81%); mp: 185–187 °C; as a pale yellow crystals (MeOH). IR (KBr, cm−1) v 3430 (NH), 1637 (C=O). 1H-NMR (DMSO-d6): δ 1.90 (m, 2H, CH2), 2.41–3.20 (m, 4H, 2CH2), 6.99–7.90 (m, 9H, Ar-H), 8.50 (br s, 1H, NH, D2O exchangeable). MS m/z (%): 296 [M + 1]+ (5), 295 [M]+ (25), 219 (10), 205 (40), 160 (30), 92 (100), 77 (50). Anal. Calcd. for C18H17NOS (295.40): C, 73.19; H, 5.80; N, 4.74; S, 10.85. Found: C, 73.44; H, 5.89; N, 4.62; S, 10.79.
6,7,8,9-Tetrahydro-6-(mercapto(phenylamino)methylene)-3-nitrobenzo[7]annulen-5-one 4b. Yield: (0.27 g, 79%); mp: 215–217 °C; yellow crystals (EtOH). IR (KBr, cm−1): v 3433 (NH), 1663 (C=O). 1H-NMR (DMSO-d6): δ 1.94–3.30 (m, 6H, 3CH2), 7.01–8.27 (m, 8H, Ar-H), 9.20 (br s, 1H, NH, D2O exchangeable). 13H-NMR (DMSO-d6): δ 25.41, 25.60, 39.90, 115.68, 119.98, 120.39, 124.39, 129.51, 130.88, 138.88, 139.05, 142.92, 147.15, 155.41, 189.93. MS m/z (%): 341 [M + 1]+ (3), 340[M]+ (15), 338 (100), 262 (35), 205 (60). Anal. Calcd. for C18H16N2O3S (340.40): C, 63.51; H, 4.74; N, 8.23; S, 9.42. Found: C, 63.44; H, 4.69; N, 7.97; S, 9.48.
3.1.2. General Procedure for Preparation of the S-Methylated Thioamide Derivatives 5a,b
To a stirred solution of the thioamide derivatives 4a,b (1 mmol) and potassium carbonate (0.14 g, 1 mmol) in DMF (10 mL), iodomethane (0.28 g, 2 mmol) was added and stirring was continued for another 12 h. The mixture was poured onto crushed ice and the solid product obtained was filtered off, washed with water, dried, and finally recrystallized from the prober solvent to afford colorless crystals of compounds 5a,b.
6,7,8,9-Tetrahydro-6-(methylthio(phenylamino)methylene)benzo[7]annulen-5-one 5a. Yield: (0.26 g, 84%); mp: 195–197 °C; buff powder (MeOH/dioxan). IR (KBr, cm−1): v 3400(NH), 1674 (C=O). 1H-NMR (DMSO-d6): δ 1.77–2.51 (m, 6H, 3CH2), 3.08 (s, 3H, CH3), 6.86–7.79 (m, 9H, Ar-H), 8.31 (br s, 1H, NH, D2O exchangeable). MS m/z (%): 310 [M + 1]+ (8), 309 [M]+ (45), 261 (30), 217 (20), 115 (70), 91 (50), 77 (100). Anal. Calcd. for C19H19NOS (309.43): C, 73.75; H, 6.19; N, 4.53; S, 10.36. Found: C, 73.52; H, 6.10; N, 4.62; S, 10.44.
6,7,8,9-Tetrahydro-6-(methylthio(phenylamino)methylene)-3-nitrobenzo[7]annulen-5-one 5b. Yield: (0.31 g, 84%); mp: 225–227 °C; pale yellow crystals (EtOH/DMF). IR (KBr, cm−1): v 3430 (NH), 1664 (C=O). 1H-NMR (DMSO-d6): δ 1.77 (m, 2H, CH2), 2.30–2.51 (m, 4H, 2CH2), 3.30 (s, 3H, CH3), 7.18–8.57 (m, 8H, Ar-H), 9.01 (br s, 1H, NH, D2O exchangeable). MS m/z (%): 355 [M + 1]+ (5), 354 [M]+ (25), 205 (35), 159 (40), 93 (100), 77(70). Anal. Calcd. for C19H18N2O3S (354.42): C, 64.39; H, 5.12; N, 7.90; S, 9.05. Found: C, 64.26; H, 5.03; N, 7.78; S, 9.14.
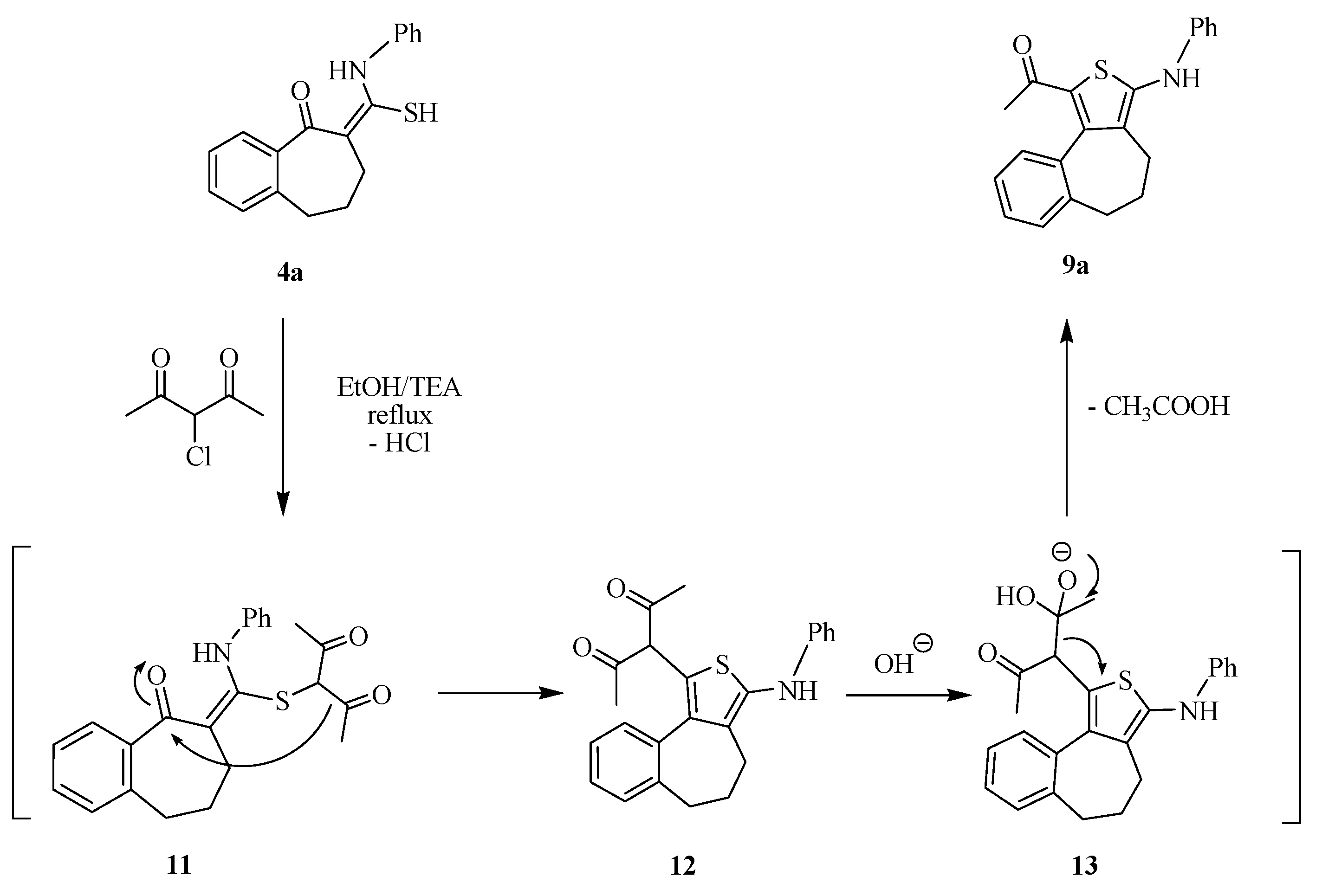
3.1.3. Reaction of Thioamide Derivatives 4 with α-Halo Carbonyl Compounds: General Procedure for the Preparation of 9a–c
Method A: To a solution of the thioamide derivatives 4a and 4b (1 mmol) and 1 mmol of chloroacetone 6 or phenacyl chloride 7 in EtOH (10 mL), 0.2 mL of triethylamine were added. The reaction mixture was refluxed for 10–15 h and then allowed to cool. The solid product obtained was filtered off, washed with EtOH, dried, and finally recrystallized from the prober solvent to afford the corresponding thiophenes 9a–c, respectively.
By the same method, 4a (1 mmol) was reacted with 3-chloroacetyl acetone (1 mmol) to afford 9a, which is identical in all respects (mp, TLC and spectra) in comparison with an authentic sample that obtained from the reaction of 4a and chloroacetone.
Method B: To a mixture of the thioamide derivatives 4a,b (1 mmol) and chloroacetone 6 or phenacyl chloride 7 (1 mmol) in DMF (5 mL), 0.19 g potassium carbonate was added. The reaction mixture was stirred at ambient temperature for 10 h, and then poured onto ice cold water acidified with 1N HCl. The solid product obtained was filtered off, washed with water, dried and finally recrystallized from the prober solvent to afford products identical in all respect with compounds 9a–d.
(2-Acetyl-5-phenylimimno)thiophen[c,f]benzo[7]anulene 9a. Yield: (0.29 g, 87%); mp: 187–190 °C; buff powder (MeOH). IR (KBr, cm−1): v 3430 (NH), 1691(C=O). 1H-NMR (DMSO-d6): δ 1.72 (m, 2H, CH2), 2.15–2.64 (m, 4H, 2CH2), 2.80 (s, 3H, CH3), 6.97–7.59 (m, 9H, Ar-H), 8.60 (s, 1H, NH, D2O exchangeable). MS m/z (%): 334 [M + 1]+ (7), 333 [M]+ (35), 308 (50), 293 (15), 194 (20), 118 (100), 92 (15), 77 (40). Anal. Calcd. for C21H19NOS (333.45): C, 75.64; H, 5.74; N, 4.20; S, 9.62. Found: C, 75.32; H, 5.61; N, 4.05; S, 9.74.
(2-Acetyl-5-phenylimino)thiophen[c,f]-3-nitrobenzo[7]anulene 9b. Yield: (0.30 g, 80%); mp: 200–202 °C; yellow crystals (EtOH). IR (KBr, cm−1): v 3428 (NH), 1654 (C=O). 1H-NMR (DMSO-d6): δ 1.72 (m, 2H, CH2), 2.35–2.591 (m, 4H, 2CH2), 3.30 (s, 3H, CH3), 6.68–8.72 (m, 8H, Ar-H), 9.53 (s, 1H, NH, D2O exchangeable). 13H-NMR (DMSO-d6): δ 21.35, 28.23, 34.12, 39.45, 116.30, 119.35, 122.21, 123.10, 125.50, 129.60, 135.90, 136.10, 138.50, 141.33, 143.31, 145.21, 150.13, 187.21. MS m/z (%): 379 [M + 1]+ (4), 378 [M]+ (25), 343 (10), 258 (40), 212 (100), 200 (35), 142 (70), 91 (65), 77 (60). Anal. Calcd. for C21H18N2O3S (378.44): C, 66.65; H, 4.79; N, 7.40; S, 8.47. Found: C, 66.51; H, 4.73; N, 7.47; S, 8.55.
(2-Benzoyl-5-phenylimino)thiophen[c,f]benzo[7]anulen (9c). Yield: (0.31 g, 81%); mp: 195–198 °C; yellow powder (EtOH). IR (KBr, cm−1): v 3425 (NH), 1681 (C=O). 1H-NMR (DMSO-d6): δ 1.82–2.84 (m, 6H, 3CH2), 6.97–7.59 (m, 14H, Ar-H), 8.60 (s, 1H, NH, D2O exchangeable). MS m/z (%): 396 [M + 1]+ (10), 395 [M]+ (45), 303 (25), 293 (15), 199 (30), 115 (100), 92 (77), 77 (40). Anal. Calcd. for C26H21NOS (395.52): C, 78.95; H, 5.35; N, 3.54; S, 8.11. Found: C, 78.84; H, 5.28; N, 3.62; S, 8.15.
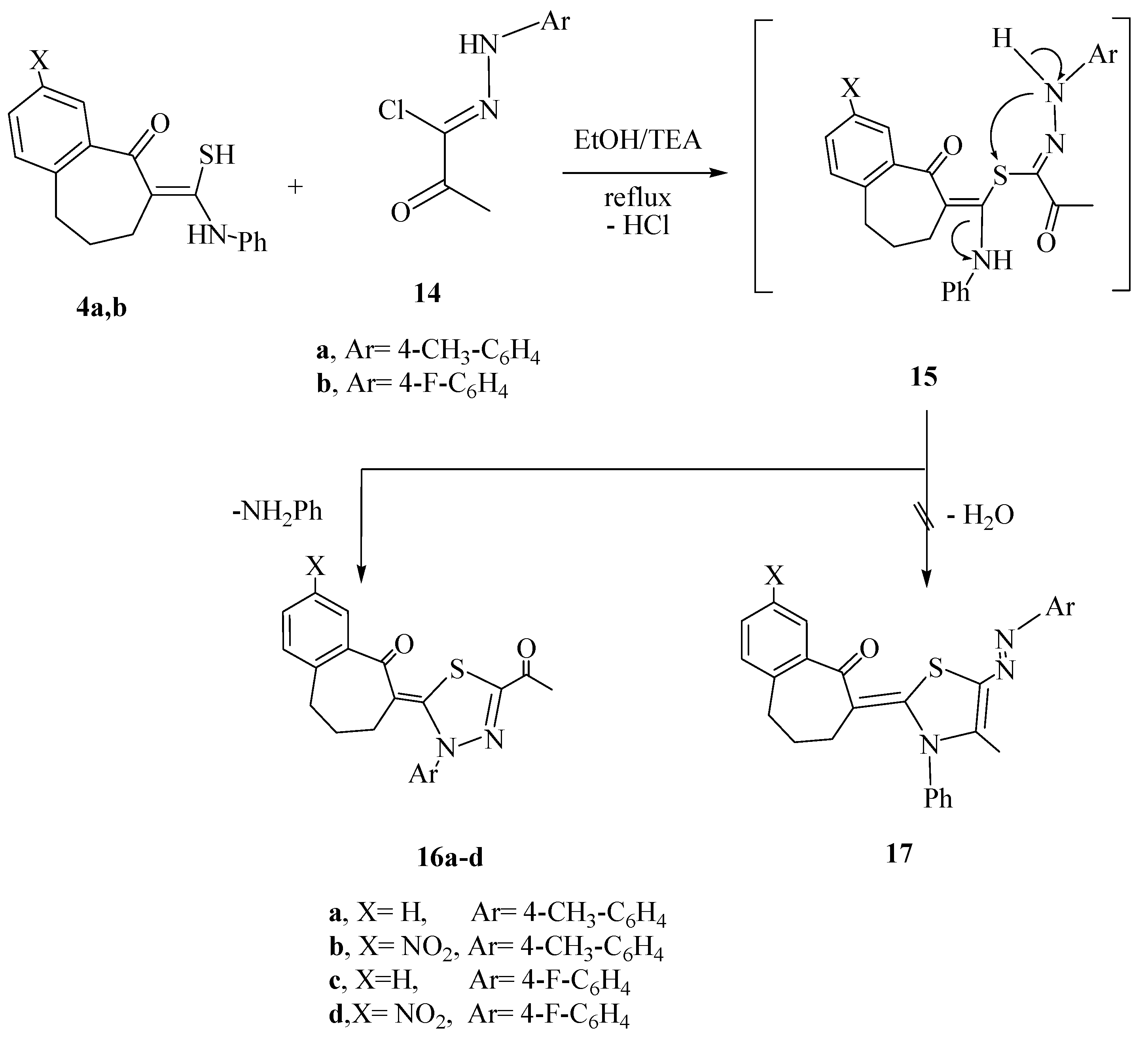
3.1.4. Reactions of Thioamide Derivatives 4a,b with C-Acetyl-N-arylhydrazonoyl Chlorides 14a,b and C-Ethoxycarbonyl-N-arylhydrazonoyl chlorides 18a,b
The reactions of the thioamide derivatives 4a and 4b with hydrazonoyl chlorides 14a,b and/or 18a,b, were carried out as described above for the synthesis of thiophene derivatives (method A), to afford the corresponding 1,3,4-thiadiazol derivatives 16a–d and 19a–d, respectively.
6-(5-Acetyl-3-p-tolyl-1,3,4-thiadiazol-2(3H)-ylidene)-6,7,8,9-tetrahydrobenzo[7]-annulen-5-one 16a. Yield: (0.3 g, 79%); mp: 225–227 °C; yellow powder (MeOH/dioxan). IR (KBr, cm−1): v 1668, 1653 (C=O). 1H-NMR (DMSO-d6): δ 1.57 (m, 2H, CH2), 2.40–2.46 (m, 4H, 2CH2), 3.10 (s, 3H, CH3), 3.43 (s, 3H, CH3), 7.10–7.50 (m, 8H, Ar-H). MS m/z (%): 377 [M + 1]+ (9), 376 [M]+ (75), 187 (45), 160 (20), 91 (80), 86 (100). Anal. Calcd. for C22H20N2O2S (376.47): C, 70.19; H, 5.35; N, 7.44; S, 8.52 . Found: C, 69.98; H, 5.19; N, 7.66; S, 8.47.
6-(5-Acetyl-3-p-tolyl-1,3,4-thiadiazol-2(3H)-ylidene)-6,7,8,9-tetrahydro-3-nitrobenzo[7]-annulen-5-one 16b. Yield: (0.38 g, 90%); mp: 260–262 °C; brown crystals (EtOH/dioxan). IR (KBr, cm−1): v 1690, 1645 (C=O). 1H-NMR (DMSO-d6): δ 1.70 (m, 2H, CH2), 2.40–2.46 (m, 4H, 2CH2), 3.10 (s, 3H, CH3), 3.43 (s, 3H, CH3), 7.30–7.90 (m, 7H, Ar-H). MS m/z (%): 423 [M + 2]+ (11), 421 [M]+ (55), 287 (15), 203 (30), 159 (20), 91 (35). Anal. Calcd. For C22H19N3O4S (421.47): C, 62.69; H, 4.54; N, 9.97; S, 7.61. Found: C, 62.35; H, 4.39; N, 10.14; S, 7.41.
6-(5-Acetyl-3-(4-fluorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)-6,7,8,9-tetrahydrobenzo[7]-annulen-5-one 16c. Yield: (0.32 g, 85%); mp: 240–242 °C; gray powder (dioxan). IR (KBr, cm−1): v 1671, 1653 (C=O). 1H-NMR (DMSO-d6): δ 1.53 (m, 2H, CH2), 2.38–2.46 (m, 4H, 2CH2), 3.43 (s, 3H, CH3), 6.76–7.40 (m, 8H, Ar-H). 13H-NMR (DMSO-d6): δ 23.13, 23.80, 25.41, 38.90, 116.44, 117.60, 119.90, 126.23, 128.75, 130.67, 134.28, 136.13, 140.35, 145.24, 151.20, 154.31, 187.23, 195.25.MS m/z (%): 381 [M + 1]+ (9), 380 [M]+ (45), 187 (25), 160 (27), 91 (85), 86 (100). Anal. Calcd. For C21H17N2O2S (380.44): C, 66.30; H, 4.50; N, 7.36; S, 8.43. Found: C, 66.11; H, 4.37; N, 7.55; S, 8.64.
6-(5-Acetyl-3-(4-fluorophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)-6,7,8,9-tetrahydro-3-nitrobenzo-[7]annulen-5-one 16d. Yield: (0.35 g, 87%); mp: 275–277 °C; green crystals (EtOH/DMF). IR (KBr, cm−1): v 1680, 1655 (C=O). 1H-NMR (DMSO-d6): δ 1.57 (m, 2H, CH2), 2.03–2.56 (m, 4H, 2CH2), 3.43 (s, 3H, CH3), 6.76–8.40 (m, 7H, Ar-H). MS m/z (%): 425 [M]+ (30) 245 (45), 171 (20), 95 (100), 77 (60). Anal. calcd. for C21H16N3O4S (425.43): C, 59.29; H, 3.79; N, 9.88; S, 7.54. Found: C, 59.09; H, 3.67; N, 10.17; S, 7.73.
Ethyl 4,5-Dihydro-5-(5,6-dihydro-9-oxo-5H-benzo[7]annulen-8(7H)-ylidene)-4-phenyl-1,3,4-thiadiazole-2-carboxylate 19a. Yield: (0.31 g, 79%); mp: 218–220 °C; brown crystals (EtOH/DMF). IR (KBr, cm−1): v 1718, 1675 (C=O). 1H-NMR (DMSO-d6): δ 1.71 (m, 2H, CH2), 2.36–2.80 (m, 6H, 3CH2), 4.2 (q, J = 7.1 Hz, 2H, CH3), 6.76–7.20 (m, 9H, Ar-H), 13H-NMR (DMSO-d6): δ 13.80, 21.35, 23.85, 35.55, 60.25, 116.30, 118.82, 126.62, 128.70, 129.60, 130.60, 133.00, 134.72, 140.40, 141.80, 154.50, 161.12, 178.35. MS m/z (%): 393 [M + 1]+ (6), 392 [M]+ (25), 346 (20), 194 (15), 242 (45), 150 (30), 92 (100), 77 (80), 65 (60). Anal. Calcd. for C22H20N2O3S (392.47): C, 67.33; H, 5.14; N, 7.14; S, 8.17. Found: C, 67.12; H, 4.98; N, 7.35; S, 8.34.
Ethyl 4,5-Dihydro-5-(6,7-dihydro-2-nitro-9-oxo-5H-benzo[7]annulen-8(9H)-ylidene)-4-phenyl-1,3,4-thiadiazole-2-carboxylate 19b. Yield: (0.33 g, 75%); mp: 227–229 °C; buff crystals (EtOH/dioxan). IR (KBr, cm−1): v 1722, 1638 (C=O). 1H-NMR (DMSO-d6): δ 1.50 (t, J = 7.0 Hz, 3H, CH3), 1.71–2.91 (m, 6H, 3CH2), 4.16 (q, J = 7.1 Hz, 2H, CH2), 6.83–7.49 (m, 8H, Ar-H), 13H-NMR (DMSO-d6): δ 21.11, 23.15, 40.33, 46.39, 64.90, 125.44, 127.60, 127.90, 128.23, 128.75, 133.67, 136.28, 139.13, 141.35, 196.24. MS m/z (%): 438 [M + 1] (6), 437 [M]+ (30), 392 (20), 364 (15), 288 (35), 203 (50), 125 (100), 77 (60). Anal. Calcd. for C22H19N3O5S (437.47): C, 60.40; H, 4.38; N, 9.61; S, 7.33. Found: C, 59.88; H, 4.26; N, 9.85; S, 7.51.
Ethyl 4,5-Dihydro-5-(5,6-dihydro-9-oxo-5H-benzo[7]annulen-8(7H)-ylidene)-4-p-tolyl-1,3,4-thiadiazole-2-carboxylate 19c. Yield: (0.29 g, 71%); mp: 192–195 °C; gray crystals (EtOH/DMF). IR (KBr, cm−1): v 1715, 1661 (C=O). 1H-NMR (DMSO-d6): δ 1.30 (t, J = 6.9 Hz, 3H, CH3), 2.23 (s, 3H, CH3), 2.46–2.92 (m, 4H, 2CH2), 3.53 (q, J = 7.1 Hz, 2H, CH2), 4.27 (m, 2H, CH2), 6.76–7.50 (m, 8H, Ar-H). MS m/z (%): 407 [M + 1]+ (4), 406 [M]+ (15), 333 (25), 315 (100), 242 (45), 158 (30), 91 (50). Anal. calcd. for C23H22N2O3S (406.50): C, 67.96; H, 5.46; N, 6.89; S, 7.89. Found: C, 67.52; H, 5.28; N, 7.06; S, 8.13.
Ethyl 4,5-Dihydro-5-(6,7-dihydro-3-nitro-9-oxo-5H-benzo[7]annulen-8(9H)-ylidene)-4-p-tolyl-1,3,4-thiadiazole-2-carboxylate 19d. Yield: (0.37 g, 82%); mp: 235–2375 °C; pale yellow crystals (EtOH/DMF). IR (KBr, cm−1): v 1735, 1670 (C=O). 1H-NMR (DMSO-d6): δ 1.35 (t, J = 6.9 Hz,3H,CH3), 1.91–2.61 (m, 6H, 3CH2), 3.28 (s, 3H, CH3), 4.37 (q, J = 7.1 Hz, 2H, CH2), 7.22–8.28 (m, 7H, Ar-H), 13H-NMR (DMSO-d6): δ 13.35, 23.55, 25.90, 26.11, 38.23, 61.25, 116.32, 121.23, 125.75, 126.61, 127.67, 129.22, 135.13, 141.35, 143.43, 148.20, 148.60, 155.62, 162.20, 185.32. MS m/z (%): 452 [M + 1]+ (12), 451 [M]+ (50), 360 (15), 234 (61), 199 (36), 125 (29), 77 (100). Anal. calcd. for C23H21N3O5S (451.49): C, 61.01; H, 4.43; N, 9.54; S, 7.10. Found: C, 61.18; H, 4.69; N, 9.31; S, 7.31.
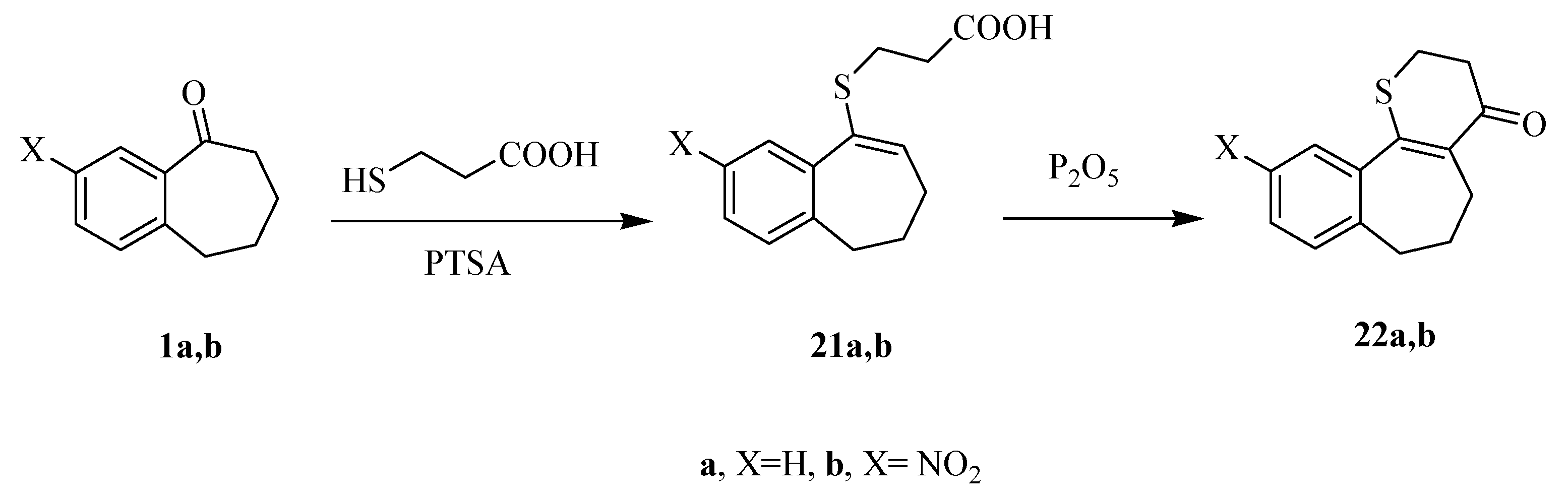
3.1.5. General Procedure for the Preparation of Compounds 21a,b
A mixture of the 1a,b (5 mmol), mercaptoacetic acid (5 mmol) and p-tolunesulfonic acid (PTSA) in dry benzene (50 mL) was refluxed for 72 h and allowed to cool to room temperature then diluted with water (30 mL). The mixture was washed with saturated NaHCO3 followed by 0.1N HCl solution. The organic layer was separated and dried over anhydrous sodium sulfate, then evaporated under reduced pressure. The solid products were collected by filtration, washed with EtOH, dried and recrystallized from the prober solvent to afford compounds 21a,b.
3-((E)-6,7-Dihydro-5H-benzo[7]annulen-9-ylthio)propanoic acid 21a. Yield: (0.21 g, 84%); mp: 193–195 °C; White crystals (MeOH). IR (KBr, cm−1): v 1722 (C=O). 1H-NMR (DMSO-d6): δ 1.65 (m, 2H, CH2), 1.96 (t, J = 6.7 Hz, 2H, CH2), 2.55–3.14 (m, 6H, 3CH2), 7.10–7.50 (m, 5H, Ar-H + CH=C), 11.5 (s, 1H, OH). MS m/z (%): 249 [M + 1]+ (25), 248 [M]+ (100), 175 (36), 143 (75). Anal. Calcd. for C14H16 O2S (248.34): C, 67.71; H, 6.49; S, 12.91. Found: C, 67.55; H, 6.33; S, 13.17.
3-((E)-6,7-Dihydro-3-nitro-5H-benzo[7]annulen-9-ylthio)propanoic acid 21b. Yield: (0.35 g, 86%); mp: 208–210 °C; pale yellow crystals (EtOH). IR (KBr, cm−1): v 1735 (C=O). 1H-NMR (DMSO-d6): δ 1.65 (m, 2H, CH2), 1.69 (t, J = 6.9 Hz, 2H, CH2), 2.55–2.90 (m, 4H, 2CH2), 3.14 (t, J = 7.0 Hz, 2H, CH2), 7.33–8.50 (m, 4H, Ar-H + CH=C), 12.3 (s, 1H, OH). MS m/z (%): 294[M + 1]+ (25), 293[M]+ (100), 220 (70), 188 (40), 143 (36). Anal. Calcd. for C14H15NO4S (293.34): C, 57.32; H, 5.15; N, 477; S, 10.93. Found: C, 57.44; H, 5.04; N, 4.87; S, 11.16.
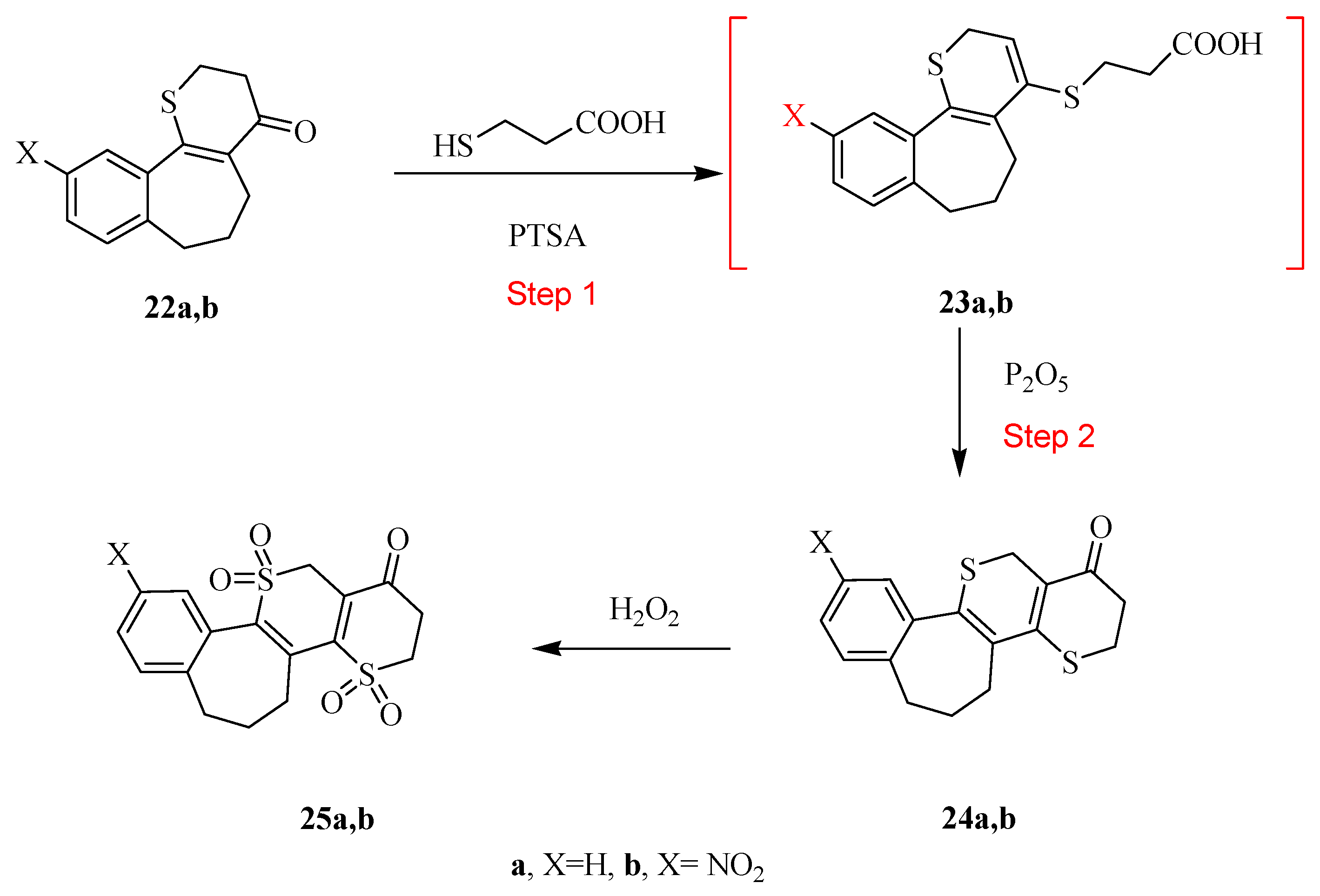
3.1.6. General Procedure for the Preparation of Compounds 22a,b
To a solution of the appropriate mercaptopropanoic acid derivatives 21a,b in benzene (thiophene free) (100 mL), phosphorus pentoxide (1 mmol) was added. The resulting mixture was refluxed for 72 h and allowed to cool to room temperature then diluted with water (30 mL), and washed with a saturated NaHCO3 solution, then we separated the water and HCl was added. The solid products that formed were collected by filtration, washed with water, dried, and recrystallized from the ethanol to afford the newly tricyclic compounds 22a,b.
2,3-Dihydrothiopyran-4-one[b,f]-benzo[7]-9,10,11-trihydroanulene 22a. Yield: (0.20 g, 86%); mp: 210–212 °C; buff powder (EtOH). IR (KBr, cm−1): v 1695 (C=O). 1H-NMR (DMSO-d6): δ 2.05 (m, 2H, CH2), 2.26–2.50 (m, 4H, 2CH2), 2.80 (m, 2H, CH2), 3.41 (m, 2H, CH2), 7.01–7.50 (m, 4H, Ar-H).MS m/z (%): 231 [M + 1]+ (25), 230 [M]+ (100), 214 (36), 142 (75). Anal. Calcd. for C14H14OS (230.33): C, 73.01; H, 6.13; S, 13.92. Found: C, 72.79; H, 5.95; S, 14.26.
2,3-Dihydrothiopyran-4-one[b,f]-3-nitrobenzo[7]9,10,11-trihydroanulene 22b. Yield: (0.23 g, 83%); mp: 227–230 °C; gray crystals (Dioxane). IR (KBr, cm−1): v 1683 (C=O). 1H-NMR (DMSO-d6): δ 2.15–2.70 (m, 6H, 3CH2), 3.25 (m, 2H, CH2), 4.07 (t, J = 7.0 Hz, 2H, CH2), 7.71–8.47 (m, 3H, Ar-H).). 13H-NMR (DMSO-d6): δ 23.50, 25.9, 28.80, 39.20, 38.50, 120.40, 122.50, 123.99, 130.60, 135.55, 145.01, 148.60, 151.7, 198.90. MS m/z (%): 276 [M + 1]+ (23), 275 [M]+ (100), 219 (70), 186 (40), 115 (36), 63 (29). Anal. calcd. for C14H13NO3S (275.32): C, 61.07; H, 4.76; N, 5.09; S, 11.65. Found: C, 60.93; H, 4.63; N, 5.21; S, 11.89.
3.1.7. General Procedure for the Preparation of Compounds 24a,b
A mixture of the compounds 22a and/or 22b (2 mmol), mercaptoacetic acid (2 mmol) and PTSA (2 mmol) in dry benzene (100 mL) was refluxed for 24 h and allowed to cool to room temperature. Phosphorus pentoxide (1 mmol) was then added and the resulting mixture was refluxed for additional 5 h and allowed to cool. The mixture was then diluted with water (30 mL), washed by saturated NaHCO3 and 0.1N HCl. The organic layer was separated and dried over anhydrous sodium sulfate, then evaporated under reduced pressure. The solid products that formed were collected by filtration, washed with water, dried, and recrystallized from the prober solvent to afford compounds 24a,b.
2,3-Dihydrothiopyrano[3,2-b]thiopyran-4(8H)-one[d,f]benzo[7]anulen 24a. Yield: (0.23 g, 86%); mp: 280–282 °C; white crystal (EtOH/Dioxan). IR (KBr, cm−1): v 1692 (C=O). 1H-NMR (DMSO-d6): δ 2.25–2.50 (m, 6H, 3CH2), 3.25–3.38 (m, 4H, 2CH2), 3.73 (s, 2H, CH2), 7.30–7.57 (m, 4H, Ar-H). 13H-NMR (DMSO-d6): δ 24.1, 24.9, 35.50, 38.40, 38.90, 126.00, 126.7, 128.01, 128.7, 132.0, 135.17, 138.99, 196.03.MS m/z (%): 301 [M + 1]+ (23), 300 [M]+ (100), 211 (50), 142 (35). Anal. calcd. for C17H16OS2 (300.44): C, 67.96; H, 5.37; S, 21.35. Found: C, 67.84; H, 5.26; S, 21.66.
2,3-Dihydrothiopyrano[3,2-b]thiopyran-4(8H)-one[d,f]-3-nitrobenzo[7]anulene 24b. Yield: (0.31 g, 90%); mp: >300 °C; brown crystals (EtOH/DMF). IR (KBr, cm−1): v 1698 (C=O). 1H-NMR (DMSO-d6): δ 2.30–2.78 (m, 6H, 3CH2), 3.27 (t, J = 6.4 Hz, 2H, CH2), 3.33 (t, J = 6.8 Hz, 2H, CH2), 3.89 (s, 2H, CH2), 7.64–8.37 (m, 3H, Ar-H). MS m/z (%): 346 [M + 1]+ (19), 345 [M]+ (100), 299 (15), 257 (40), 209 (20), 60 (25). Anal. Calcd. for C17H15NO3S2 (345.44): C, 59.11; H, 4.38; N, 4.05; S, 18.56. Found: C, 58.97; H, 4.26; N, 4.15; S, 18.86.
3.1.8. General Procedure for the Preparation of Compounds 25a,b
A mixture of the compound 24a and/or 24b (1 mmol) and hydrogen peroxide (2 mmol) was refluxed for 24 h and allowed to cool to room temperature then diluted with water (30 mL). The solid products that formed were collected by filtration, washed with water, dried and recrystallized from the ethanol to afford compounds 25a,b.
2,3-Dihydro-dioxothiopyrano[3,2-b]dioxothiopyran-4(8H)-one[d,f]benzo[7]anulen 25a. Yield (0.23 g, 65%); mp: > 300 °C; gray crystals (Dioxan). IR (KBr, cm−1): v 1689 (C=O). 1H-NMR (DMSO-d6): δ 1.76 (m, 2H, CH2), 2.15–2.30 (m, 4H, 2CH2), 2.63 (s, 2H, CH2), 3.27–3.52 (m, 4H, 2CH2), 7.01–7.47 (m, 4H, Ar-H). MS m/z (%): 366 [M + 2]+ (3), 364 [M]+ (25), 246 (30), 142 (70), 115 (100). Anal. Calcd. for C17H16O5S2 (364.44): C, 56.03; H, 4.43; S, 17.60. Found: C, 55.76; H, 4.28; S, 18.04.
2,3-Dihydro-dioxothiopyrano[3,2-b]dioxothiopyran-4(8H)-one[d,f]-3-nitrobenzo[7]anulene 25b. Yield (0.30 g, 75%); mp: > 300 °C; buff crystals (Dioxan). IR (KBr, cm−1): v 1695 (C=O). 1H-NMR (DMSO-d6): δ 1.90 (m, 2H, CH2), 2.15–2.30 (m, 4H, 2CH2), 2.53 (s, 2H, CH2), 3.27–4.09 (m, 4H, 2CH2), 7.71–8.47 (m, 3H, Ar-H). MS m/z (%): 410 [M + 1]+ (10), 409 [M]+ (100), 289 (15), 187 (40). Anal. Calcd. for C17H15NO7S2 (409.43): C, 49.87; H, 3.69; N, 3.42; S, 15.66. Found: C, 49.63; H, 3.57; N, 3.52; S, 15.89.