Design, Synthesis and in Vivo Evaluation of Novel Glycosylated Sulfonylureas as Antihyperglycemic Agents
Abstract
:1. Introduction
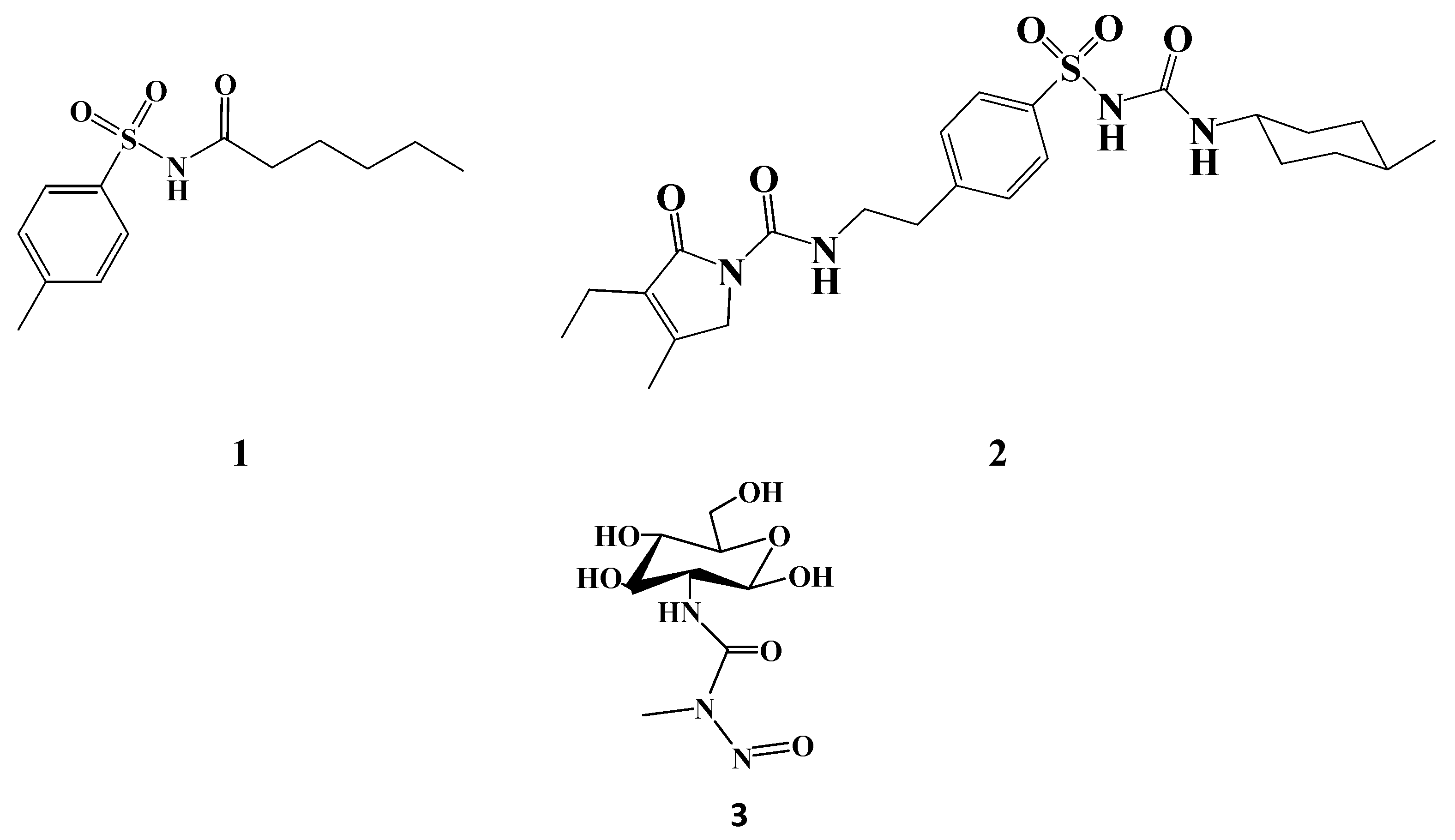
2. Results and Discussion
2.1. Chemistry
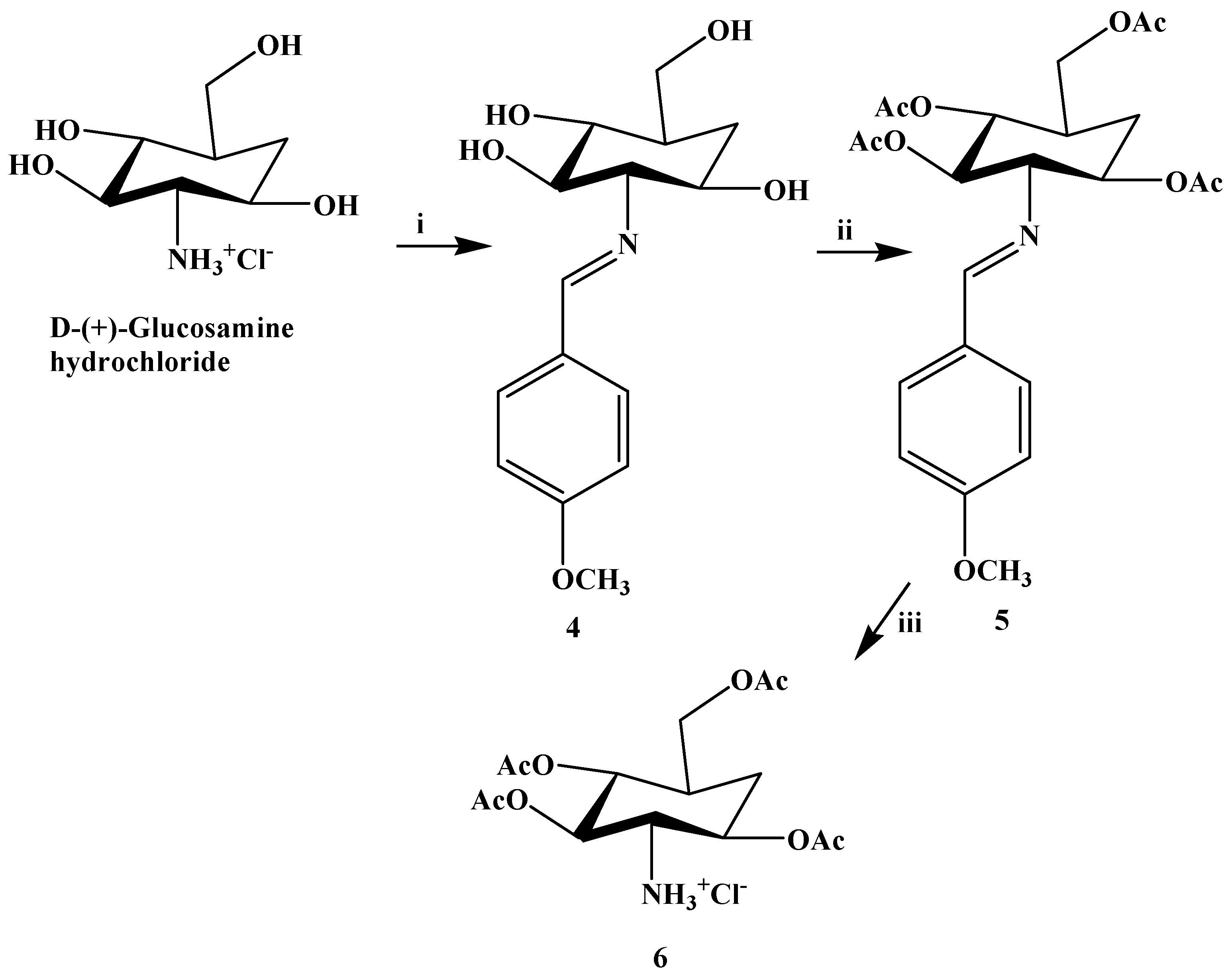

2.2. In Vivo Evaluation
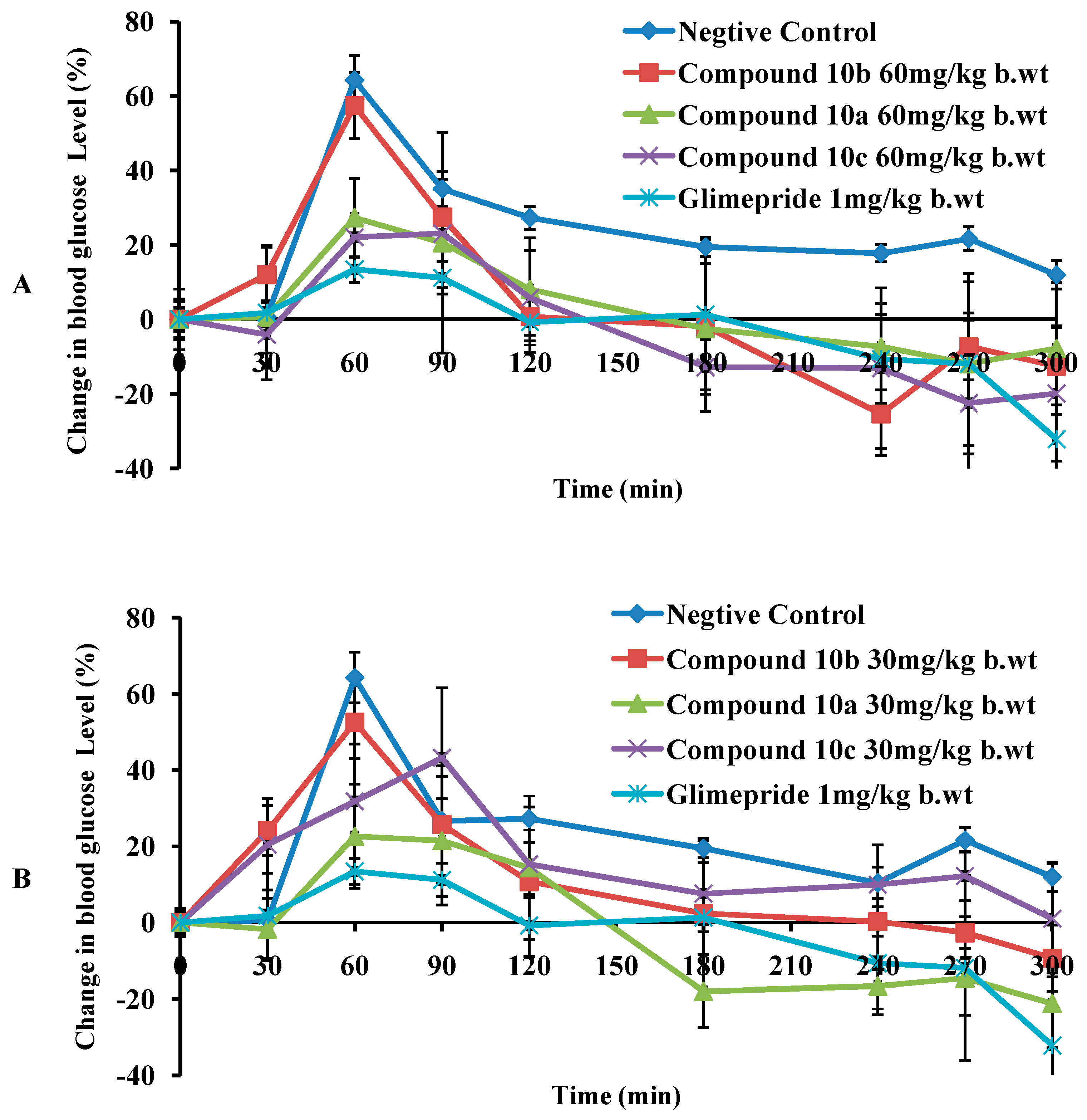
| Compound (Dose) | Time (min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 180 | 240 | 270 | 300 | ||
| A | 10a (60 mg/kg b.wt) | - | - | - | - | - | - | ** | - | - |
| 10a (30 mg/kg b.wt) | - | - | - | - | - | * | * | * | ** | |
| 10b (60 mg/kg b.wt) | - | - | - | - | ** | ** | *** | ** | * | |
| 10b (30 mg/kg b.wt) | - | - | - | - | ** | ** | ** | * | * | |
| 10b (7.5 mg/kg b.wt) | - | - | - | - | * | ** | - | - | ||
| 10c (60 mg/kg b.wt) | - | - | - | - | - | * | ** | * | - | |
| 10c (30 mg/kg b.wt) | - | - | - | - | * | - | - | - | - | |
| Glimepiride 1 mg/kg b.wt | - | *** | - | - | - | - | - | - | * | |
| B | 10a (60 mg/kg b.wt) | - | - | * | - | * | - | * | * | - |
| 10a (30 mg/kg b.wt) | - | - | * | - | - | *** | * | - | ** | |
| 10b (60 mg/kg b.wt) | - | - | * | - | * | * | * | * | - | |
| 10b (30 mg/kg b.wt) | - | - | - | - | * | ** | * | * | - | |
| 10b (7.5 mg/kg b.wt) | - | - | - | - | * | ** | * | - | - | |
| 10c (60 mg/kg b.wt) | - | - | - | - | - | ** | - | - | - | |
| 10c (30 mg/kg b.wt) | - | - | - | - | * | ** | * | - | - | |
Diabetic Mice (Group B)
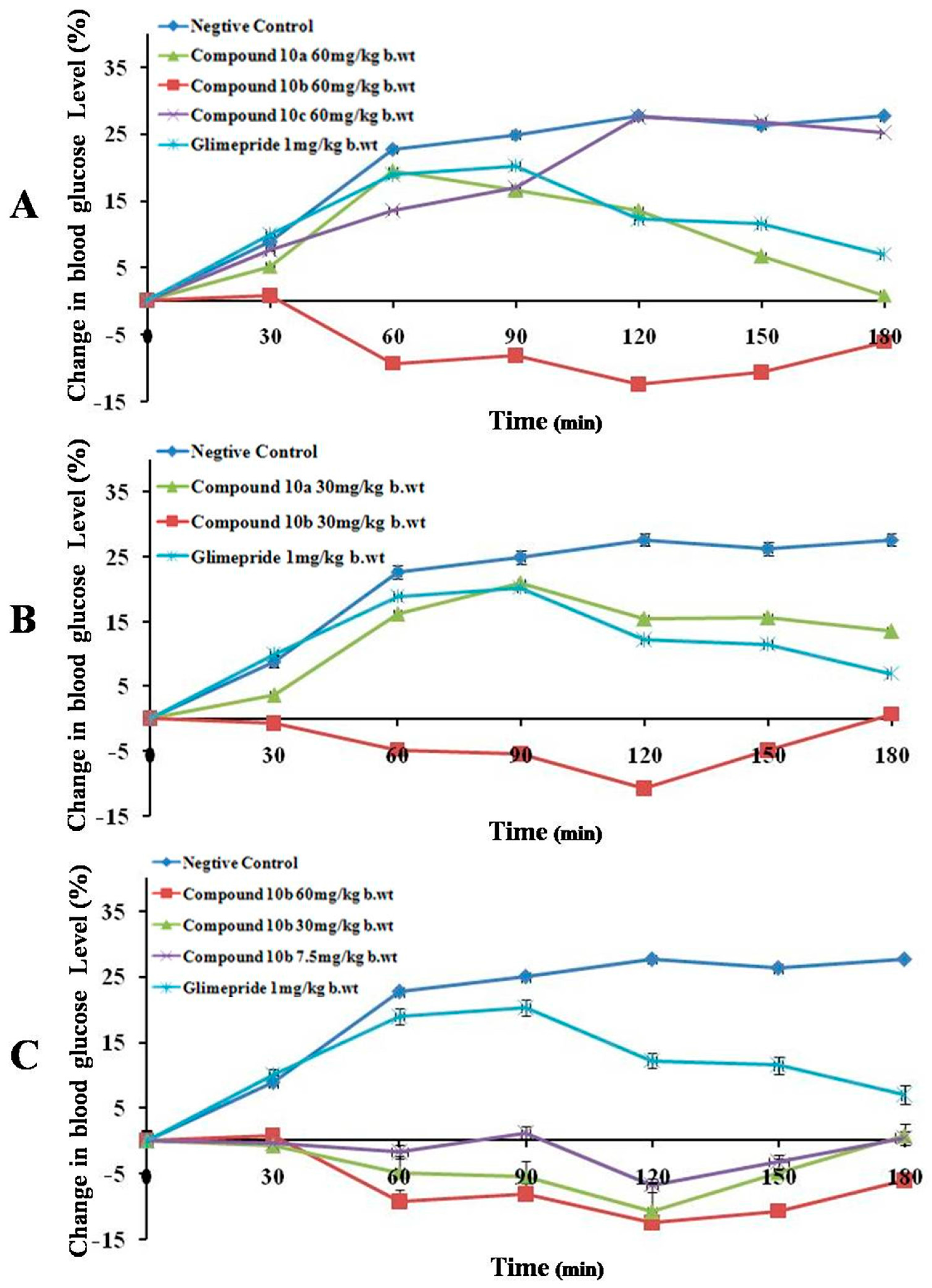
| Compound (Dose) | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | ||
| A | 10a (60 mg/kg b.wt) | *** | *** | *** | *** | *** | *** | *** |
| 10a (30 mg/kg b.wt) | - | - | - | - | - | - | - | |
| 10b(60 mg/kg b.wt) | *** | *** | *** | *** | *** | *** | *** | |
| 10b (30 mg/kg b.wt) | - | * | *** | *** | *** | *** | *** | |
| 10b (7.5 mg/kg b.wt) | - | - | ** | *** | *** | ** | ** | |
| 10c (60 mg/kg b.wt) | - | - | ** | ** | *** | *** | *** | |
| Glimepiride 1 mg/kg b.wt | ** | ** | ** | ** | *** | *** | *** | |
| B | 10a (60 mg/kg b.wt) | - | - | - | - | - | - | - |
| 10a (30 mg/kg b.wt) | - | * | * | * | * | * | - | |
| 10b (60 mg/kg b.wt) | - | - | - | - | - | - | - | |
| 10b (30 mg/kg b.wt) | - | - | - | - | - | - | - | |
| 10b (7.5 mg/kg b.wt) | - | - | - | - | - | - | - | |
| 10c (60 mg/kg b.wt) | - | - | - | - | - | - | - | |
3. Experimental Section
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of N-(Arylsulfonyl)carbamoylides 8a–c
3.2.2. Yields, Melting Points, Analytical and Spectroscopic Data of 8a–c
3.2.3. General Procedure for the Preparation of Arylsulfonylureates 9a–c
3.2.4. General Procedure for Deacetylationof Compounds 10a–c
3.3. In Vivo Testing
3.3.1. Chemicals
3.3.2. Animals
3.3.3. Oral Glucose Tolerance Test
3.3.4. Induction of Diabetes in Mice
3.3.5. Blood Collection and Determination of Blood Glucose
3.3.6. Statistical Analysis
3.4. High Performance Liquid Chromatography (HPLC)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yeap, S.K.; Liang, W.S.; Beh, B.K.; Ho, W.Y.; Yousr, A.N.; Alitheen, N.B. In vivo antidiabetic and acute toxicity of spray-dried Vernonia amygdalina water extract. Int. Food Res. J. 2013, 20, 613–616. [Google Scholar]
- World Health Organization. Global Health Estimates: Deaths by Cause, Age, Sex and Country, 2000–2012. Geneva, WHO, 2014; Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 27 October 2015).
- Global Status Report on Non Communicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/nmh/publications/ncd_report2010/en/ (accessed on 23 October 2015).
- Zimmet, P.; Alberti, K.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A.; Bonadonna, R.C.; Ferrannini, E. Pathogenesis of NIDDM-a balanced overview. Diabetes Care 1992, 15, 318–368. [Google Scholar] [CrossRef] [PubMed]
- Rendell, M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs 2004, 64, 1339–1358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Verma, D. Antidiabetic andantihyperlipidemic effect of Morinda citrofolia and Coccinia indica in alloxan induced diabetic rats. Pharmacologyonline 2011, 2, 307–311. [Google Scholar]
- Kasiviswanath, R.; Ramesh, A.; Kumar, K.E. Hypoglycemic and antihyperglycemic effect of Gmelina asiatica Linn. in normal and in alloxan induced diabetic rats. Biol. Pharm. Bull. 2005, 28, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.D. Glyburide and glipizide, second-generation oral sulfonylurea hypoglycemic agents. Clin. Pharm. 1984, 3, 473–485. [Google Scholar] [PubMed]
- Del Prato, S.; Pulizzi, N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism 2006, 55, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Shammi, G.; Jitendra, K.R.; Narang, R.K.; Rajesh, K.S. Sulfonyl ureas for antidiabetic therapy, an overview for glipizide. Int. J. Pharm. Pharm. Sci. 2010, 2, 1–6. [Google Scholar]
- Hosseinzadeh, N.; Seraj, S.; Bakhshi-Dezffoli, M.E.; Hasani, M.; Khoshneviszadeh, M.; Fallah-Bonekohal, S.; Abdollahi, M.; Foroumadi, A.; Shafiee, A. Synthesis and antidiabetic evaluation of benzenesulfonamide derivatives. Iran. J. Pharm. Res. 2013, 12, 325–330. [Google Scholar] [PubMed]
- Faidallah, H.M.; Khan, K.A. Synthesis and biological evaluation of new barbituric and thiobarbituric acid fluoro analogs of benzenesulfonamides as antidiabetic and antibacterial agents. J. Fluor. Chem. 2012, 142, 96–104. [Google Scholar] [CrossRef]
- Mariappan, G.; Saha, B.P.; Datta, S.; Kumar, D.; Haldar, P.K. Design, synthesis and antidiabetic evaluation of oxazolone derivatives. J. Chem. Sci. 2011, 123, 335–341. [Google Scholar] [CrossRef]
- Iqbal, Z.; Hameed, S.; Ali, S.; Tehseen, Y.; Shahid, M.; Iqbal, J. Synthesis, characterization, hypoglycemic and aldose reductase inhibition activity of arylsulfonylspiro[fluorene-9,5′-imidazolidine]-“2′,4′-diones. Eur. J. Med. Chem. 2015, 98, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Shafi, S.; Ali, Y.; Alam, P.; Pasha, M.A.; Dhulap, A.; Bano, S.; et al. Novel benzenesulfonylureas containing thiophenylpyrazoline moiety as potential antidiabetic and anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 5298–5303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Zhang, Y.A.; Wu, G.Z.; Zhou, J.P.; Huang, W.L.; Hu, X.W. Synthesis and biological evaluation of sulfonylurea and thiourea derivatives substituted with benzenesulfonamide groups as potential hypoglycemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Wargo, K.A.; Banta, W.M. A comprehensive review of the loop diuretics: Should furosemide be first line? Ann. Pharmacother. 2009, 43, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.C.; Liu, X.H.; Li, Y.H.; Wang, S.H.; Li, Z.M. Synthesis and herbicidal activity of novel sulfonylureas containing thiadiazol moiety. Chem. Res. Chin. Univ. 2008, 24, 32–35. [Google Scholar] [CrossRef]
- Sohn, H.; Lee, K.S.; Ko, Y.K.; Ryu, J.W.; Woo, J.C.; Koo, D.W.; Shin, S.J.; Ahn, S.J.; Shin, A.R.; Song, C.H.; et al. In vitro and ex vivo activity of new derivatives of acetohydroxyacid synthase inhibitors against Mycobacterium tuberculosis and non-tuberculous mycobacteria. Int. J. Antimicrob. Agents 2008, 31, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Saczewski, F.; Kuchnio, A.; Samsel, M.; Lobocka, M.; Kiedrowska, A.; Lisewska, K.; Saczewski, J.; Gdaniec, M.; Bednarski, P.J. Synthesis of novel aryl(heteroaryl)sulfonyl ureas of possible biological interest. Molecules 2010, 15, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, R.; Marucchini, C.; Orena, M.; Zadra, C. Molecular structure and stereoelectronic properties of herbicide sulphonylureas. Bioorg. Med. Chem. 2002, 10, 1019–1024. [Google Scholar] [CrossRef]
- Rostom, S.A. Synthesis and in vitro antitumor evaluation of some indeno[1,2-c]pyrazol(in)es substituted with sulfonamide, sulfonylurea(-thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg. Med. Chem. 2006, 14, 6475–6485. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.; Rodrigues, J.; Gamboa de Dominguez, N.; Charris, J.; Gut, J.; Rosenthal, P.J.; Dominguez, J.N. Synthesis and evaluation of sulfonylurea derivatives as novel antimalarials. Eur. J. Med. Chem. 2007, 42, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Mastrolorenzo, A.; Scozzafava, A.; Supuran, C.T. Antifungal activity of silver and zinc complexes of sulfa drug derivatives incorporating arylsulfonylureido moieties. Eur. J. Pharm. Sci. 2000, 11, 99–107. [Google Scholar] [CrossRef]
- Szafrański, K.; Sławiński, J. Synthesis of novel 1-(4-substituted pyridine-3-sulfonyl)-3-phenylureas with potential anticancer activity. Molecules 2015, 20, 12029–12044. [Google Scholar] [CrossRef] [PubMed]
- Sławiński, J.; Szafrański, K.; Vullo, D.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Synthesis of Heterocyclic 4-Substituted Pyridine-3-Sulfonamide Derivatives and Their Inhibition of the Human Cytosolic Isozymes I and II and Transmembrane Tumor-Associated Isozymes IX and XII. Eur. J. Med. Chem. 2013, 69, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Vavra, J.J.; Deboer, C.; Dietz, A.; Hanka, L.J.; Sokolski, W.T. Streptozotocin, a new antibacterial antibiotic. Antibiot. Annu. 1958, 7, 230–235. [Google Scholar]
- Pathak, S.; Dorfmueller, H.C.; Borodkin, V.S.; van Aalten, D.M.F. Chemical dissection of the link between streptozotocin, O-Glcnac, and pancreatic cell death. Chem. Biol. 2008, 15, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.; Saltz, L. Islet cell tumors of the pancreas—The medical oncologist’s perspective. Surg. Clin. N. Am. 2001, 81, 527–542. [Google Scholar] [CrossRef]
- Ran, C.; Pantazopoulos, P.; Medarova, Z.; Moore, A. Synthesis and testing of beta-cell-specific streptozotocin-derived near-infrared imaging probes. Angew. Chem. Int. Ed. 2007, 46, 8998–9001. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.B. N-acetylglucosamine (GlcNAc) functions in cell signaling. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef] [PubMed]
- King, C. Some reactions of p-toluenesulfonyl isocyanate. J. Org. Chem. 1960, 25, 352–356. [Google Scholar] [CrossRef]
- Irie, H.; Nishimura, M.; Yoshida, M.; Ibuka, T. Lewis acid catalysed preparation of some carbamates and sulphonylureas. Application to the determination of enantiomeric purity of chiral alcohols. J. Chem. Soc. Perkin Trans. 1989, 1, 1209–1210. [Google Scholar] [CrossRef]
- Cervelló, J.; Sastre, T. An improved method for the synthesis of sulfonylureas. Synthesis 1990, 1990, 221–222. [Google Scholar] [CrossRef]
- Andreani, A.; Rambaldi, M.; Leoni, A.; Locatelli, A.; Andreani, F.; Gehret, J.C. Synthesis of imidazo[2,1-b]thiazoles as herbicides. Pharm. Acta Helv. 1996, 71, 247–252. [Google Scholar] [CrossRef]
- McKay, M.J.; Nguyen, H.M. Recent developments in glycosyl urea synthesis. Carbohydr. Res. 2014, 385, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Saczewski, F.; Kornicka, A.; Brzozowski, Z. 4-dimethylaminopyridinium carbamoylides as stable and non-hazardous substitutes of arylsulfonyl and heteroaryl isocyanates. Green Chem. 2006, 8, 647–656. [Google Scholar] [CrossRef]
- Abbas, M.A.; Hameed, S.; Farman, M.; Kressler, J.; Mahmood, N. Conjugates of degraded and oxidized hydroxyethyl starch and sulfonylureas: Synthesis, characterization, and in vivo antidiabetic activity. Bioconjugate Chem. 2015, 26, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Strukil, V.; Mottillo, C.; Friscic, T. Mechanosynthesis of pharmaceutically relevant sulfonyl-(thio)ureas. Chem. Commun. 2014, 50, 5248–5250. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Iwai, T.; Ishino, Y. Solvent-assisted thiocarboxylation of amines and alcohols with carbon monoxide and sulfur under mild conditions. Tetrahedron 2005, 61, 9157–9163. [Google Scholar] [CrossRef]
- Sonoda, N.; Mizuno, T.; Murakami, S.; Kondo, K.; Ogawa, A.; Ryu, I.; Kambe, N. Selenium-catalyzed synthesis of S-alkyl thiocarbamates from amines, carbon monoxide, sulfur, and alkyl halides. Chem. Int. Ed. Engl. 1989, 28, 452–453. [Google Scholar] [CrossRef]
- Knolker, H.J.; Braxmeier, T.; Schlechtingen, G. A novel method for the synthesis of isocyanates under mild conditions. Angew. Chem. Int. Ed. Engl. 1995, 34, 2497–2500. [Google Scholar] [CrossRef]
- Knolker, H.J.; Braxmeier, T.; Schlechtingen, G. Isocyanates, Part 2. Synthesis of symmetrical and unsymmetrical ureas by DMAP-catalyzed reaction of alkyl- and arylamines with di-tert-butyldicarbonate. Synlett 1996, 1996, 502–504. [Google Scholar] [CrossRef]
- Knolker, H.J.; Braxmeier, T. Isocyanates, Part 3. Synthesis of carbamates by DMAP-catalyzed reaction of amines with di-tert-butyldicarbonate and alcohols. Tetrahedron Lett. 1996, 37, 5861–5864. [Google Scholar] [CrossRef]
- Grzyb, J.A.; Batey, R.A. Carbamoylimidazolium salts as diversification reagents: An application to the synthesis of tertiary amides from carboxylic acids. Tetrahedron Lett. 2003, 44, 7485–7488. [Google Scholar] [CrossRef]
- Ishii, H.; Goyal, M.; Ueda, M.; Takeuchi, K.; Asai, M. Oxidative carbonylation of phenol to diphenyl carbonate catalyzed by Pd dinuclear complex bridged with pyridylphosphine ligand. J. Mol. Catal. A Chem. 1999, 148, 289–293. [Google Scholar] [CrossRef]
- Liberek, B.; Melcer, A.; Osuch, A.; Wakiec, R.; Milewski, S.; Wisniewski, A. N-alkyl derivatives of 2-amino-2-deoxy-d-glucose. Carbohydr. Res. 2005, 340, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Myszka, H.; Bednarczyk, D.; Najder, M.; Kaca, W. Synthesis and induction of apoptosis in B cell chronic leukemia by diosgenyl 2-amino-2-deoxy-β-d-glucopyranoside hydrochloride and its derivatives. Carbohydr. Res. 2003, 338, 133–141. [Google Scholar] [CrossRef]
- Sączewski, J.; Gdaniec, M. The structure and theoretical study of 4-dimethylaminopyridinium n-(arylsulfonyl)carbamoylides. J. Mol. Struct. 2009, 921, 13–17. [Google Scholar] [CrossRef]
- Fairweather, J.K.; Liu, L.; Karoli, T.; Ferro, V. Synthesis of disaccharides containing 6-deoxy-α-l-talose as potential heparan sulfate mimetics. Molecules 2012, 17, 9790–9802. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, M.; Perez, M.; Colovray-Gotteland, V.; Halazy, S. A simple one-pot preparation of N,N′-unsymmetrical ureas from N-Boc protected primary anilines and amines. Synlett 1996, 1996, 507–508. [Google Scholar] [CrossRef]
- Fowler, P.; Bernet, B.; Vasella, A. A 1H-NMR spectroscopic investigation of the conformation of the acetamido group in some derivatives of N-acetyl-d-allosamine and -d-glucosamine. Helv. Chim. Acta 1996, 79, 269–287. [Google Scholar] [CrossRef]
- Stella, J.; Krishnamoorthy, P.; Mohamed, A.J. Hypoglycemic effect of Vitex agnus castus in streptozotocin induced. Asian J. Biochem. Pharm. Res. 2011, 1, 206–212. [Google Scholar]
- Mohamed, M.S.; Ali, S.A.; Abdelaziz, D.H.; Fathallah, S.S. Synthesis and evaluation of novel pyrroles and pyrrolopyrimidines as anti-hyperglycemic agents. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Munoz, A.; Zhang, X.; Duefer, M.; Drews, G.; Krippeit-Drews, P.; Aguilar-Bryan, L. ABCC8 and ABCC9: Abc transporters that regulate K(+) channels. Pflugers Arch. 2007, 453, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Wied, S. The sulfonylurea drug, glimepiride, stimulates glucose-transport, glucose-transporter translocation, and dephosphorylation in insulin-resistant rat adipocytes in vitro. Diabetes 1993, 42, 1852–1867. [Google Scholar] [CrossRef] [PubMed]
- Bahr, M.; Vonholtey, M.; Muller, G.; Eckel, J. Direct stimulation of myocardial glucose-transport and glucose transporter-1 (GLUT1) and GLUT4 protein expression by the sulfonylurea glimepiride. Endocrinology 1995, 136, 2547–2553. [Google Scholar] [PubMed]
- Priyadarshini, L.; Mazumder, P.B.; Choudhury, M.D. Acute toxicity and oral glucosetolerance test of ethanol and methanol extracts of antihyperglycaemic plant Cassia alata Linn. IOSR J. Pharm. Biol. Sci. 2014, 9, 43–46. [Google Scholar]
- Kasabri, V.; Afifi, F.U.; Hamdan, I. In vitro and in vivo acute antihyperglycemic effects of five selected indigenous plants from Jordan used in traditional medicine. J. Ethnopharmacol. 2011, 133, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Risco, S.; Ruiz, T.; Zarzuelo, A. Hypoglycemic activity of Salvia lavandulifolia. Planta Med. 1986, 52, 260–262. [Google Scholar] [CrossRef]
- Nirupa, G.; Tripathi, U.M. RP-HPLC analytical method development and validation for simultaneous estimation of three drugs: Glimepiride, pioglitazone, and metformin and its pharmaceutical dosage forms. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 9a–c and 10a–c are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suaifan, G.A.R.Y.; Shehadeh, M.B.; Darwish, R.M.; Al-Ijel, H.; Abbate, V. Design, Synthesis and in Vivo Evaluation of Novel Glycosylated Sulfonylureas as Antihyperglycemic Agents. Molecules 2015, 20, 20063-20078. https://doi.org/10.3390/molecules201119676
Suaifan GARY, Shehadeh MB, Darwish RM, Al-Ijel H, Abbate V. Design, Synthesis and in Vivo Evaluation of Novel Glycosylated Sulfonylureas as Antihyperglycemic Agents. Molecules. 2015; 20(11):20063-20078. https://doi.org/10.3390/molecules201119676
Chicago/Turabian StyleSuaifan, Ghadeer A. R. Y., Mayadah B. Shehadeh, Rula M. Darwish, Hebah Al-Ijel, and Vincenzo Abbate. 2015. "Design, Synthesis and in Vivo Evaluation of Novel Glycosylated Sulfonylureas as Antihyperglycemic Agents" Molecules 20, no. 11: 20063-20078. https://doi.org/10.3390/molecules201119676






